Early and Delayed Impact of Nanosilver on the Glutamatergic NMDA Receptor Complex in Immature Rat Brain
Abstract
:1. Introduction
2. Results
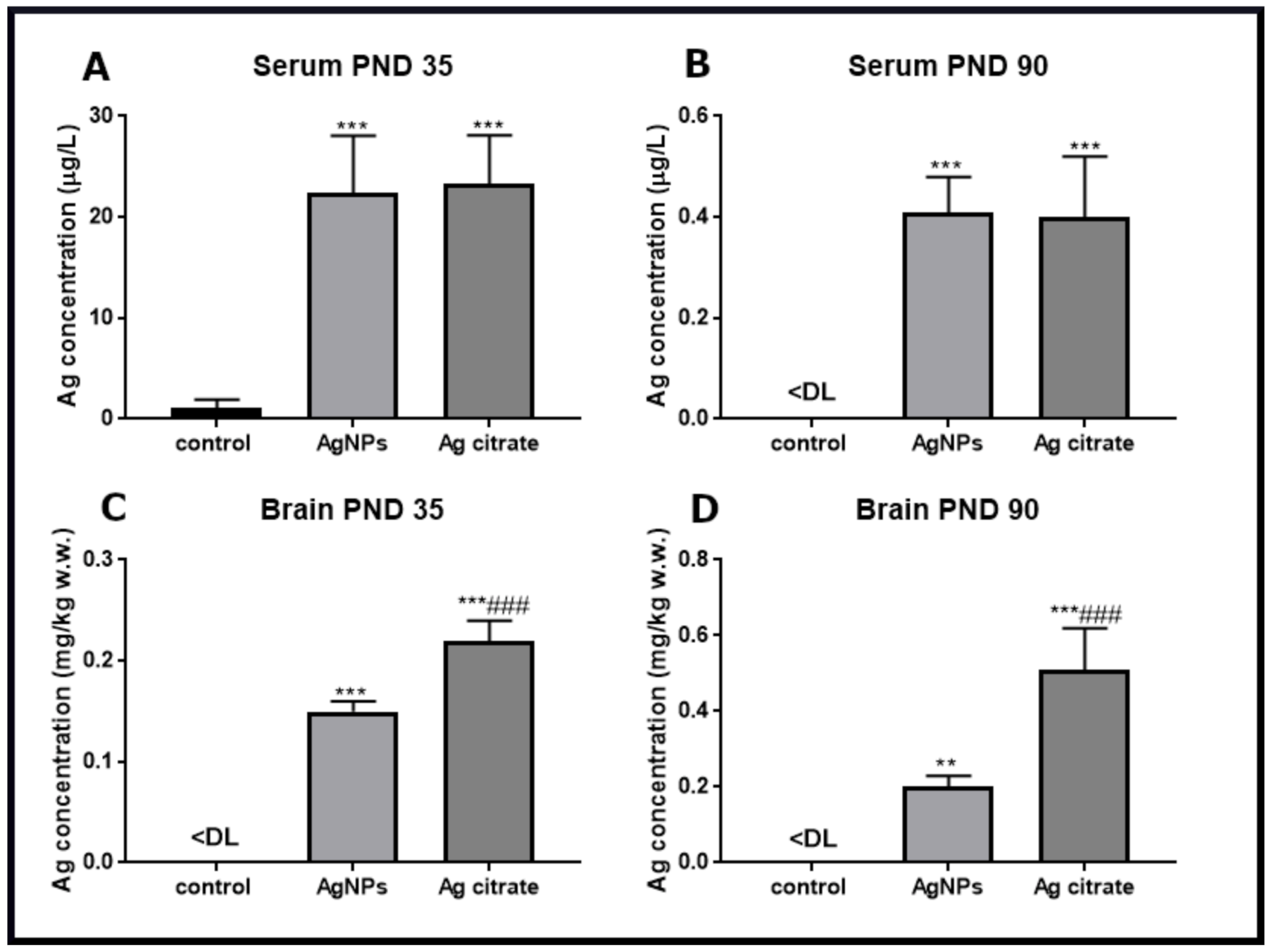
2.1. Silver Accumulates in the Brain of Exposed Rats and Induces Ultrastructural Alterations in Synapses Early after Exposure
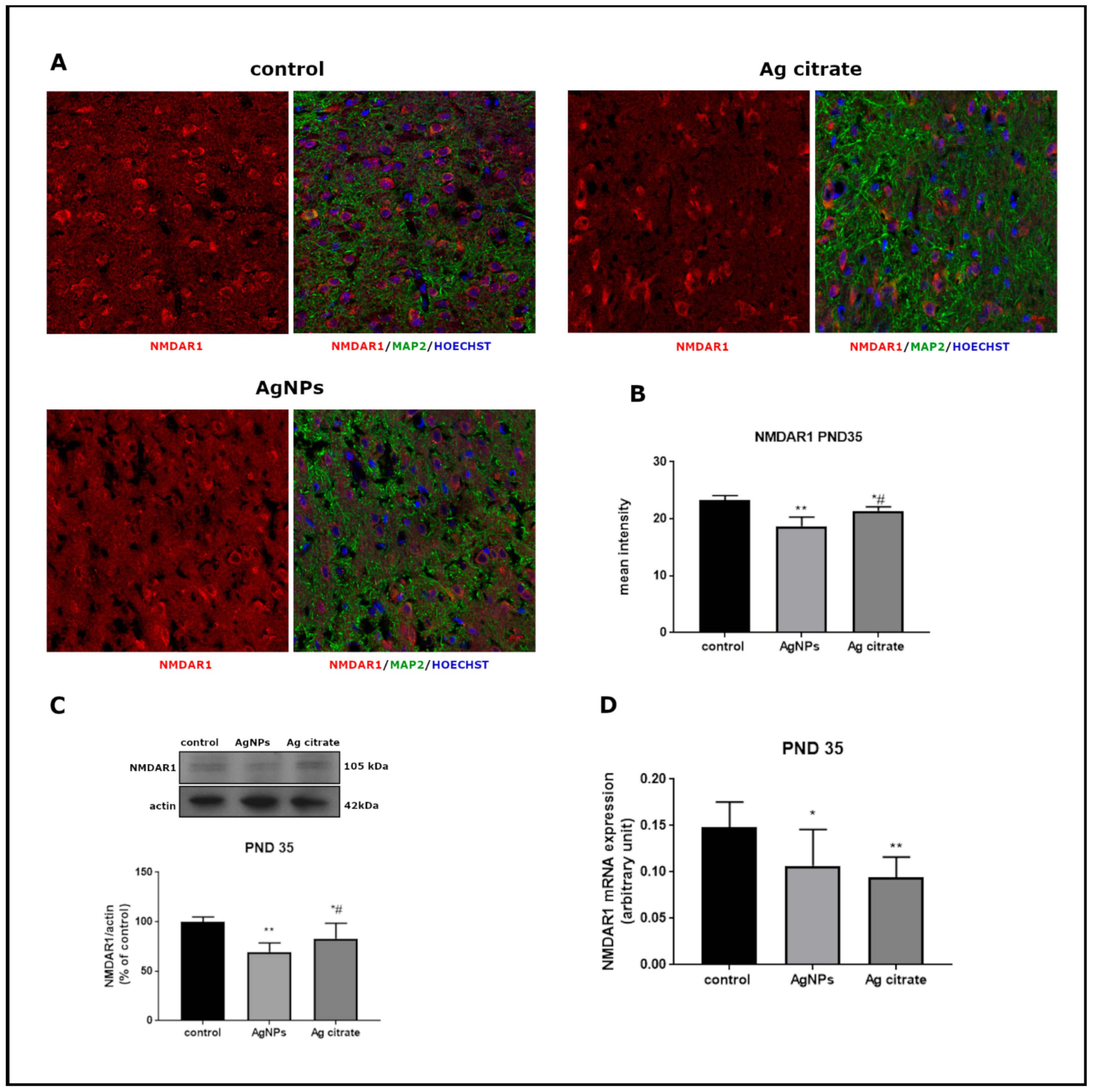
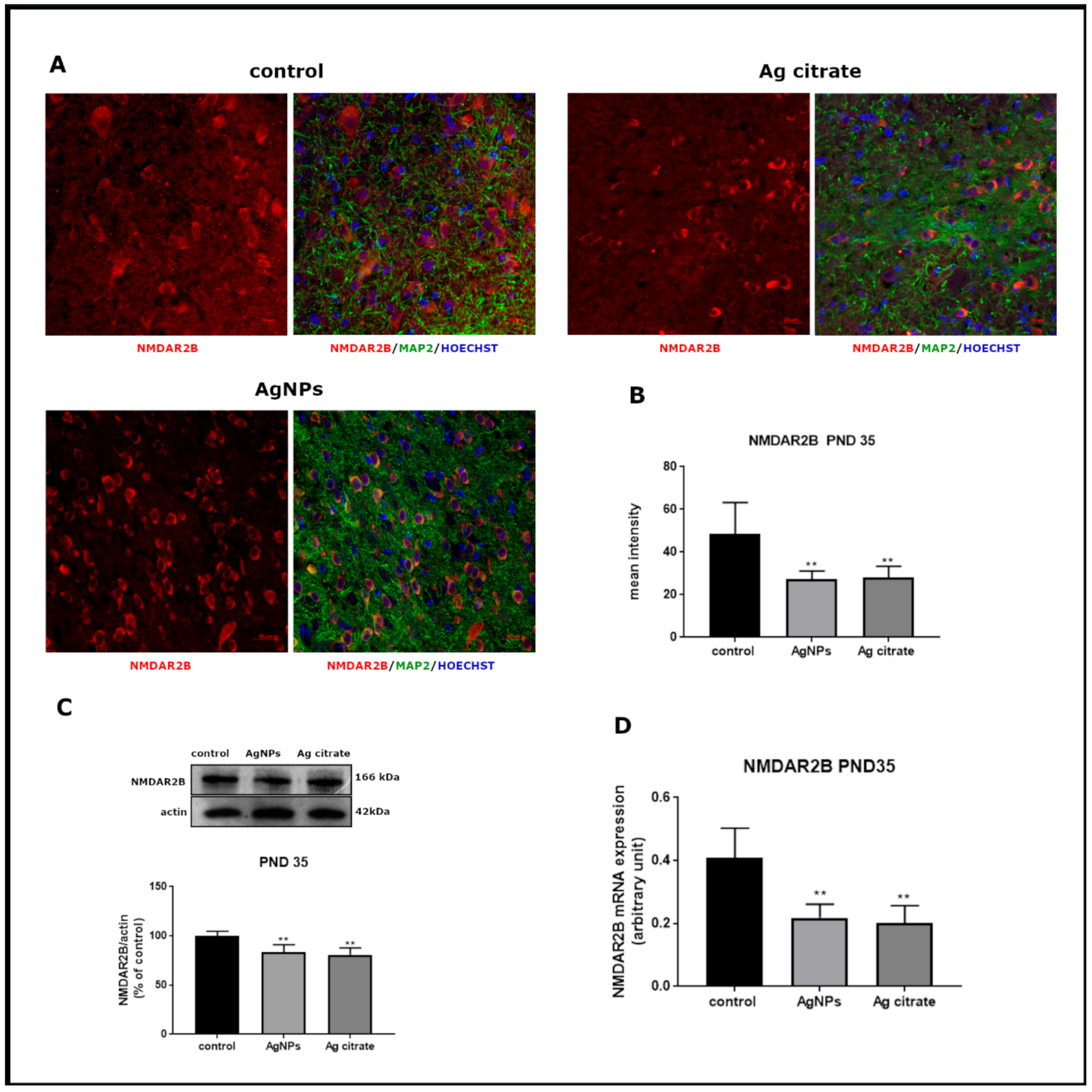
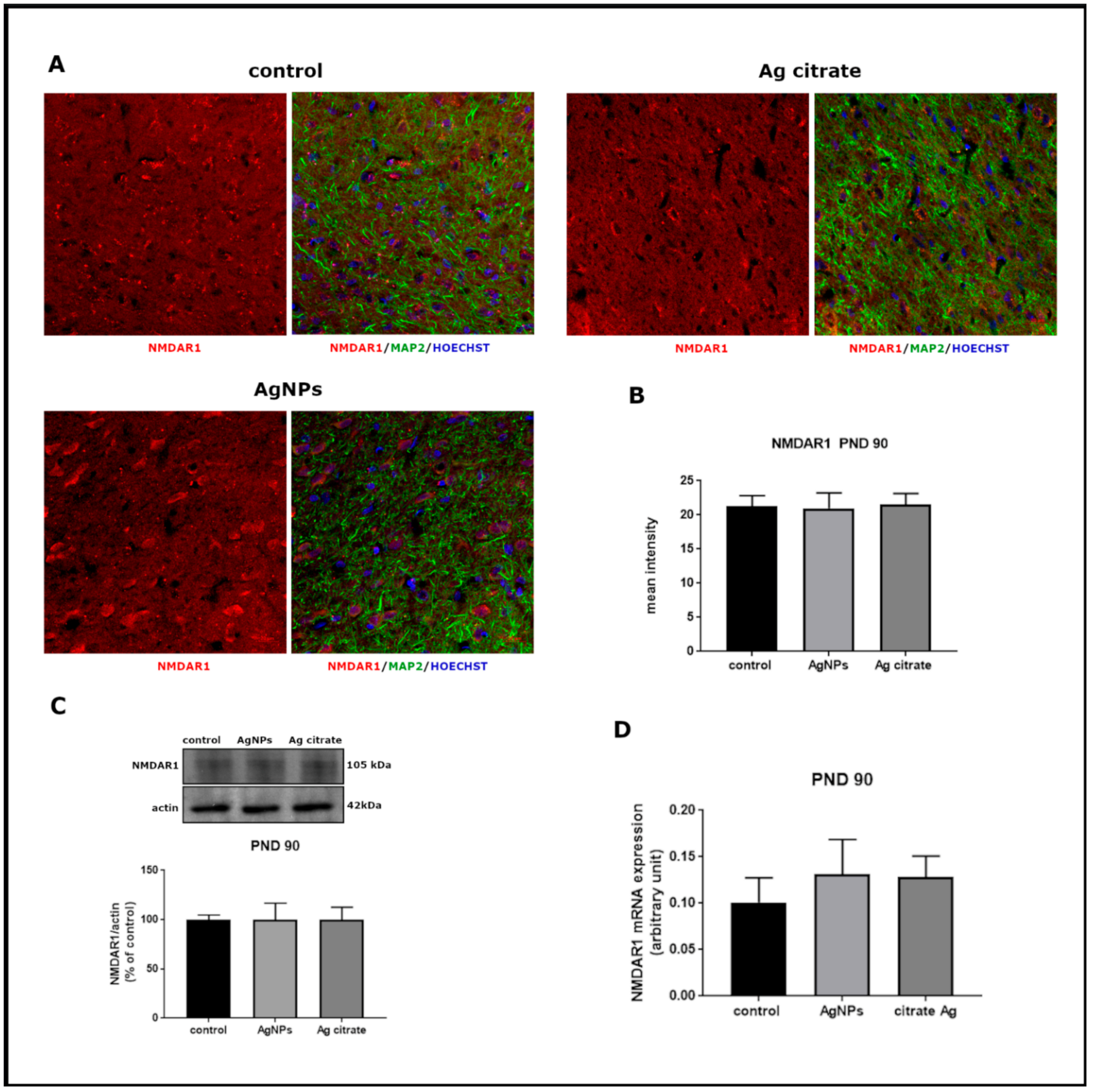
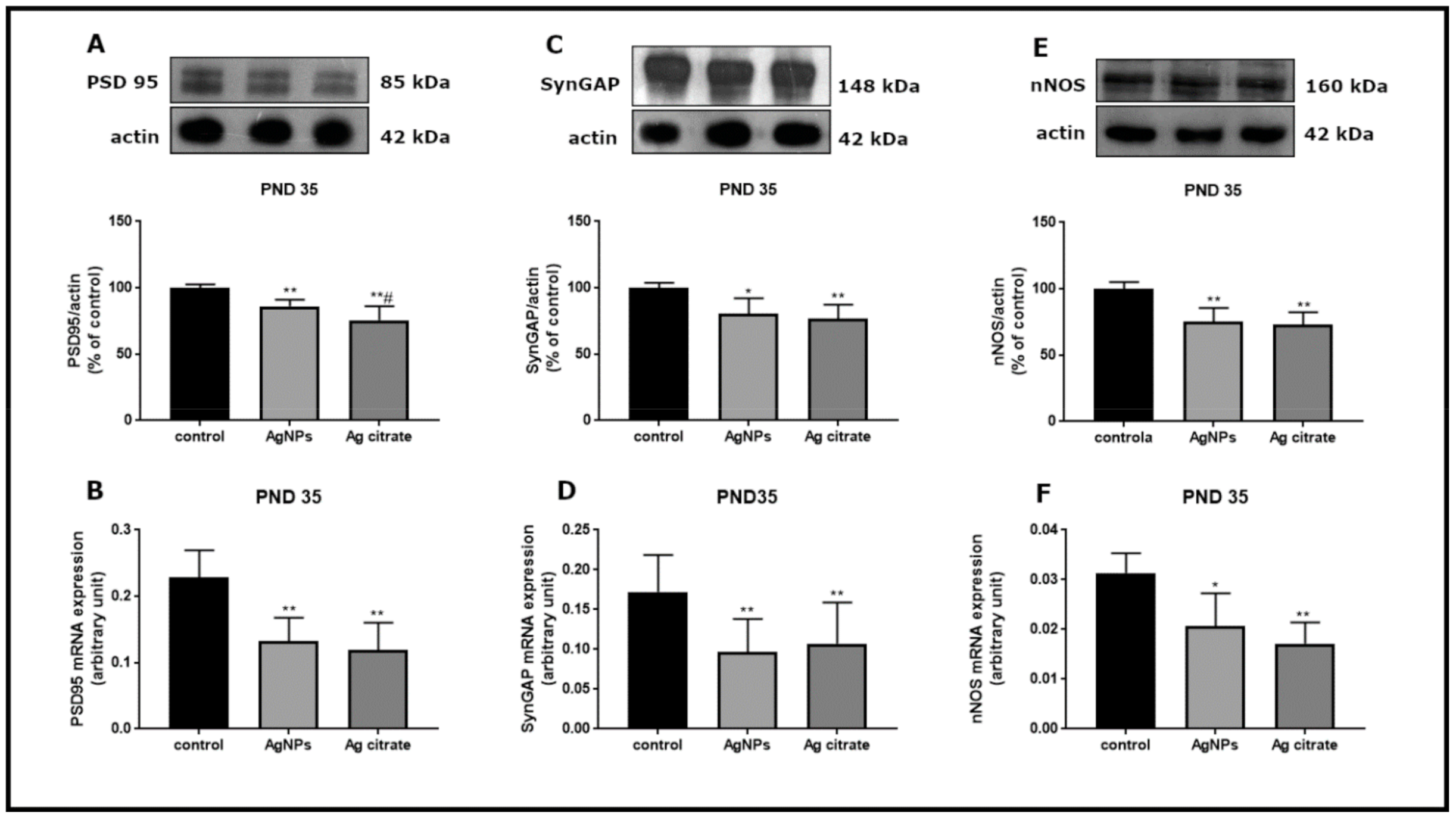
2.2. Silver Influences the Expression of Selected Proteins Related to the NMDA Receptor Complex
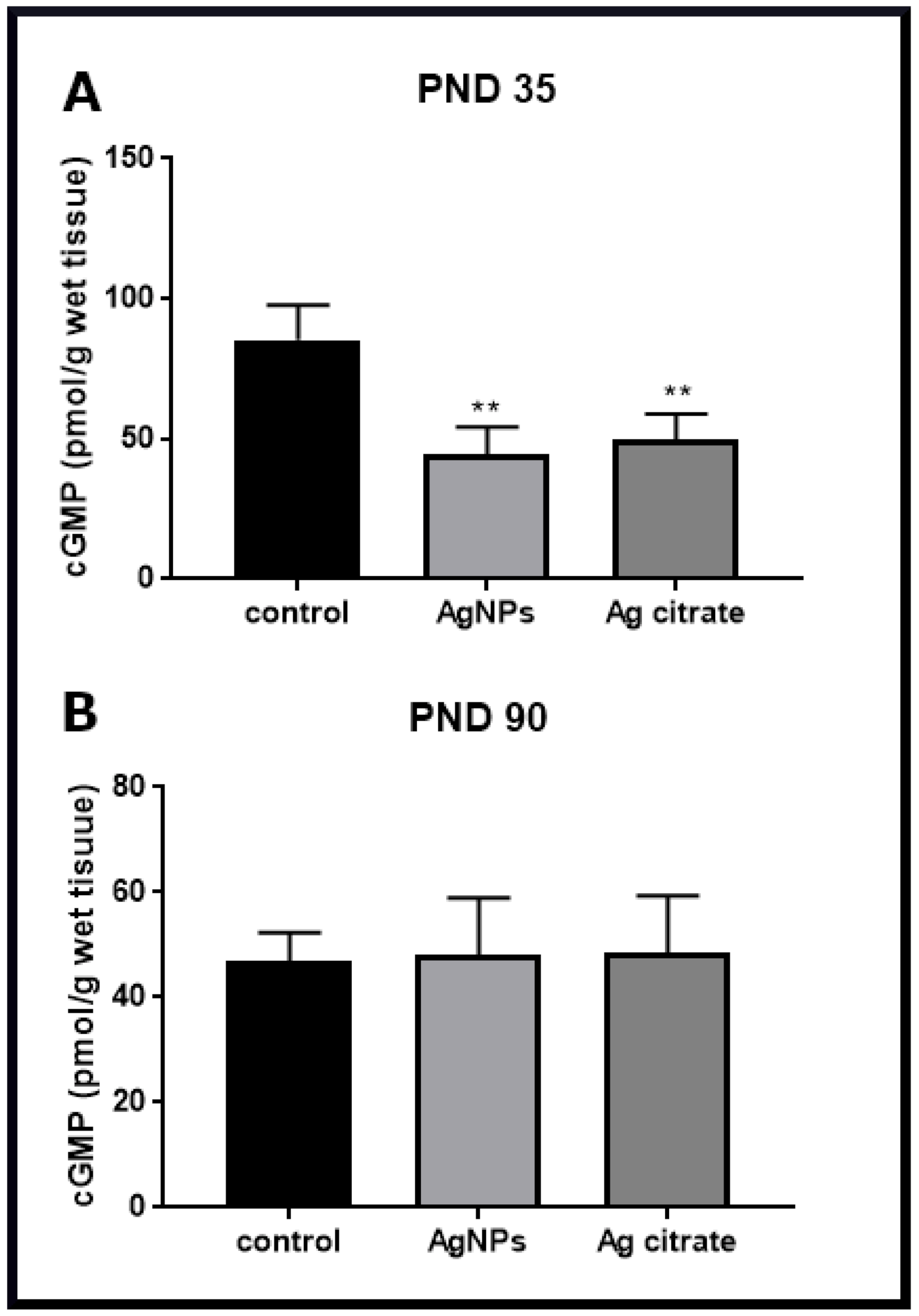
2.3. Silver Down-Regulates the nNOS-NO-cGMP Pathway
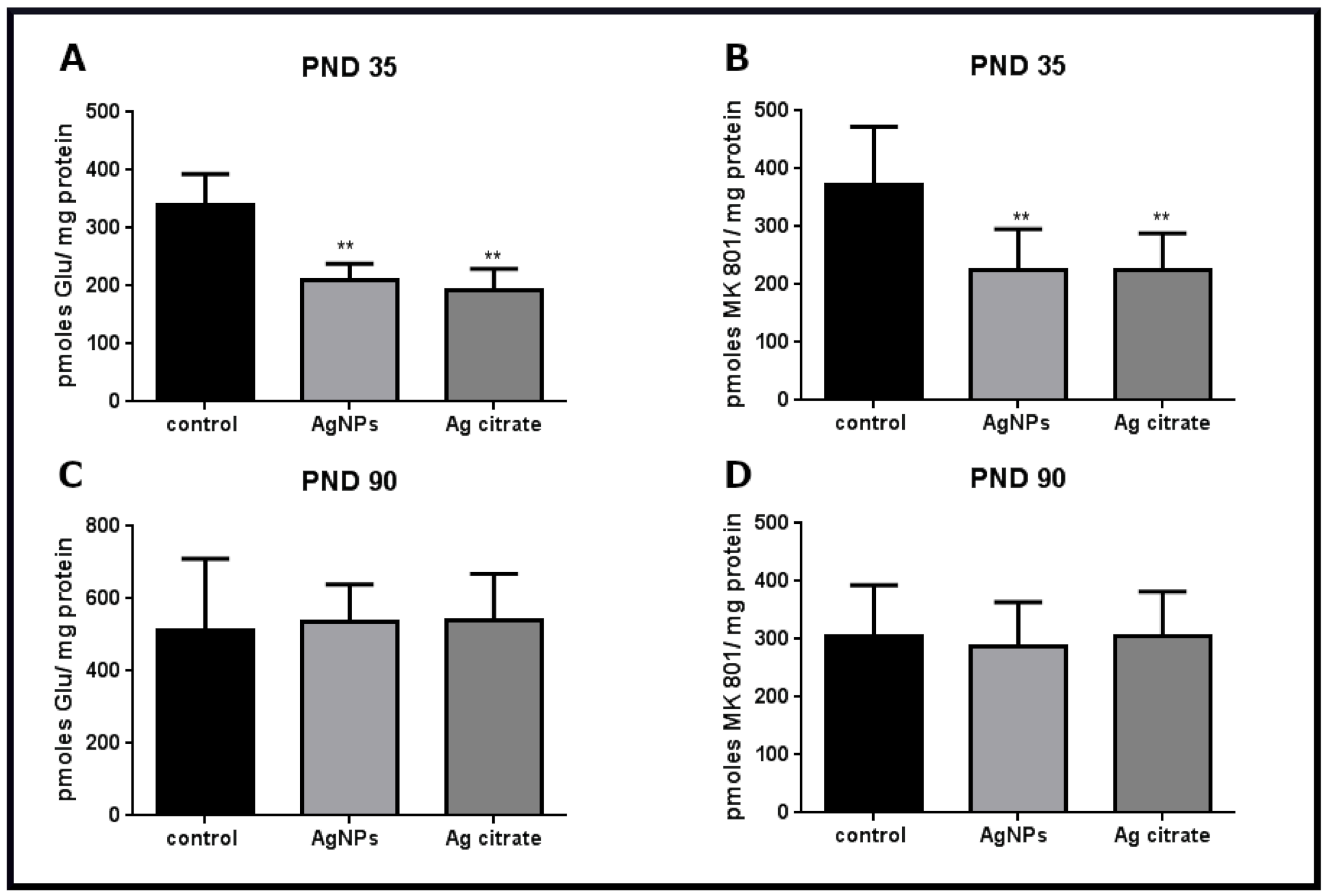
2.4. Silver Influences the Binding of Ligands to the NMDA Receptor
3. Discussion
3.1. AgNPs/Ag+ Alters the Ultrastructure of the Synapses in Developing Brain Tissue of Exposed Rats
3.2. NMDA Receptor-Dependent Cell-Death Pathway in Silver-Exposed Rat Brains
3.3. Physiological Significance of Down-Regulation of the NMDA Receptor Protein Complex by AgNPs/Ag+
4. Materials and Methods
4.1. Silver Nanoparticles
4.2. Animals and Experimental Design
4.3. Ultrastructural Analysis of Synapses in Brain Samples
4.4. Immunohistochemical Procedure and Microscopic Analysis
4.5. Western Blot Analysis
4.6. Analysis of Gene Expression by qPCR
4.7. Preparation of Membrane Fractions and Receptor Binding Assay
4.8. cGMP Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strużyńska, L.; Skalska, J. Mechanisms Underlying Neurotoxicity of Silver Nanoparticles. Adv. Exp. Med. Biol. 2018, 1048, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Skalska, J.; Frontczak-Baniewicz, M.; Strużyńska, L. Synaptic degeneration in rat brain after prolonged oral exposure to silver nanoparticles. Neurotoxicology 2015, 46, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Sałek, M.; Gewartowska, M.; Sidoryk-Węgrzynowicz, M.; Strużyńska, L. Early Postnatal Exposure to a Low Dose of Nanoparticulate Silver Induces Alterations in Glutamate Transporters in Brain of Immature Rats. Int. J. Mol. Sci. 2020, 21, 8977. [Google Scholar] [CrossRef]
- Breitenberg, V.; Shütz, A. Cortex: Statistics and Geometry of Neuronal Connectivity, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Hassel, B.; Dingledine, R. Glutamate and glutamate receptors. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 342–366. [Google Scholar]
- Kaizuka, T.; Takumi, T. Postsynaptic density proteins and their involvement in neurodevelopmental disorders. J. Biochem. 2018, 163, 447–455. [Google Scholar] [CrossRef]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.K.; Takamiya, K.; Huganir, R.L. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J. Neurosci. 2003, 23, 1119–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Sheng, M. NMDA receptors in nervous system diseases. Neuropharmacology 2013, 74, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemińska, E.; Stafiej, A.; Strużyńska, L. The role of the glutamatergic NMDA receptor in nanosilver-evoked neurotoxicity in primary cultures of cerebellar granule cells. Toxicology 2014, 315, 38–48. [Google Scholar] [CrossRef]
- Tang, S.; Wang, M.; Germ, K.E.; Du, H.-M.; Sun, W.-J.; Gao, W.-M.; Mayer, G.D. Health implications of engineered nanoparticles in infants and children. World J. Pediatr. 2015, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Wan, Z.; Xi, T. Influence of silver nanoparticles on neurons and blood-brain barrier via subcutaneous injection in rats. Appl. Surf. Sci. 2008, 255, 502–504. [Google Scholar] [CrossRef]
- Cameron, S.J.; Hosseinian, F.; Willmor, W.G. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, M.G.; Bardelli, D.; Chiu, M.; Bussolati, O. Changes in the expression of the glutamate transporter EAAT3/EAAC1 in health and disease. Cell. Mol. Life Sci. 2014, 71, 2001–2015. [Google Scholar] [CrossRef]
- Scimemi, A.; Tian, H.; Diamond, J.S. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. J. Neurosci. 2009, 29, 14581–14595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.A.; Shi, Y.; Usui, H.; During, M.J.; Sakimura, K.; Nicoll, R. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: Single-cell NMDA receptor subunit deletion in vivo. Neuron 2011, 71, 1085–1101. [Google Scholar] [CrossRef] [Green Version]
- Dosemeci, A.; Weinberg, R.J.; Reese, T.S.; Tao-Cheng, J.-H. The Postsynaptic Density: There Is More than Meets the Eye. Front. Synaptic Neurosci. 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martone, M.E.; Jones, Y.Z.; Young, S.J.; Ellisman, M.H.; Zivin, J.A.; Hu, B.R. Modification of postsynaptic densities after transient cerebral ischemia: A quantitative and three-dimentional ultrastructural study. J. Neurosci. 1999, 19, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Otmakhov, N.; Tao-Cheng, J.H.; Carpenter, S.; Asrican, B.; Dosemeci, A.; Reese, T.S.; Lisman, J. Persistent Accumulation of Calcium/Calmodulin-Dependent Protein Kinase II in Dendritic Spines after Induction of NMDA Receptor-Dependent Chemical Long-Term Potentiation. J. Neurosci. 2004, 24, 9324–9331. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the lipid and protein corona formation on different sized polymeric nanoparticles. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; MacDonald, J.E.; Tymianski, M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284, 1845–1848. [Google Scholar] [CrossRef]
- Fan, X.; Jin, W.J.; Wang, Y.T. The NMDA receptor complex: A multifunctional machine at the glutamatergic synapse. Front. Cell. Neurosci. 2014, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Dawson, V.; Dawson, T.M.; London, E.D.; Bredt, D.S.; Snyder, S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical culture. Proc. Natl. Acad. Sci. USA 1991, 88, 6368–6371. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [Green Version]
- Knott, A.B.; Bossy-Wetzel, E. Nitric Oxide in Health and Disease of the Nervous System. Antioxid. Redox Signal. 2009, 11, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Christopherson, K.S.; Hillier, B.J.; Lim, W.A.; Bredt, D.S. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 1999, 274, 27467–27473. [Google Scholar] [CrossRef] [Green Version]
- Aarts, M.; Liu, Y.; Liu, L.; Besshoh, S.; Arundine, M.; Gurd, J.W.; Wang, Y.T.; Salter, M.W.; Tymianski, M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 2002, 298, 846–850. [Google Scholar] [CrossRef]
- Doyle, D.A.; Lee, A.; Lewis, J.; Kim, E.; Sheng, M.; MacKinnon, R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell 1996, 85, 1067–1076. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, F.; Xu, H.-B.; Luo, C.-X.; Wu, H.-Y.; Zhu, M.M.; Lu, W.; Ji, X.; Zhou, Q.-G.; Zhu, D.Y. Treatment of cerebral ischemia by disrupting ischemia induced interaction of nNOS with PSD-95. Nat. Med. 2010, 16, 160. [Google Scholar] [CrossRef]
- Lai, T.W.; Shyu, W.C.; Wang, Y.T. Stroke intervention pathways: NMDA receptors and beyond. Trends Mol. Med. 2011, 17, 266–275. [Google Scholar] [CrossRef]
- Flint, A.C.; Maisch, U.S.; Weishaupt, J.H.; Monyer, H. NR2A Subunit Expression Shortens NMDA Receptor Synaptic Currents in Developing Neocortex. J. Neurosci. 1997, 17, 2469–2476. [Google Scholar] [CrossRef]
- Forrest, D.; Yuzaki, M.; Soares, H.D.; Ng, L. Targeted disruption of NMDAR 1 gene abolishes NMDA response and results in neonatal death. Neuron 1994, 13, 325–338. [Google Scholar] [CrossRef]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An Embarrassment of Riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Lüschner, C.; Malenka, R.C. NMDA Receptor-Dependent Long-Term Potentiation and Long-Term Depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, 1–15. [Google Scholar]
- Pigott, B.M.; Garthwaite, J. Nitric Oxide Is Required for L-Type Ca2+ Channel-Dependent Long-Term Potentiation in the Hippocampus. Front. Synaptic Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, L.E.; Chen, H.J.; Sokolova, I.; Knuesel, I.; Kennedy, M.B. SynGAP regulates spine formation. J. Neurosci. 2004, 24, 8862–8872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.G.; Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Kamenetz, F.; Tomita, T.; Hsieh, H.; Seabrook, G.; Borchelt, D.; Iwatsubo, T.; Sisodia, S.; Malinow, R. APP processing and synaptic function. Neuron 2003, 37, 925–937. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Orzelska-Górka, J.; Struzyńska, L.; Kędzierska, E.; Biała, G. Response of immature rats to a low dose of nanoparticulate silver: Alterations in behavior, cerebral vasculature-related transcriptome and permeability. Ecotoxicol. Environ. Saf. 2021, 208, 111416. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Reynolds, I.J. [3H](+) MK801 Radioligand Binding Assay at the N-Methyl-D-Aspartate Receptor. Curr. Protoc. Pharmacol. 2001, 11, 1.20.1–1.20.8. [Google Scholar]
- Reynolds, I.J.; Murphy, S.N.; Miller, R.J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc. Natl. Acad. Sci. USA 1987, 84, 7744–7748. [Google Scholar] [CrossRef] [Green Version]
- Sulkowski, G.; Dąbrowska-Bouta, B.; Salińska, E.; Strużyńska, L. Modulation of glutamate transport and receptor binding by glutamate receptor antagonists in EAE rat brain. PLoS ONE 2014, 9, e113954. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska-Bouta, B.; Sulkowski, G.; Sałek, M.; Frontczak-Baniewicz, M.; Strużyńska, L. Early and Delayed Impact of Nanosilver on the Glutamatergic NMDA Receptor Complex in Immature Rat Brain. Int. J. Mol. Sci. 2021, 22, 3067. https://doi.org/10.3390/ijms22063067
Dąbrowska-Bouta B, Sulkowski G, Sałek M, Frontczak-Baniewicz M, Strużyńska L. Early and Delayed Impact of Nanosilver on the Glutamatergic NMDA Receptor Complex in Immature Rat Brain. International Journal of Molecular Sciences. 2021; 22(6):3067. https://doi.org/10.3390/ijms22063067
Chicago/Turabian StyleDąbrowska-Bouta, Beata, Grzegorz Sulkowski, Mikołaj Sałek, Małgorzata Frontczak-Baniewicz, and Lidia Strużyńska. 2021. "Early and Delayed Impact of Nanosilver on the Glutamatergic NMDA Receptor Complex in Immature Rat Brain" International Journal of Molecular Sciences 22, no. 6: 3067. https://doi.org/10.3390/ijms22063067





