Adenosine Receptor Agonist HE-NECA Enhances Antithrombotic Activities of Cangrelor and Prasugrel in vivo by Decreasing of Fibrinogen Density in Thrombus
Abstract
1. Introduction
2. Results
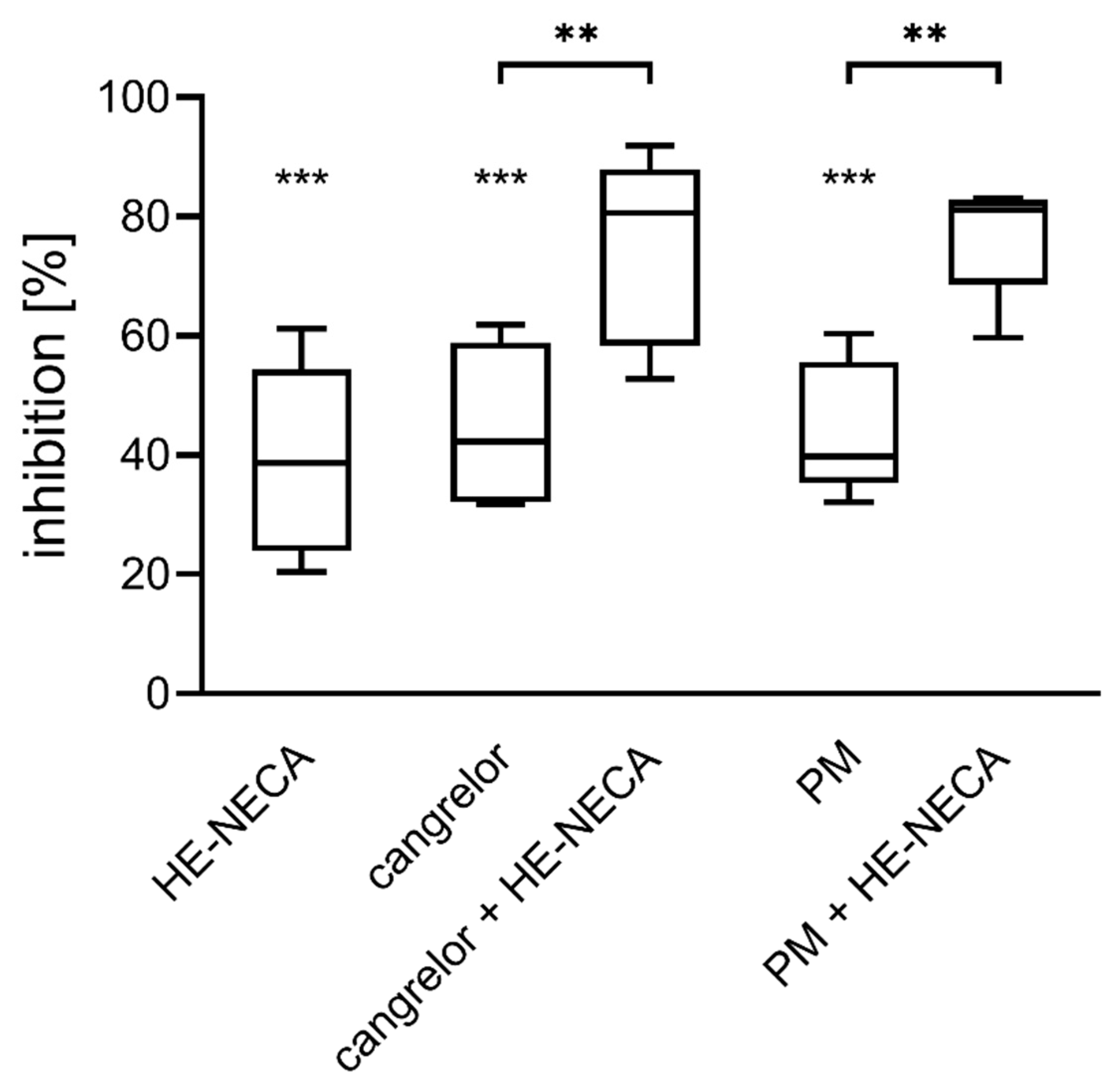
2.1. Combined Effect of HE-NECA and P2Y12 Inhibitors on Platelet Aggregation in Whole Blood
2.2. Ferric Chloride-Induced Thrombosis—Microscopy
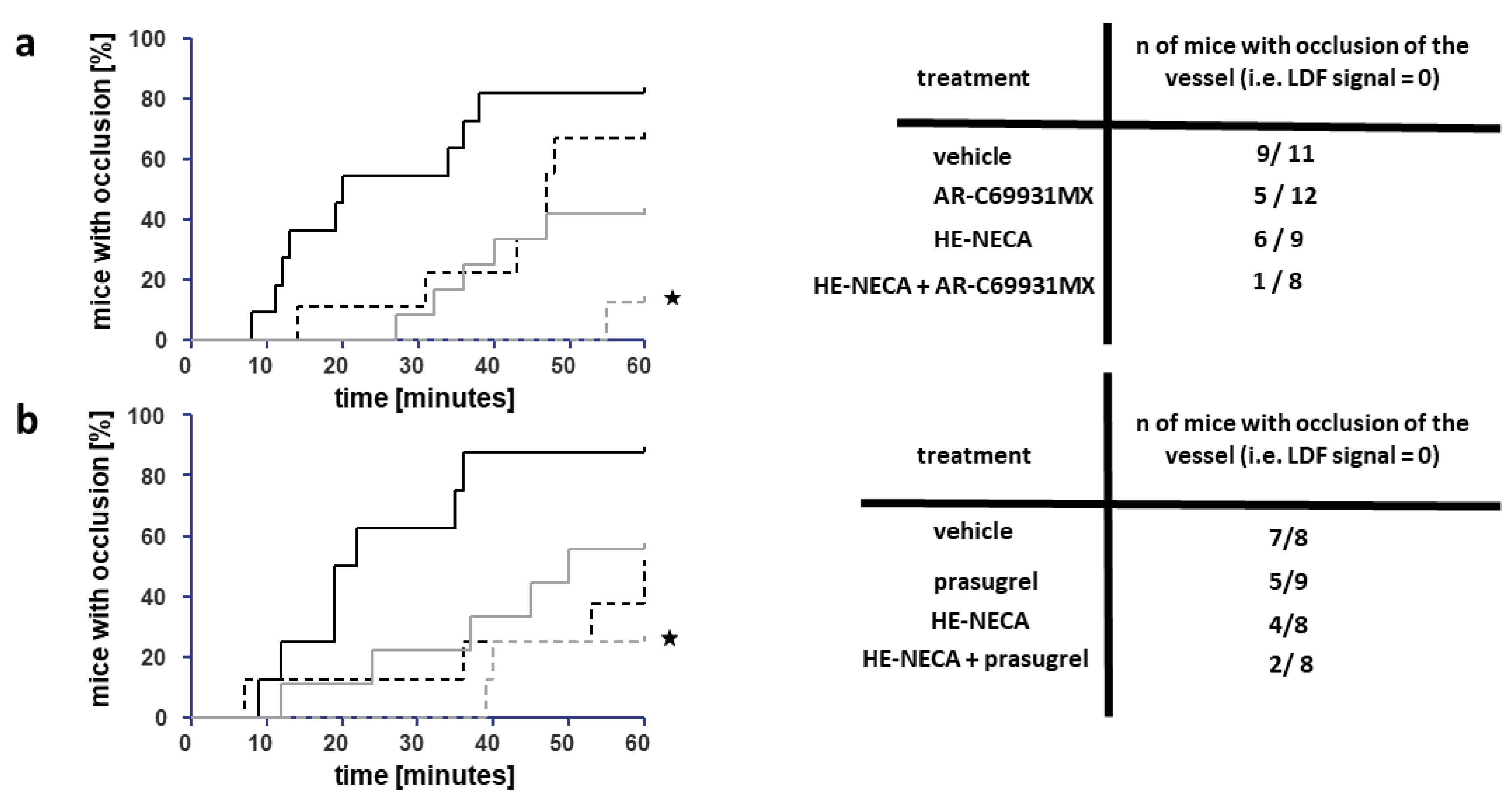
2.3. Ferric Chloride-Induced Thrombosis—Laser Doppler Flowmetry
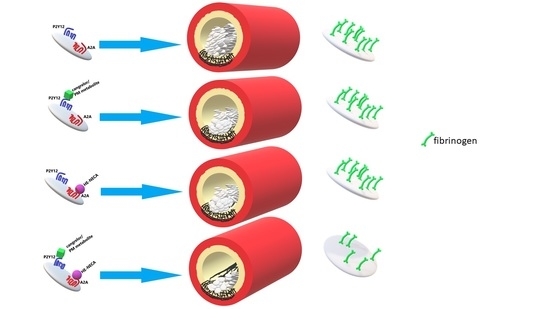
2.4. Platelet Activation and Thrombus Formation In Vitro
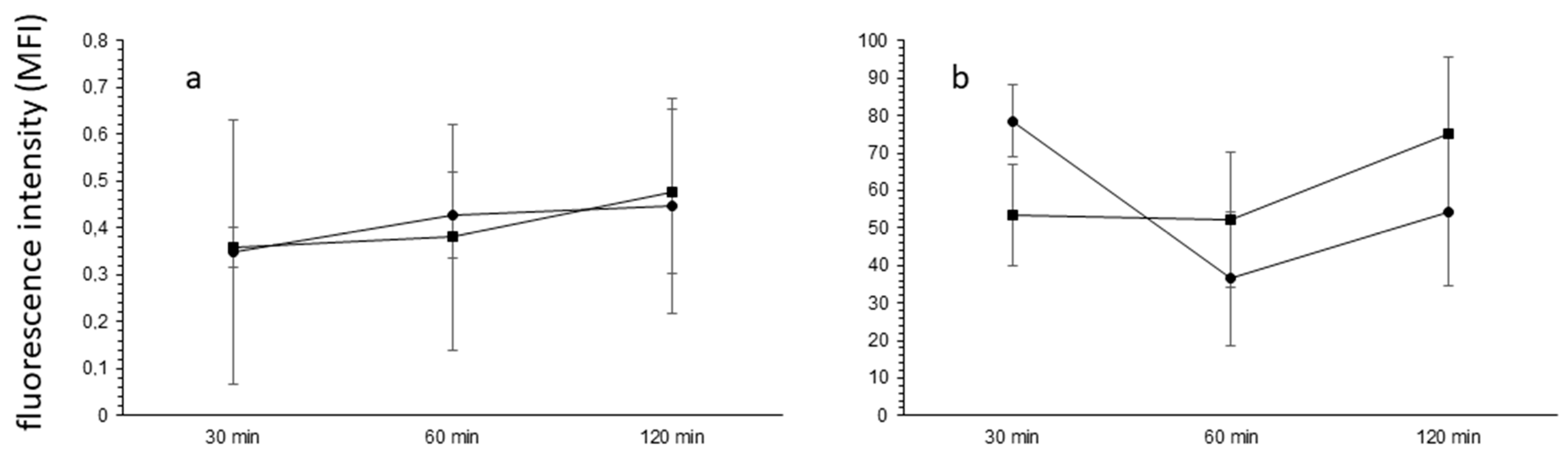
2.5. The Blood–Brain Barrier Permeability
2.6. Blood Pressure Assessment
3. Discussion
3.1. The Antithrombotic Activity of HE-NECA in Combination with Cangrelor or Prasugrel
3.2. HE-NECA Effect on Blood Pressure
3.3. HE-NECA Effect on the Blood–Brain Barrier Permeability
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Animals
5.3. Blood Donors
5.4. Platelet Aggregometry Measured in Whole Blood
5.5. HE-NECA and Cangrelor Administration
5.6. HE-NECA and Prasugrel Administration
5.7. Measurement of Thrombus Formation with the Use of Intravital Microscopy
5.8. Measurement of Thrombus Formation with the Use of Laser Doppler Flowmetry (LDF)
5.9. Assessment of HE-NECA and P2Y12 Inhibitors Effect on Thrombus Formation under Shear Stress In Vitro
5.10. The Blood–Brain Barrier Permeability In Vivo
5.11. Blood Pressure Assessment
5.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston-Cox, H.; Koupenova, M.; Yang, D.; Corkey, B.; Gokce, N.; Farb, M.G.; Lebrasseur, N.; Ravid, K. The A2b Adenosine Receptor Modulates Glucose Homeostasis and Obesity. PLoS ONE 2012, 7, e40584. [Google Scholar] [CrossRef] [PubMed]
- Johnston-Cox, H.A.; Ravid, K. Adenosine and blood platelets. Purinergic Signal. 2011, 7, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wolska, N.; Rozalski, M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. Int. J. Mol. Sci. 2019, 20, 5475. [Google Scholar] [CrossRef] [PubMed]
- Sandoli, D.; Chiu, P.J.; Chintala, M.; Dionisotti, S.; Ongini, E. In vivo and ex vivo effects of adenosine A1 and A2 receptor agonists on platelet aggregation in the rabbit. Eur. J. Pharmacol. 1994, 259, 43–49. [Google Scholar] [CrossRef]
- Fuentes, E.; Badimon, L.; Caballero, J.; Padro, T.; Vilahur, G.; Alarcón, M.; Pérez, P.; Palomo, I. Protective mechanisms of adenosine 5′-monophosphate in platelet activation and thrombus formation. Thromb. Haemost. 2014, 111, 491–507. [Google Scholar] [CrossRef]
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic Acid Inhibits Human Platelet Activation and Thrombus Formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef]
- Fuentes, E.; Pereira, J.; Mezzano, D.; Alarcón, M.; Caballero, J.; Palomo, I. Inhibition of Platelet Activation and Thrombus Formation by Adenosine and Inosine: Studies on Their Relative Contribution and Molecular Modeling. PLoS ONE 2014, 9, e112741. [Google Scholar] [CrossRef]
- Pan, C.; Wei, X.; Ye, J.; Liu, G.; Zhang, S.; Zhang, Y.; Du, H.; Ding, Z. BF066, a Novel Dual Target Antiplatelet Agent without Significant Bleeding. PLoS ONE 2012, 7, e40451. [Google Scholar] [CrossRef]
- Boncler, M.; Wzorek, J.; Wolska, N.; Polak, D.; Watala, C.; Rozalski, M. Adenosine receptor agonists deepen the inhibition of platelet aggregation by P2Y12 antagonists. Vasc. Pharmacol. 2019, 113, 47–56. [Google Scholar] [CrossRef]
- Wolska, N.; Boncler, M.; Polak, D.; Wzorek, J.; Przygodzki, T.; Gapinska, M.; Watala, C.; Rozalski, M. Adenosine Receptor Agonists Exhibit Anti-Platelet Effects and the Potential to Overcome Resistance to P2Y12 Receptor Antagonists. Molecules 2019, 25, 130. [Google Scholar] [CrossRef]
- Przygodzki, T.; Wolska, N.; Talar, M.; Polak, D.; Gapinska, M.; Watala, C. Comparison of different microscopy approaches to quantification of inhibitory effect on thrombus formation under flow conditions by the example of adenosine receptor agonist HE-NECA. J. Pharmacol. Toxicol. Methods 2018, 94, 94–104. [Google Scholar] [CrossRef]
- Tantry, U.; Chaudhary, R.; Kubica, J.; Bliden, K.; Gurbel, P.A. Cangrelor for the treatment of patients with Arterial Thrombosis. Expert Opin. Pharmacother. 2018, 19, 1389–1398. [Google Scholar] [CrossRef]
- Kupka, D.; Sibbing, D. De-Escalation of P2Y12 Receptor Inhibitor Therapy after Acute Coronary Syndromes in Patients Undergoing Percutaneous Coronary Intervention. Korean Circ. J. 2018, 48, 863–872. [Google Scholar] [CrossRef]
- Koo, M.H.; Nawarskas, J.J.; Frishman, W.H. Prasugrel: A new antiplatelet drug for the prevention and treatment of cardiovascular disease. Cardiol. Rev. 2008, 16, 314–318. [Google Scholar] [CrossRef]
- Wolska, N.; Kassassir, H.; Luzak, B.; Watala, C.; Rozalski, M. Adenosine Receptor Agonists Increase the Inhibition of Platelet Function by P2Y12 Antagonists in a cAMP- and Calcium-Dependent Manner. Pharmaceuticals 2020, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Mogami, H.; Murakami, Y.; Nakamura, T.; Kanayama, N.; Konno, H.; Urano, T. Real-time analysis of platelet aggregation and procoagulant activity during thrombus formation in vivo. Pflüger’s Archiv für die Gesammte Physiologie des Menschen und der Tiere 2008, 456, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Portaluppi, F.; Gessi, S.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: Functional and biochemical aspects. Circulation 2000, 102, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Portaluppi, F.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation 1999, 99, 2499–2502. [Google Scholar] [CrossRef]
- Lev, E.I.; Arikan, M.E.; Vaduganathan, M.; Alviar, C.L.; Tellez, A.; Mathuria, N.; Builes, A.; Granada, J.F.; Del Conde, I.; Kleiman, N.S. Effect of caffeine on platelet inhibition by clopidogrel in healthy subjects and patients with coronary artery disease. Am. Heart J. 2007, 154, 694.e1–694.e7. [Google Scholar] [CrossRef]
- Darbousset, R.; Delierneux, C.; Mezouar, S.; Hego, A.; Lecut, C.; Guillaumat, I.; Riederer, M.A.; Evans, R.J.; Dignat-George, F.; Panicot-Dubois, L.; et al. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood 2014, 124, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Grahl, L.; Von Bruehl, M.-L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef]
- Von Brühl, M.-L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Bynoe, M.S. A2A Adenosine Receptor Regulates the Human Blood-Brain Barrier Permeability. Mol. Neurobiol. 2014, 52, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-C.; Yang, Y.L.; Liao, K.H.; Lai, T.W. Adenosine receptor agonist NECA increases cerebral extravasation of fluorescein and low molecular weight dextran independent of blood-brain barrier modulation. Sci. Rep. 2016, 6, 23882. [Google Scholar] [CrossRef]
- Zavala-Tecuapetla, C.; Orozco-Suarez, S.; Manjarrez, J.; Cuellar-Herrera, M.; Vega-Garcia, A.; Buzoianu-Anguiano, V. Activation of adenosine receptors modulates the efflux transporters in brain capillaries and restores the anticonvulsant effect of carbamazepine in carbamazepine resistant rats developed by window-pentylenetetrazole kindling. Brain Res. 2020, 1726, 146516. [Google Scholar] [CrossRef]
- Jackson, S.; Anders, N.M.; Mangraviti, A.; Wanjiku, T.M.; Sankey, E.W.; Liu, A.; Brem, H.; Tyler, B.; Rudek, M.A.; Grossman, S.A. The effect of regadenoson-induced transient disruption of the blood–brain barrier on temozolomide delivery to normal rat brain. J. Neuro-Oncol. 2015, 126, 433–439. [Google Scholar] [CrossRef]
- Liu, Y.; Alahiri, M.; Ulloa, B.; Xie, B.; Sadiq, S.A. Adenosine A2A receptor agonist ameliorates EAE and correlates with Th1 cytokine-induced blood brain barrier dysfunction via suppression of MLCK signaling pathway. Immun. Inflamm. Dis. 2017, 6, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; George, R.T.; Lodge, M.A.; Piotrowski, A.; Wahl, R.L.; Gujar, S.K.; Grossman, S.A. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J. Neuro-Oncol. 2017, 132, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Weingart, J.; Nduom, E.K.; Harfi, T.T.; George, R.T.; McAreavey, D.; Ye, X.; Anders, N.M.; Peer, C.; Figg, W.D.; et al. The effect of an adenosine A2A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS 2018, 15, 2. [Google Scholar] [CrossRef]
- Alexopoulos, D. Prasugrel resistance: Fact or fiction. Platelets 2011, 23, 83–90. [Google Scholar] [CrossRef]
- Warlo, E.M.K.; Arnesen, H.; Seljeflot, I. A brief review on resistance to P2Y12 receptor antagonism in coronary artery disease. Thromb. J. 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Thackaberry, E.A.; Wang, X.; Schweiger, M.; Messick, K.; Valle, N.; Dean, B.; Sambrone, A.; Bowman, T.; Xie, M. Solvent-based formulations for intravenous mouse pharmacokinetic studies: Tolerability and recommended solvent dose limits. Xenobiotica 2013, 44, 235–241. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Pappas, C.; Sfantou, D.; Lekakis, J. Cangrelor in Percutaneous Coronary Intervention: Current Status and Perspectives. J. Cardiovasc. Pharmacol. Ther. 2017, 23, 13–22. [Google Scholar] [CrossRef]
- Ye, Y.; Birnbaum, G.D.; Perez-Polo, J.R.; Nanhwan, M.K.; Nylander, S.; Birnbaum, Y. Ticagrelor Protects the Heart Against Reperfusion Injury and Improves Remodeling After Myocardial Infarction. Arter. Thromb. Vasc. Biol. 2015, 35, 1805–1814. [Google Scholar] [CrossRef]
- Ohno, K.; Tomizawa, A.; Jakubowski, J.A.; Mizuno, M.; Sugidachi, A. Prevention of occlusive arterial thrombus formation by a single loading dose of prasugrel suppresses neointimal hyperplasia in mice. Thromb. Res. 2015, 136, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nieman, M.; Gupta, A.S. Ferric Chloride-induced Murine Thrombosis Models. J. Vis. Exp. 2016, 115, e54479. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mosawy, S.; Jackson, D.E.; Woodman, O.L.; Linden, M.D. The flavonols quercetin and 3′,4′-dihydroxyflavonol reduce platelet function and delay thrombus formation in a model of type 1 diabetes. Diabetes Vasc. Dis. Res. 2014, 11, 174–181. [Google Scholar] [CrossRef]
- Speich, H.E.; Bhal, V.; Houser, K.H.; Caughran, A.T.; Lands, L.T.; Houng, A.K.; Bäckström, J.; Enerbäck, M.; Reed, G.L.; Jennings, L.K. Signaling via P2Y12 May Be Critical for Early Stabilization of Platelet Aggregates. J. Cardiovasc. Pharmacol. 2014, 63, 520–527. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Kramkowski, K.; Mogielnicki, A.; Chabielska, E.; Cylwik, D.; Buczko, W. The effect of ‘tissue’ and ‘plasma’ angiotensin converting enzyme inhibitors on overall haemostatic potentials in rats. Thromb Res. 2006, 117, 557–561. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, D.; Talar, M.; Wolska, N.; Wojkowska, D.W.; Karolczak, K.; Kramkowski, K.; Bonda, T.A.; Watala, C.; Przygodzki, T. Adenosine Receptor Agonist HE-NECA Enhances Antithrombotic Activities of Cangrelor and Prasugrel in vivo by Decreasing of Fibrinogen Density in Thrombus. Int. J. Mol. Sci. 2021, 22, 3074. https://doi.org/10.3390/ijms22063074
Polak D, Talar M, Wolska N, Wojkowska DW, Karolczak K, Kramkowski K, Bonda TA, Watala C, Przygodzki T. Adenosine Receptor Agonist HE-NECA Enhances Antithrombotic Activities of Cangrelor and Prasugrel in vivo by Decreasing of Fibrinogen Density in Thrombus. International Journal of Molecular Sciences. 2021; 22(6):3074. https://doi.org/10.3390/ijms22063074
Chicago/Turabian StylePolak, Dawid, Marcin Talar, Nina Wolska, Dagmara W. Wojkowska, Kamil Karolczak, Karol Kramkowski, Tomasz A. Bonda, Cezary Watala, and Tomasz Przygodzki. 2021. "Adenosine Receptor Agonist HE-NECA Enhances Antithrombotic Activities of Cangrelor and Prasugrel in vivo by Decreasing of Fibrinogen Density in Thrombus" International Journal of Molecular Sciences 22, no. 6: 3074. https://doi.org/10.3390/ijms22063074
APA StylePolak, D., Talar, M., Wolska, N., Wojkowska, D. W., Karolczak, K., Kramkowski, K., Bonda, T. A., Watala, C., & Przygodzki, T. (2021). Adenosine Receptor Agonist HE-NECA Enhances Antithrombotic Activities of Cangrelor and Prasugrel in vivo by Decreasing of Fibrinogen Density in Thrombus. International Journal of Molecular Sciences, 22(6), 3074. https://doi.org/10.3390/ijms22063074