Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin
Abstract
1. Introduction
2. Results and Discussion
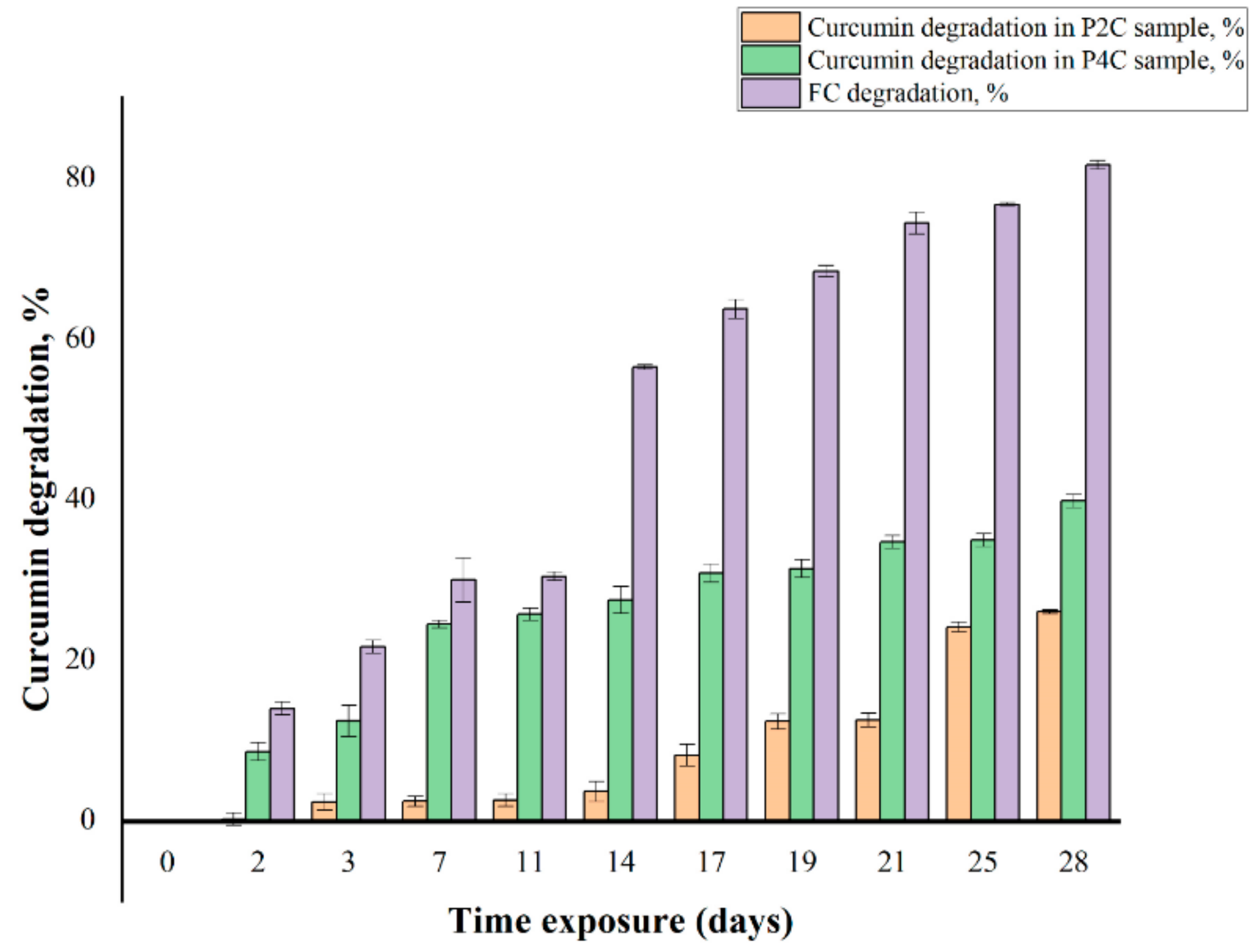
2.1. Photostability of Curcumin
2.2. Stability of Curcumin at Complexation with Metal Ions
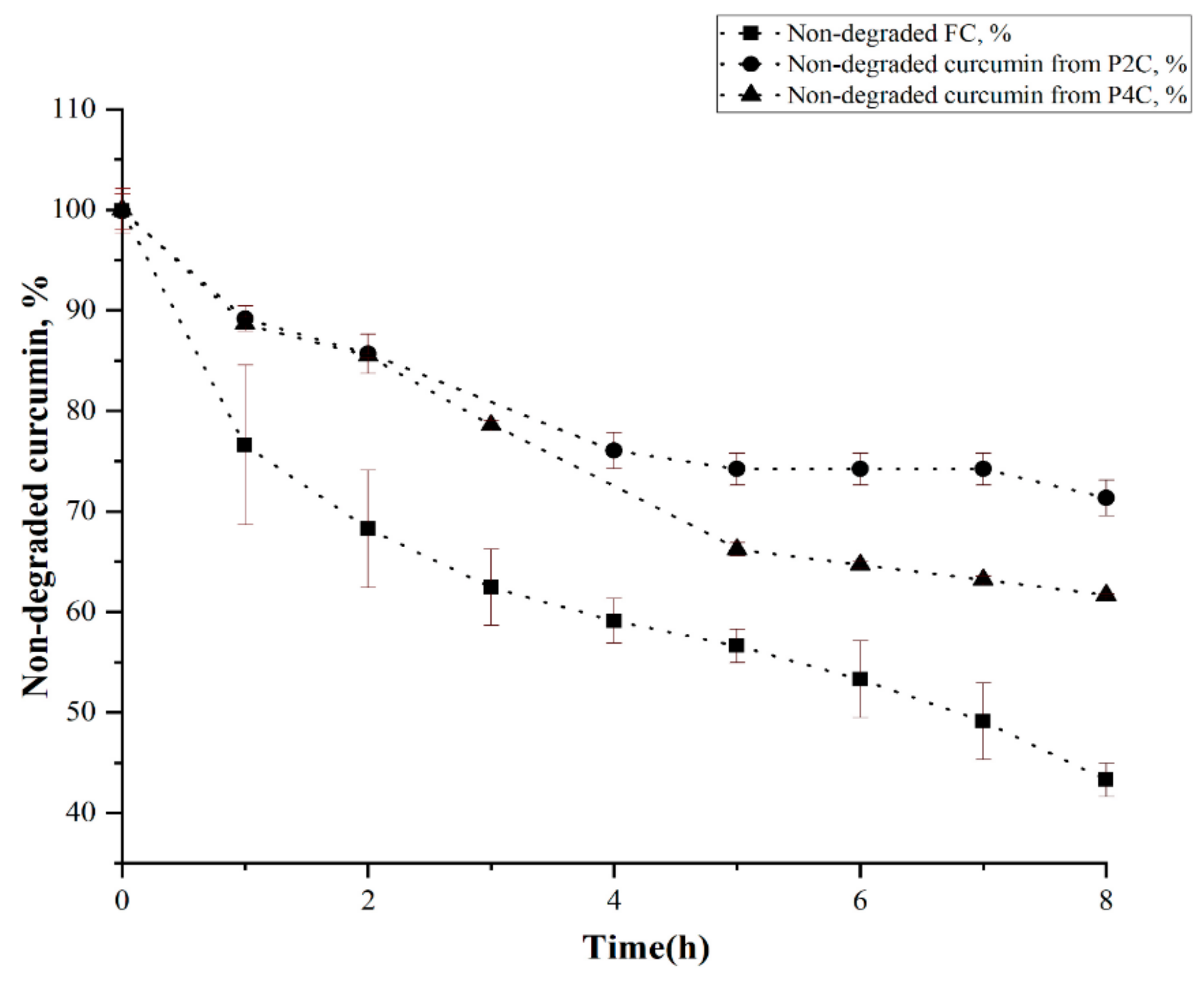
2.3. Curcumin Stability at Different pH Values
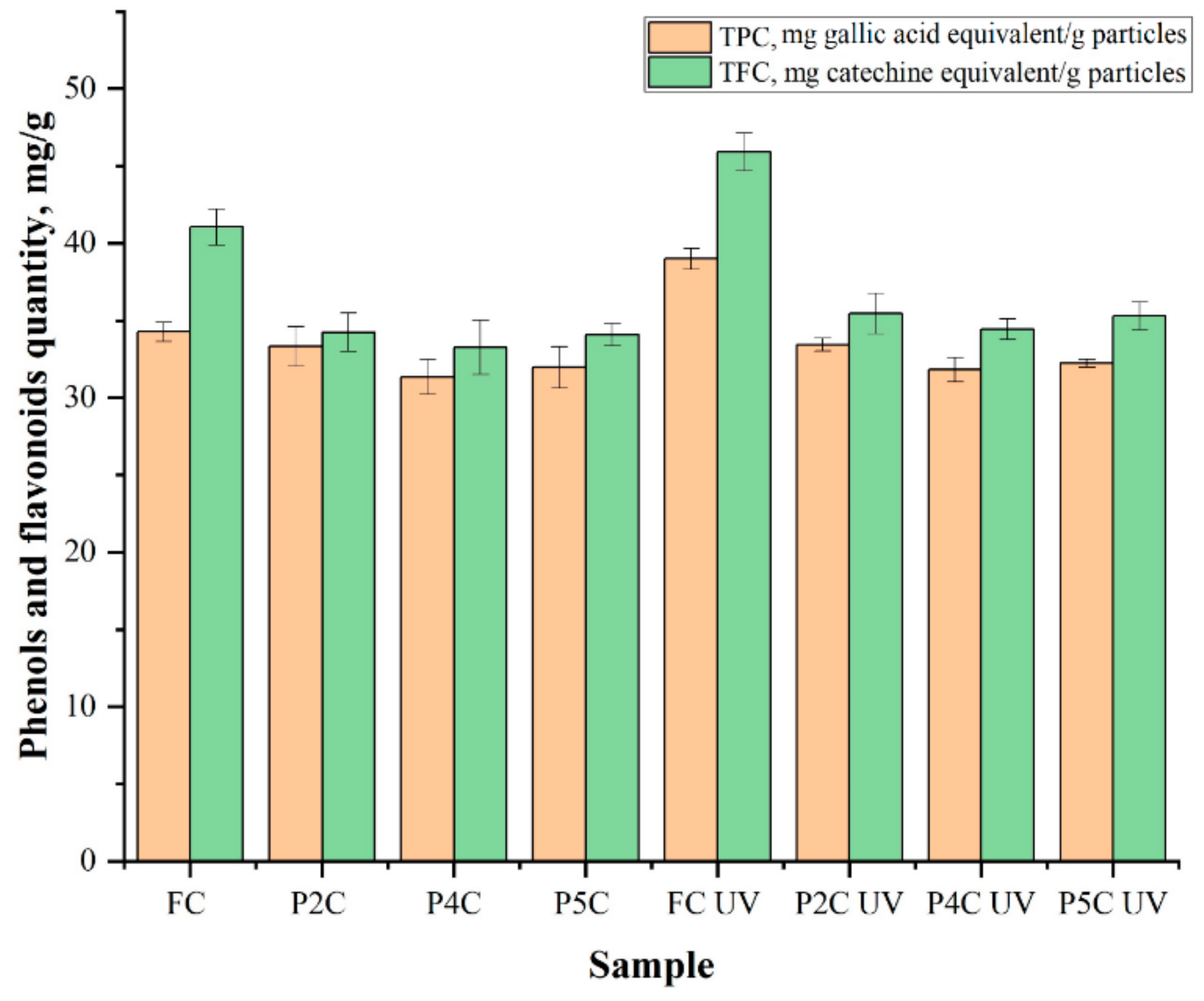
2.4. Determination of Total Phenol (TPC) and Flavonoid (TFC) Content of Curcumin
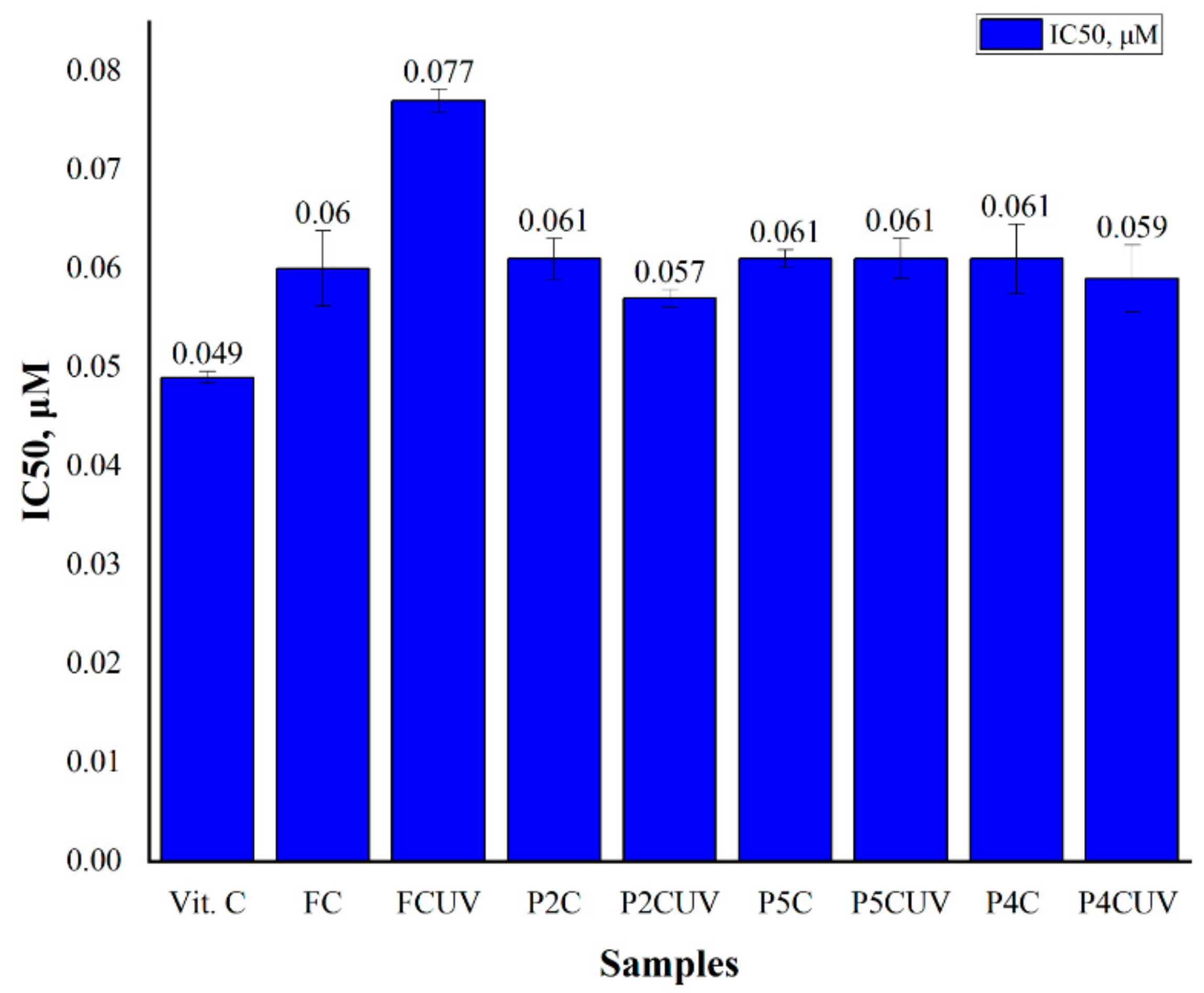
2.5. Antioxidant Activity
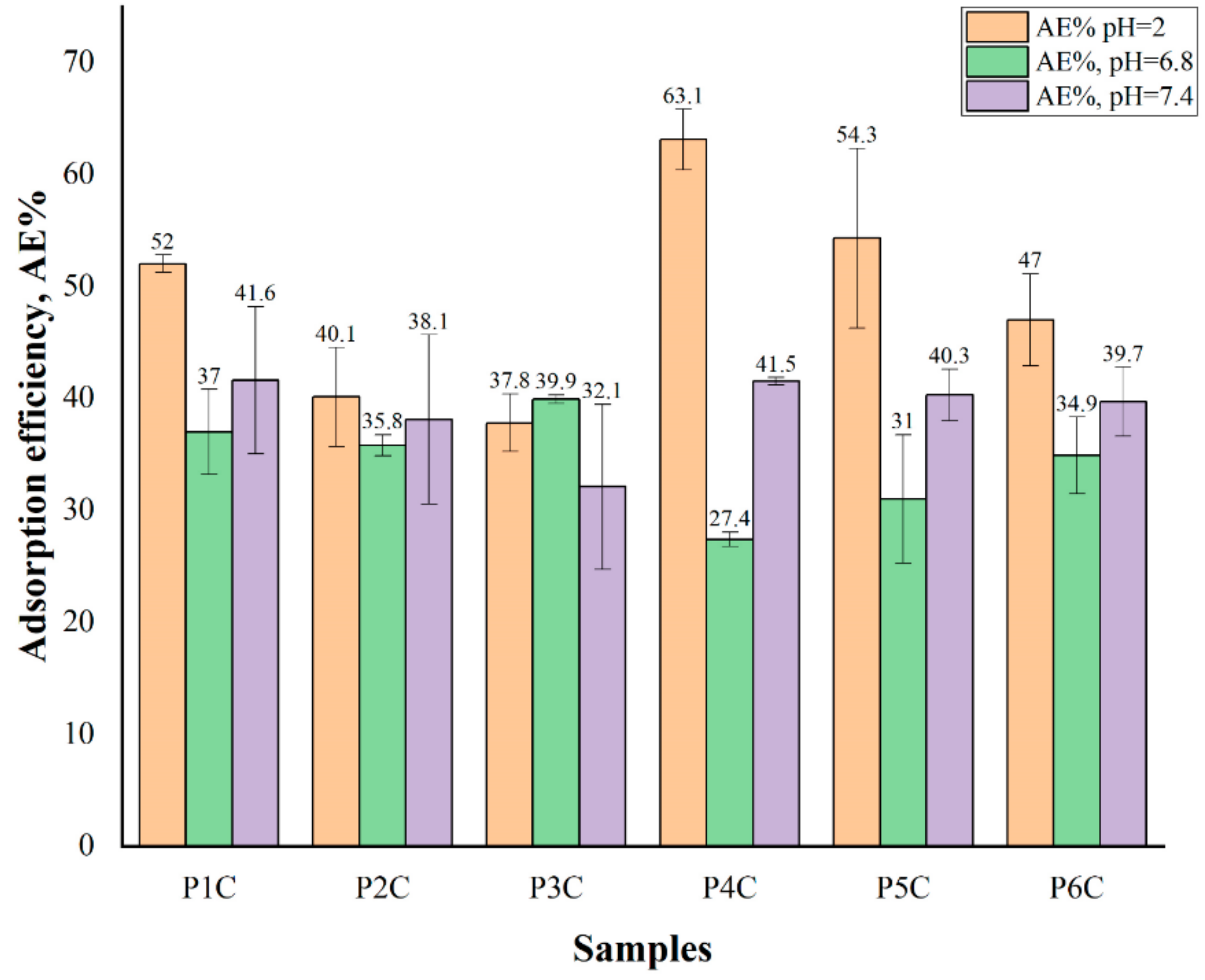
2.6. Proteins’ Adsorption
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Complex Particles
3.3. Characterization Methods
3.3.1. FTIR Spectroscopy
3.3.2. Photostability of Curcumin
3.3.3. Curcumin Stability at Complexation with Metal Ions
3.3.4. Curcumin Stability at Different pH Values
3.3.5. Determination of the Total Phenols (TPC) and Flavonoids (TFC) Content
Determination of the Total Flavonoids Content (TFC)
3.3.6. Antioxidant Activity Determination
3.3.7. Determination of Protein Adsorption
3.3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Sarmiento-Aguilar, A.; Toledo-Mauriño, J.J.; Bozada-Gutiérrez, K.E.; Bosques-Padilla, F.J.; Martínez-Vázquez, M.A.; Marroquín-Jiménez, V.; García-Figueroa, R.; Jaramillo-Buendía, C.; MirandaCordero, R.M.; et al. Incidence and prevalence of inflammatory bowel disease in Mexico from a nationwide cohort study in a period of 15 years (2000–2017). Medicine 2019, 98, e16291. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sole, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation, I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 2483–2492. [Google Scholar] [CrossRef]
- Kinney, S.R.M.; Carlson, L.; Ser-Dolansky, J.; Thompson, C.; Shah, S.; Gambrah, A.; Xing, W.; Schneider, S.S.; Mathias, C.B. Curcumin ingestion inhibits mastocytosis and suppresses intestinal anaphylaxis in a murine model of food allergy. PLoS ONE 2015, 10, e0132467. [Google Scholar] [CrossRef]
- Larasatil, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018, 8, 2039. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Haddad, M.; Sauvain, M.; Deharo, E. Curcuma as a parasiticidal agent: A review. Planta Med. 2011, 77, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Buescher, R.W. Kinetics of alkaline degradation of the food pigments curcumin and curcuminoids. J. Food Sci. 1997, 62, 267–269. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Degradation of curcumin dye in aqueous solution and on Ag nanoparticles studied by ultraviolet-visible absorption and surface-enhanced Raman spectroscopy. Appl. Spectrosc. 2006, 60, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.A.; Arya, M.; Momin, S.A. Structure activity relationship of tautomers of curcumin: A review. Ukr. Food J. 2019, 8, 45–60. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photoch. Photobio. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Khurana, A.; Ho, C.T. High performance liquid chromatographic analysis of curcuminoids and their photo-oxidative decomposition compounds in Curcuma Longa L. J. Liq. Chromatogr. 1988, 11, 2295–2304. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Chaudhuri, S.; Sardar, S.; Choudhury, S.; Polley, N.; Lemmens, P.; Pal, S.K. Modulation of Stability and functionality of a phytoantioxidant by weakly interacting metal ions: Curcumin in aqueous solution. RSC Adv. 2015, 5, 102516–102524. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravarty, A.R. Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity. Acc. Chem. Res. 2015, 48, 2075–2083. [Google Scholar] [CrossRef]
- Wright, J.S. Predicting the antioxidant activity of curcumin and curcuminoids. J. Mol. Struct. 2002, 591, 207–217. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Stamate Cretan, M.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug delivery system based on ph-sensitive biocompatible poly(2-vinyl pyridine)-b-poly(ethylene oxide) nanomicelles loaded with curcumin and 5-fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Beloqui, A.; Coco, R.; Memvanga, P.B.; Ucakar, B.; des Rieux, A.; Preat, V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014, 473, 203–212. [Google Scholar] [CrossRef]
- Su, J.; Tao, X.; Xu, H.; Chen, J.F. Facile encapsulation of nanoparticles in nanoorganized bio-polyelectrolyte microshells. Polymer 2007, 48, 7431–7443. [Google Scholar] [CrossRef]
- Guzman-Villanueva, D.; El-Sherbiny, I.M.; Herrera-Ruiz, D.; Smyth, H.D.C. Design and in vitro evaluation of a new nano-microparticulate system for enhanced aqueous-phase solubility of curcumin. Biomed. Res. Int. 2013, 2013, 724763. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chuah, L.H.; Billa, N.; Roberts, C.J.; Burley, J.C.; Manickam, S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm. Dev. Technol. 2011, 18, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xia, S.; Tan, C.; Zhang, X. Preparation and evaluation of chitosan-calcium-gellan gum beads for controlled release of protein. Eur. Food Res. Technol. 2013, 237, 467–479. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Boni, F.I.; Ferreira, N.N.; Silva, D.S.; Campana-Filho, S.P.; Almeida, A.; Vasconcelos, T.; Gremião, M.P.D.; Cury, B.S.F.; Sarmento, B. Gellan Gum/pectin beads are safe and efficient for the targeted colonic delivery of resveratrol. Polymers 2018, 10, 50. [Google Scholar] [CrossRef]
- Zainal Ariffin, S.H.; Yeen, W.W.; Zainol Abidin, I.Z.; Megat Abdul Wahab, R.; Zainal Ariffin, Z.; Senafi, S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in the human intestine and liver cell lines. BMC Complement. Altern. Med. 2014, 14, 508–524. [Google Scholar] [CrossRef]
- Li, C.; Hein, S.; Wang, K. Chitosan-carrageenan polyelectrolyte complex for the delivery of protein drugs. Int. Sch. Res. Not. Biomater. 2013, 2013, 629807. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, Z.; Chen, F.; Luo, X.; McClements, D.J. Impact of delivery system type on curcumin stability: Comparison of curcumin degradation in aqueous solutions, emulsions, and hydrogel beads. Food Hydrocoll. 2017, 71, 187–197. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, Stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Amiji, M. Platelet adhesion and activation on an amphoteric chitosan derivative bearing sulfonate groups. Colloids Surf. B. 1998, 10, 263–271. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Liu, B. Adsorption of BSA onto sulfonated microspheres. Biochem. Eng. J. 2005, 23, 259–263. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. Available online: http://www.eurekaselect.com/node/112339/article (accessed on 1 April 2020). [CrossRef]
- Souza, C.R.A.; Osme, S.F.; Gloria, M.B.A. Stability of curcuminoid pigments in model systems. J. Food Process. Preserv. 1997, 21, 353–363. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin–synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.Q.; Niu, J.; Liu, W.; Peng, S.F.; Liu, C.M. The Stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef]
- Moussawi, R.N.; Patra, D. Modification of nanostructured ZnO surfaces with curcumin: Fluorescence-based sensing for arsenic and improving arsenic removal by ZnO. RSC Adv. 2016, 6, 17256–17268. [Google Scholar] [CrossRef]
- Khalil, M.I.; AL-Zahem, A.M.; Qunaibit, M.M. Synthesis, characterization, and antitumor activity of binuclear curcumin–metal (II) hydroxo complexes. Med. Chem. Res. 2014, 23, 1683–1689. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Ismail, E.H.; Sabry, D.Y.; Mahdy, H.; Khalil, M.M.H. Synthesis and characterization of some ternary metal complexes of curcumin with 1,10-phenanthroline and their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef]
- Bich, V.T.; Thuy, N.T.; Binh, N.T.; Huong, N.T.M.; Yen, P.N.D.; Luong, T.T. Structural and spectral properties of curcumin and metal-curcumin complex derived from turmeric (Curcuma longa). In Physics and Engineering of New Materials; Cat, D.T., Pucci, A., Wandelt, K., Eds.; Springer Proceedings in Physics; Physics and Engineering of New Materials; Springer: Berlin/Heidelberg, Germany, 2009; pp. 271–278. [Google Scholar] [CrossRef]
- Pallikkavil, R.; Basheer Ummathur, M.; Sreedharan, S.; Krishnankutty, K. Synthesis, characterization and antimicrobial studies of Cd(II), Hg(II), Pb(II), Sn(II) and Ca(II) complexes of curcumin. Main Group Met. Chem. 2013, 36, 123–127. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Zebib, B.; Mouloungui, Z.; Noirot, V. Stabilization of curcumin by complexation with divalent cations in glycerol/water system. Bioinorg Chem. Appl. 2010, 2010, 292760. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Si, J.; Kang, C.; Chung, B.; Chung, D.; Kim, D. Facile preparation of water soluble curcuminoids extracted from turmeric (Curcuma longa L.) powder by using steviol glucosides. Food Chem. 2017, 214, 366–373. [Google Scholar] [CrossRef]
- Giri, A.; Goswami, N.; Sasmal, C.; Polley, N.; Majumdar, D.; Sarkar, S.; Bandyopadhyay, S.N.; Singhac, A.; Pal, S.K. Unprecedented catalytic activity of Mn3O4 nanoparticles: Potential lead of a sustainable therapeutic agent for hyperbilirubinemia. RSC Adv. 2014, 4, 5075–5079. [Google Scholar] [CrossRef]
- Daniel-da-Silva, A.L.; Lopes, A.B.; Gil, A.M.; Correia, R.N. Synthesis and characterization of porous κ-carrageenan/calcium phosphate nanocomposite scaffolds. J. Mater. Sci. 2007, 42, 8581–8591. [Google Scholar] [CrossRef]
- Varghese, J.S.; Chellappa, N.N.; Fathima, N. Gelatin-Carrageenan Hydrogels: Role of Pore Size Distribution on Drug Delivery Process. Colloids Surf. B Biointerfaces 2014, 113, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Young, S.; Dunstan, D.E.; Alany, R.G. Texture and rheological characterization of kappa and iota carrageenan in the presence of counterions. Carbohydr. Polym. 2010, 82, 69–77. [Google Scholar] [CrossRef]
- Li, J.; Yang, B.; Qian, Y.; Wang, Q.; Han, R.; Hao, T.; Shu, Y.; Zhang, Y.; Yao, F.; Wang, C. Iota-carrageenan/chitosan/gelatin scaffold for the osteogenic differentiation of adipose-derived MSCs in vitro. J. Biomed. Mater. Res. Part. B. 2015, 103, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Luis, P.B.; Sintim, H.O.; Schneider, C. Unraveling curcumin degradation: Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem. 2015, 290, 4817–4828. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Pistis, V.; Suzuki, T.; Tejera, N.; Pratt, D.A.; Schneider, C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J. Biol. Chem. 2011, 286, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Naksuriya, O.; van Steenbergen, M.J.; Torano, J.S.; Okonogi, S.; Hennink, W.E. A kinetic degradation study of curcumin in its free form and loaded in polymeric micelles. AAPS J. 2016, 18, 777–787. [Google Scholar] [CrossRef]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.A.R.; Song, Y. Microencapsulation and the characterization of polyherbal formulation (PHF) rich in natural polyphenolic compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 17, 2041731416648810. [Google Scholar] [CrossRef]
- Mori, M.; Hamamoto, A.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Tachibana, S.; Ikehara, T.; Nakaya, Y.; Akutagawa, M.; Kinouchi, Y. Development of a new water sterilization device with a 365 nm UV-LED. Med. Bio. Eng. Comput. 2007, 45, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Manap, M.Y.A.; Khatib, A.; Razis, A.F.A. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.K.; Srinivasan, P.; Choi, J.; Kim, J.H.; Han, S.B.; Kim, D.J.; Byun, M.W. Effect of gamma irradiation on microbial analysis, antioxidant activity, sugar content and color of ready to-use tamarind juice during storage. LWT Food Sci. Technol. 2009, 42, 101–105. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of Almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Taheri, S.; Abdullah, T.L.; Karimi, E.; Oskoueian, E.; Ebrahimi, M. Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma alismatifolia (Zingiberaceae) Leaves. Int. J. Mol. Sci. 2014, 15, 13077–13090. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, H.A.; Kim, M.R.; Hong, J. Changes in chemical stability and bioactivities of curcumin by ultraviolet radiation. Food Sci. Biotechnol. 2013, 22, 279–282. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.E. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of Carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H. Protein adsorption on polymer particles. In Encyclopedia of Surface and Colloid Science; Hubbart, A.T., Ed.; CRC Press by Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 2002; pp. 4373–4381. Available online: https://books.google.ro/books?id=GobXwAOZIxcC (accessed on 1 May 2020).
- Lima, P.H.L.; Pereira, S.V.A.; Rabello, R.B.; Rodriguez-Castellón, E.; Beppu, M.M.; Chevallier, P.; Mantovani, D.; Vieira, R.S. Blood protein adsorption on sulfonated chitosan and k-carrageenan films. Colloids Surf. B. Biointerfaces 2013, 111, 719–725. [Google Scholar] [CrossRef]
- Betancourt, T.; Pardo, J.; Soo, K.; Peppas, N.A. Characterization of pH-responsive hydrogels of poly(itaconic acid-g-ethylene glycol) prepared by UV-initiated free radical polymerization as biomaterials for oral delivery of bioactive agents. J. Biomed. Mater. Res. A. 2010, 93, 175–188. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle Anthocyanins: Antioxidant Properties and Stability to Heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef]
- Hieu, T.Q.; Thao, D.T.T. Enhancing the solubility of curcumin metal complexes and investigating some of their biological activities. J. Chem. 2019, 2019, 8082195. [Google Scholar] [CrossRef]
- Zhao, X.-Z.; Jiang, T.; Wang, L.; Yang, H.; Zhang, S.; Zhou, P. Interaction of curcumin with Zn(II) and Cu(II) ions based on experiment and theoretical calculation. J. Mol. Struct. 2010, 984, 316–325. [Google Scholar] [CrossRef]
- Kumavat, S.D.; Chaudhari, Y.S.; Borole, P.; Mishra, P.; Shenghani, K.; Duvvuri, P. Degradation studies of curcumin. Int. J. Pharm. Sci. Rev. Res. 2013, 3, 50–55. Available online: http://www.ijprr.com/File_Folder/50-55.pdf (accessed on 1 August 2019).
- Mahato, R.I.; Narang, A.S. Chemical kinetics and stability. In Pharmaceutical Dosage Forms and Drug Delivery; Mahato, R.I., Narang, A.S., Eds.; Taylor and Francis Group, 6000, Broken Sound Parkway; CRC Press: New York, NY, USA, 2011; pp. 59–74. Available online: https://books.google.ro/books?id=3g3rUu-f4qIC (accessed on 1 August 2019).
- Flynn, E. Pharmacokinetic Compartmental Modeling. In xPharm: The Comprehensive Pharmacology Reference, Reference Module Biomedical Sciences; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–5. [Google Scholar] [CrossRef]
- Blass, B.E. In vitro ADME and In vivo Pharmacokinetics. In Basic Principles of Drug Discovery and Development; Blass, B.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 245–306. [Google Scholar] [CrossRef]
- Alhakmani, F.; Kumar, S.; Khan, S.A. Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed. 2013, 3, 623–627. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Bayo Lewu, F. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Palazzo de Mello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Inês, M.; Almeida, G.S.; Barreiros, L.; Reis, S.; Segundo, M.A. Automatic aluminum chloride method for routine estimation of total flavonoids in red wines and teas. Food Anal. Methods 2012, 5, 530–539. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant. Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]






| Sample | PH | k | t1/2 (h) |
|---|---|---|---|
| FC | 3 | 0.10 ± 0.005 | 6.8 ± 0.3 |
| 6.8 | 0.11 ± 0.006 | 6.2 ± 0.3 | |
| 7.4 | 0.12 ± 0.001 | 5.7 ± 0.05 | |
| 9 | 0.32 ±0.025 | 2.1 ± 0.1 | |
| P2C | 3 | 0.051 ± 0.003 | 13.5 ± 0.8 |
| 6.8 | 0.05 ± 0.002 | 13.6 ± 0.5 | |
| 7.4 | 0.053 ± 0.002 | 13.1± 0.6 | |
| 9 | 0.059 ± 0.001 | 11.8 ± 0.3 | |
| P4C | 3 | 0.067± 0.003 | 10.35 ± 0.5 |
| 6.8 | 0.067 ± 0.001 | 10.34 ± 0.1 | |
| 7.4 | 0.065 ± 0.001 | 10.7 ± 0.2 | |
| 9 | 0.071 ±0.002 | 9.7 ± 0.3 |
| Sample * | Gellan (%) | i-Carrageenan (%) | Magnesium Acetate Solutions Concentrations (%), 100 mL | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| P1C | 100 | 0 | 1 | 97.25 |
| P2C | 2 | 91.5 | ||
| P3C | 3 | 87.23 | ||
| P4C | 70 | 30 | 2 | 85.71 |
| P5C | 80 | 20 | 2 | 90.4 |
| P6C | 90 | 10 | 2 | 94.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iurciuc, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. https://doi.org/10.3390/ijms22063075
Iurciuc C-E, Atanase LI, Jérôme C, Sol V, Martin P, Popa M, Ochiuz L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. International Journal of Molecular Sciences. 2021; 22(6):3075. https://doi.org/10.3390/ijms22063075
Chicago/Turabian StyleIurciuc (Tincu), Camelia-Elena, Leonard Ionuţ Atanase, Christine Jérôme, Vincent Sol, Patrick Martin, Marcel Popa, and Lăcrămioara Ochiuz. 2021. "Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin" International Journal of Molecular Sciences 22, no. 6: 3075. https://doi.org/10.3390/ijms22063075
APA StyleIurciuc, C.-E., Atanase, L. I., Jérôme, C., Sol, V., Martin, P., Popa, M., & Ochiuz, L. (2021). Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. International Journal of Molecular Sciences, 22(6), 3075. https://doi.org/10.3390/ijms22063075



.png)