Mode of Action of 1-Naphthylphthalamic Acid in Conspicuous Local Stem Swelling of Succulent Plant, Bryophyllum calycinum: Relevance to the Aspects of Its Histological Observation and Comprehensive Analyses of Plant Hormones
Abstract
1. Introduction
2. Results
2.1. Effect of NPA on Conspicuous Local Stem Swelling in Succulent Plants
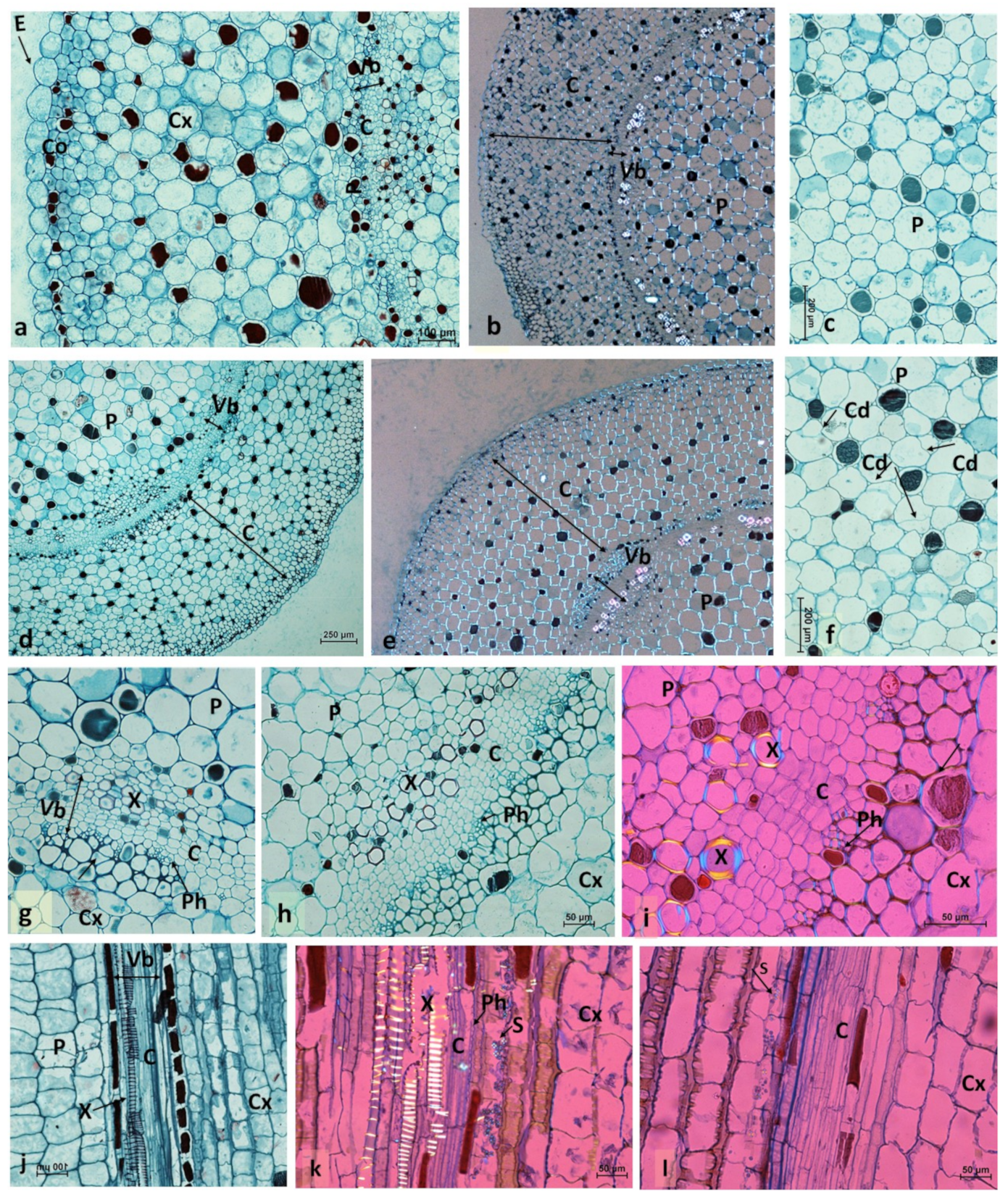
2.2. Histological Analyses of Conspicuous Local Stem Swelling in Succulent Plants
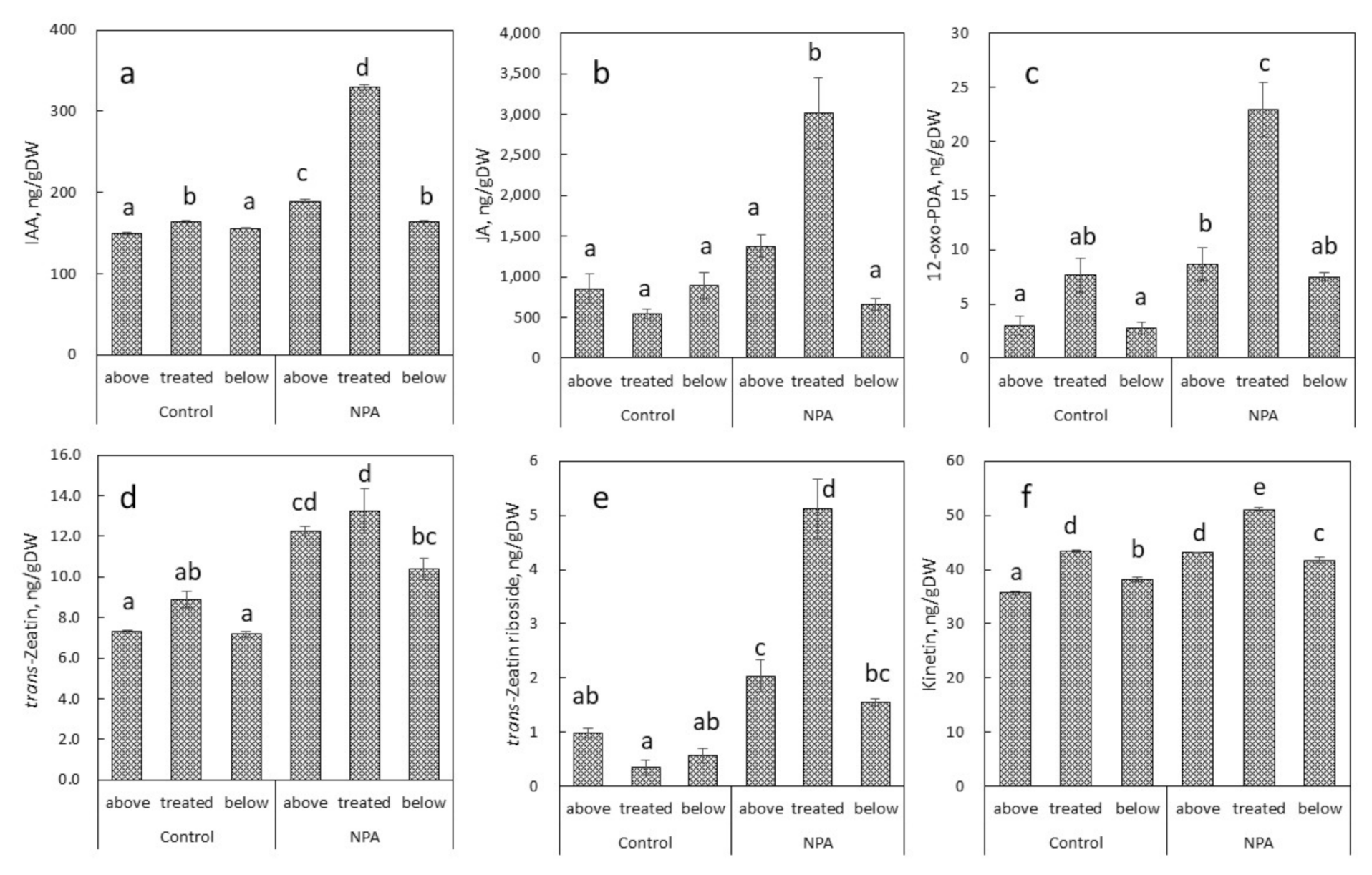
2.3. Endogenous Levels of Plant Hormones in Relation to NPA-Induced Conspicuous Local Stem Swelling in Succulent Plants
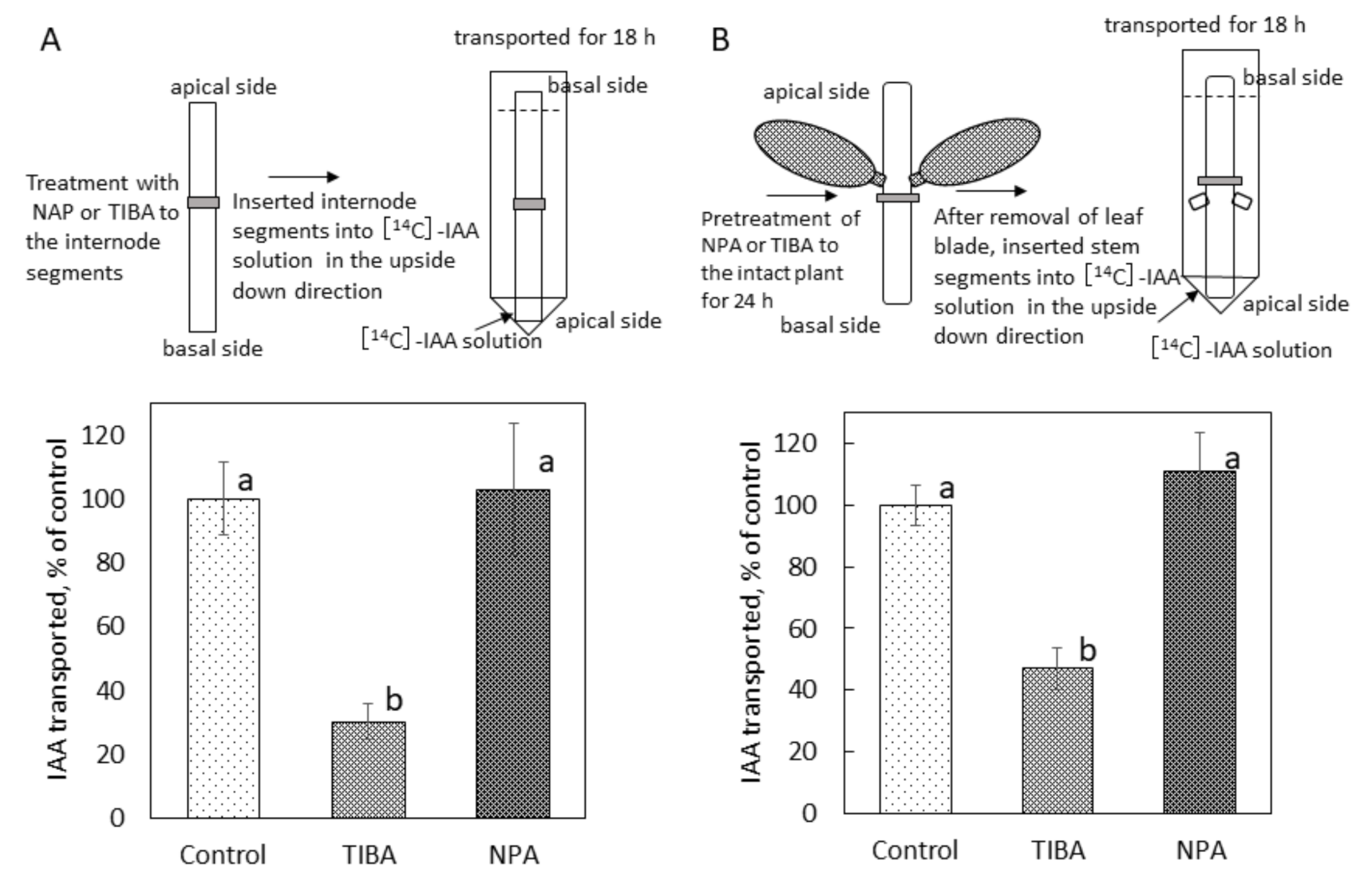
2.4. Effect of NPA on Polar Auxin Transport in Bryophyllum Calycinum Stem Segments
3. Discussion
4. Materials and Methods
4.1. Plant Materials and the Application of NPA and TIBA
4.2. Histological Analyses of Conspicuous Local Stem Swelling in Succulent Plants
4.3. Comprehensive Analyses of Endogenous Plant Hormones in Succulent Plants
4.4. Determination of Polar Auxin Transport in Bryophyllum Calycinum Stem Segments
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berleth, T.; Sachs, T. Plant morphogenesis: Long-distance coordination and local patterning. Curr. Opin. Plant Biol. 2001, 4, 57–62. [Google Scholar] [CrossRef]
- Taize, L.; Zeiger, M. Plant Physiology, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2002. [Google Scholar]
- Petrášek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef]
- Křeček, P.; Skůpa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zažímalová, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Javid, M.G.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef]
- Ueda, J.; Saniewski, M.; Miyamoto, K. Auxin, One Major Plant Hormone, in Soil. In Bioactive Compounds in Agricultural Soils; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–209. [Google Scholar]
- Saniewski, M.; Góraj, J.; Węgrzynowicz-Lesiak, E.; Miyamoto, K.; Ueda, J. Differential effects of auxin polar transport inhibitors on rooting in some Crassulaceae species. Acta Agrobot. 2014, 67, 85–92. [Google Scholar] [CrossRef]
- Kulka, R.G. Hormonal control of root development on epiphyllous plantlets of Bryophyllum (Kalanchoe) marnierianum: Role of auxin and ethylene. J. Exp. Bot. 2008, 59, 2361–2370. [Google Scholar] [CrossRef]
- Ueda, J.; Góraj-Koniarska, J.; Miyamoto, K.; Saniewski, M. Epinasty and/or hyponasty, and petiole growth in Bryophyllum calycinum: Focus on the interaction of indole-3-acetic acid and methyl jasmonate. Acta Biol. Crac. Ser. Bot. 2018, 60, 73–81. [Google Scholar]
- Ueda, J.; Miyamoto, K.; Góraj-Koniarska, J.; Saniewski, M. Petiole bending in detached leaves of Bryophyllum calycinum: Rel-evance to polar auxin transport in petioles. Acta Biol. Crac. Ser. Bot. 2018, 60, 25–33. [Google Scholar]
- Saniewski, M.; Góraj-Koniarska, J.; Gabryszewska, E.; Miyamoto, K.; Ueda, J. Differential effects of N-1-naphthylphthalamic acid (NPA) and 2,3,5-triiodobenzoic acid (TIBA) on auxin control of swelling of the shoots of Bryophyllum calycinum Salisb. Acta Agrobot. 2017, 70, 1723. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K. New Aspects of Gravity Responses in Plant Cells. Adv. Appl. Microbiol. 2003, 229, 209–244. [Google Scholar] [CrossRef]
- Guo, D.P.; Jiang, Y.T.; Zeng, G.W.; Ali, G. Stem swelling of stem mustard, as affected by temperature and growth regulators. Sci.Hortic. 1994, 60, 153–160. [Google Scholar] [CrossRef]
- Abraham, P.E.; Yin, H.; Borland, A.M.; Weighill, D.; Lim, S.D.; De Paoli, H.C.; Engle, N.; Jones, P.C.; Agh, R.; Weston, D.J.; et al. Transcript, protein and metabolite temporal dynamics in the CAM plant Agave. Nat. Plants 2016, 2, 16178. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, R.; Kępczyński, J.; Pilarska, M.; Cembrowska, D.; Menzel, D.; Samaj, J. Cytokinin and ethylene affect auxin transport-dependent rhizogenesis in hypocotyls of common ice plant (Mesembryanthemum crystallinum L.). J. Plant. Growth Regul. 2009, 28, 331–340. [Google Scholar] [CrossRef]
- Nissen, S.J.; Foley, M.E. Correlative inhibition and dormancy in root buds of leafy spurge (Euphorbia escula). Weed Sci. 1987, 35, 155–159. [Google Scholar] [CrossRef]
- Horvath, D.P. The role of specific plant organs and polar auxin transport on correlative inhibition of leafy spurge (Euphorbia escula) root buds. Can. J. Bot. 1998, 76, 1227–1235. [Google Scholar]
- Juárez, M.J.A.; Cárdenas, R.H.; Villa, J.N.S.; O’Connor, D.; Sluis, A.; Hake, S.; Ordaz-Ortiz, J.; Terry, L.; Simpson, J. Functionally different PIN proteins control auxin flux during bulbil development in Agave Tequilana. J. Exp. Bot. 2015, 66, 3893–3905. [Google Scholar] [CrossRef]
- Campanoni, P.; Blasius, B.; Nick, P. Auxin Transport Synchronizes the Pattern of Cell Division in a Tobacco Cell Line. Plant Physiol. 2003, 133, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Nonghmaithem, S.; Devulapalli, S.; Sreelakshmi, Y.; Sharma, R. Is naphthylphthalamic acid a specific phytotropin? It elevates ethylene and alteres metabolic homeostasis in tomato. Plant. Sci. 2020, 291, 110358. [Google Scholar] [CrossRef]
- Little, C.H.A.; Macdonald, J.E.; Olsson, O. Involvement of Indole-3-Acetic Acid in Fascicular and Interfascicular Cambial Growth and Interfascicular Extraxylary Fiber Differentiation in Arabidopsis thaliana Inflorescence Stems. Int. J. Plant Sci. 2002, 163, 519–529. [Google Scholar] [CrossRef]
- Suer, S.; Agusti, J.; Sanchez, P.; Schwarz, M.; Greb, T. WOX4 Imparts Auxin Responsiveness to Cambium Cells in Arabidopsis. Plant Cell 2011, 23, 3247–3259. [Google Scholar] [CrossRef]
- Ji, J.; Strable, J.; Shimizu, R.; Koenig, D.; Sinha, N.; Scanlon, M.J. WOX4 Promotes Procambial Development. Plant Physiol. 2010, 152, 1346–1356. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 home-box gene in Arabidopsis. Plant. Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.; Sung, Z.R.; Berleth, T. Responses of plant vascular systems to auxin transport inhibition. Development 1999, 126, 2979–2991. [Google Scholar]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.E.; Smith, O.E. Cytokinins and tuber induction in the potato plants. Nature 1969, 221, 279–280. [Google Scholar] [CrossRef]
- Shibaoka, H. Involvement of wall microtubule in gibberellin promotion and kinetin inhibition of stem elongation. Plant. Cell Physiol. 1974, 15, 255–263. [Google Scholar]
- Miyamoto, K.; Kato-Noguchi, H.; Hashimoto, T. Activities of growth inhibitors isolated from light-grown dwarf pea shoots. Plant Growth Regul. 1992, 11, 411–417. [Google Scholar] [CrossRef]
- Hashimoto, T. Synergistic Effect of Indoleacetic Acid and Kinetin on the Primary Thickening of Pea Stem Segments. J. Plant Res. 1961, 74, 110–117. [Google Scholar] [CrossRef]
- Katsumi, M. Physiological Effects of Kinetin Effect on the Thickening of Etiolated Pea Stem Sections. Physiol. Plant. 1962, 15, 115–121. [Google Scholar] [CrossRef]
- Vanderhoef, L.N.; Stahl, C.; Siegel, N.; Zeigber, R. The inhibitions by cytokinin of auxin-promoted elongation in excised soy-bean hypocotyl. Physiol. Plant. 1973, 29, 22–27. [Google Scholar] [CrossRef]
- Victor, T.S.; Vanderhoef, L.N. Mechanical Inhibition of Hypocotyl Elongation Induces Radial Enlargement. Plant Physiol. 1975, 56, 845–846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saniewski, M.; Węgrzynowicz-Lesiak, E.; Góraj-Koniarska, J.; Gabryszewska, E. Effect of benzyladenine (BA) on aux-in-induced stem elongation and thickening in tulip (Tulipa gesneriana L.). Acta Agrobot. 2016, 69, 1650. [Google Scholar]
- Kushwah, S.; Jones, A.M.; Laxmi, A. Cytokinin Interplay with Ethylene, Auxin, and Glucose Signaling Controls Arabidopsis Seedling Root Directional Growth. Plant Physiol. 2011, 156, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Pernisová, M.; Klima, P.; Horák, J.; Valková, M.; Malbeck, J.; Souček, P.; Reichman, P.; Hoyerová, K.; Dubová, J.; Friml, J.; et al. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc. Natl. Acad. Sci. USA 2009, 106, 3609–3614. [Google Scholar] [CrossRef]
- Hu, W.; Fagundez, S.; Katin-Grazzini, L.; Li, Y.; Li, W.; Chen, Y.; Wang, X.; Deng, Z.; Xie, S.; McAvoy, R.J. Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Hortic. Res. 2017, 4, 17071. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Kikuta, Y.; Tazaki, H.; Tsujino, Y.; Sakamura, S.; Yoshihara, T. Potato tuber-inducing activities of jasmonic acid and related compounds. Phytochemistry 1991, 30, 1435–1438. [Google Scholar] [CrossRef]
- Tung, P.; Hooker, T.S.; Tampe, P.A.; Reid, D.M.; Thorpe, T.A. Jasmonic Acid: Effects on Growth and Development of Isolated Tomato Roots Cultured In vitro. Int. J. Plant Sci. 1996, 157, 713–721. [Google Scholar] [CrossRef]
- Hentrich, M.; Böttcher, C.; Düchting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation ofYUCCA8andYUCCA9gene expression. Plant J. 2013, 74, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.C.; Goossens, A. Jasmonate signaling: A copycat of auxin signaling? Plant. Cell Environ. 2013, 36, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed]
- Pazmino, D.M.; Rodriguez-Serrano, M.; Romero-Puertas, M.C.; Sandalio, L.M. Regulation of epinasty induced by 2,4-dichlorophenoxyacetic acid in pea and Arabidopsis plants. Biol. Plant. 2014, 16, 809–818. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Saniewski, M.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Ueda, J.; Miyamoto, K. Formation of the Secondary Abscission Zone Induced by the Interaction of Methyl Jasmonate and Auxin in Bryophyllum calycinum: Relevance to Auxin Status and Histology. Int. J. Mol. Sci. 2020, 21, 2784. [Google Scholar] [CrossRef] [PubMed]
- Dziurka, M.; Janeczko, A.; Juhász, C.; Gullner, G.; Oklestková, J.; Novák, O.; Saja, D.; Skoczowski, A.; Tóbiás, I.; Barna, B. Local and systemic hormonal responses in pepper leaves during compatible and incompatible pepper-tobamovirus interactions. Plant Physiol. Biochem. 2016, 109, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Płażek, A.; Dubert, F.; Kopeć, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Wolko, B. Seed Hydropriming and Smoke Water Significantly Improve Low-Temperature Germination of Lupinus Angustifolius L. Int. J. Mol. Sci. 2018, 19, 992. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Koźmińska, A.; Hanus-Fajerska, E.; Dziurka, M.; Dziurka, K. Insight into mechanisms of multiple stresses tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019, 180, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Saniewski, M.; Dziurka, M.; Dziurka, K.; Góraj-Koniarska, J.; Ueda, J.; Miyamoto, K. Methyl jasmonate induces leaf senescence of Ginkgo biloba L.: Relevance to endogenous levels of plant hormones. Plant Growth Regul. 2020, 91, 383–396. [Google Scholar] [CrossRef]

| Plant Hormone, ng/gDW | Control (Lanolin) | NPA Treatment | ||||
|---|---|---|---|---|---|---|
| Above | Treated Area | Below | Above | Treated Area | Below | |
| Abscisic acid | 163.3 ± 2.9 b | 204.9 ± 6.2 b | 211.0 ± 4.8 b,c | 209.6 ± 3.7 a | 260.1 ± 17.0 a | 239.7 ± 5.7 c |
| Salicylic acid | 1509 ± 157 b,c | 1520 ± 134 a,b,c | 2254 ± 288 a | 2332 ± 70 b,c | 4631 ± 787 c | 1667 ± 106 a,b |
| cis-Zeatin | 5.5 ± 0.17 a | 7.1 ± 0.16 c | 6.2 ± 0.07 b | 6.8 ± 0.22 b,c | 8.7 ± 0.27 d | 7.1 ± 0.07 c |
| cis-Zeatin riboside | 0.1 ± 0.06 a | 0.2 ± 0.16 a | 0.0 ± 0.01 a | 0.5 ± 0.16 a | 1.2 ± 0.0.37 b | 0.5 ± 0.12 a |
| Gibberellin A1 | 13.8 ± 0.8 c | 18.1 ± 1.4 b | 23.5 ± 1.1 c | 14.6 ± 1.2 a | 20.9 ± 0.9 a | 21.7 ± 1.3 c |
| Gibberellin A4 | 5.0 ± 1.0 a | 6.7 ± 0.9 a | 6.3 ± 1.8 a | 9.4 ±1.8 a | 9.7 ± 2.7 b | 8.4 ± 2.7 a |
| Gibberellin A19 | 35.5 ± 1.9 b | 44.7 ± 0.7 a | 48.9 ± 0.8 a | 53.5 ± 0.3 b | 47.4 ± 2.9 b | 52.5 ± 2.7 b |
| Gibberellin A44 | 3.0 ± 0.1 b | 4.1 ± 0.1 b | 5.1 ± 0.2 a | 2.5 ± 0.2 b | 4.8 ± 0.5 b | 3.8 ± 0.4 b |
| Gibberellin A15 | 1.4 ± 0.1 b | 9.2 ± 0.2 a | 1.1 ± 0.1 b | 1.6 ± 0.1 b | 1.2 ± 0.5 a | 2.5 ± 0.2 a |
| Gibberellin A53 | 21.5 ± 2.1 b | 54.2 ± 4.7 c | 106.7 ± 2.6 c | 55.6 ± 2.6 a | 63.3 ± 14.3 d | 129.8 ± 9.7 b,c |
| Gibberellin A9 | 35.3 ± 2.7 a,b | 43.7 ± 0.9 b,c | 41.0 ± 5.7 a | 39.4 ± 1.6 d | 40.3 ± 3.2 c | 49.2 ± 1.6 b,c |
| Gibberellin A8 | 50.7 ± 11.7 a,b | 64.9 ± 29.6 b,c | 72.9 ± 8.2 a | 72.1 ± 9.9 c | 79.2 ± 16.8 c | 76.0 ± 18.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasek-Ciolakowska, A.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Saniewski, M.; Ueda, J.; Miyamoto, K. Mode of Action of 1-Naphthylphthalamic Acid in Conspicuous Local Stem Swelling of Succulent Plant, Bryophyllum calycinum: Relevance to the Aspects of Its Histological Observation and Comprehensive Analyses of Plant Hormones. Int. J. Mol. Sci. 2021, 22, 3118. https://doi.org/10.3390/ijms22063118
Marasek-Ciolakowska A, Dziurka M, Kowalska U, Góraj-Koniarska J, Saniewski M, Ueda J, Miyamoto K. Mode of Action of 1-Naphthylphthalamic Acid in Conspicuous Local Stem Swelling of Succulent Plant, Bryophyllum calycinum: Relevance to the Aspects of Its Histological Observation and Comprehensive Analyses of Plant Hormones. International Journal of Molecular Sciences. 2021; 22(6):3118. https://doi.org/10.3390/ijms22063118
Chicago/Turabian StyleMarasek-Ciolakowska, Agnieszka, Michał Dziurka, Urszula Kowalska, Justyna Góraj-Koniarska, Marian Saniewski, Junichi Ueda, and Kensuke Miyamoto. 2021. "Mode of Action of 1-Naphthylphthalamic Acid in Conspicuous Local Stem Swelling of Succulent Plant, Bryophyllum calycinum: Relevance to the Aspects of Its Histological Observation and Comprehensive Analyses of Plant Hormones" International Journal of Molecular Sciences 22, no. 6: 3118. https://doi.org/10.3390/ijms22063118
APA StyleMarasek-Ciolakowska, A., Dziurka, M., Kowalska, U., Góraj-Koniarska, J., Saniewski, M., Ueda, J., & Miyamoto, K. (2021). Mode of Action of 1-Naphthylphthalamic Acid in Conspicuous Local Stem Swelling of Succulent Plant, Bryophyllum calycinum: Relevance to the Aspects of Its Histological Observation and Comprehensive Analyses of Plant Hormones. International Journal of Molecular Sciences, 22(6), 3118. https://doi.org/10.3390/ijms22063118






