Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes
Abstract
1. Introduction
2. Results
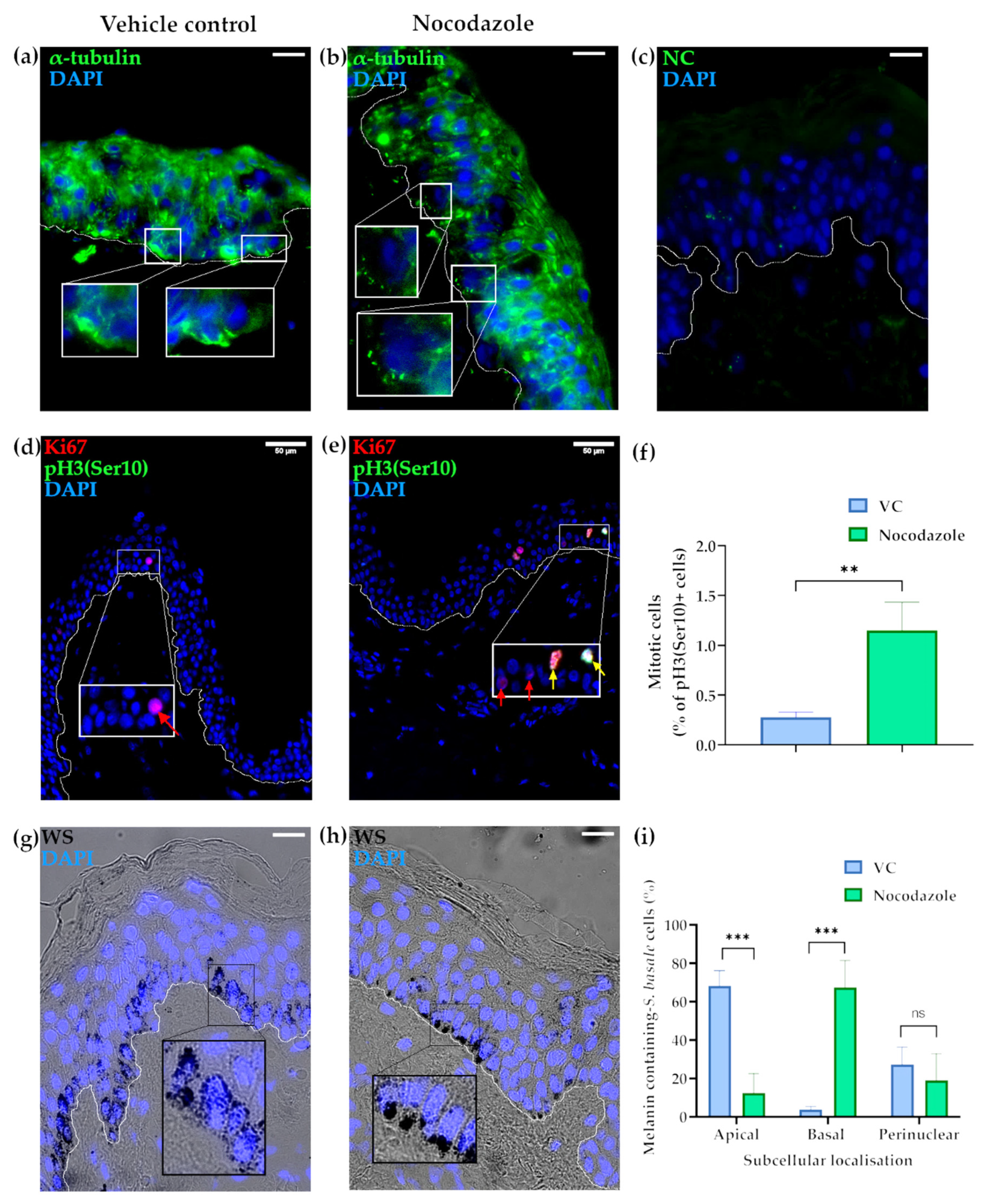
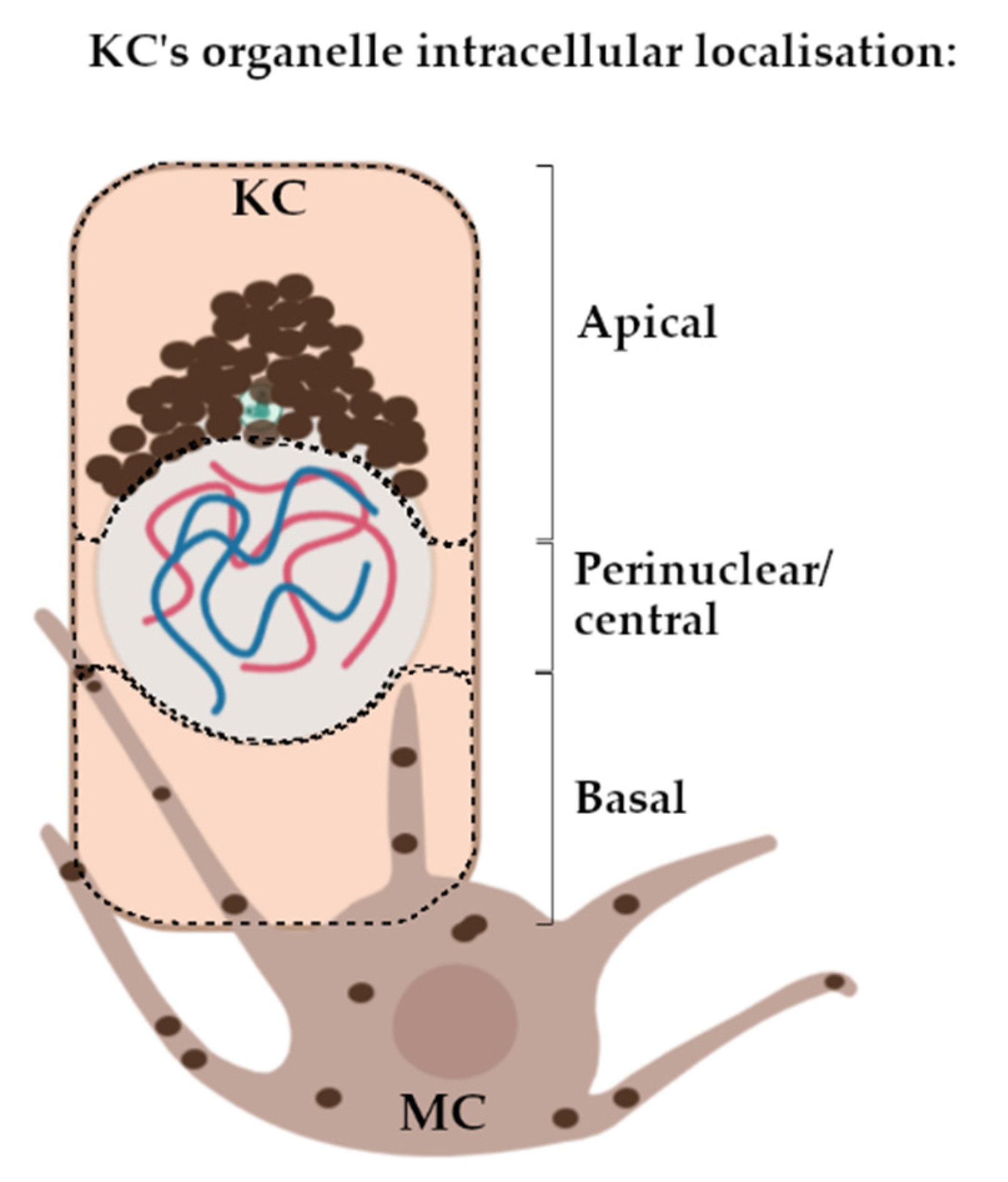
2.1. Microtubules Are Involved in the Localisation of Melanin into Supranuclear Caps in S. basale KCs
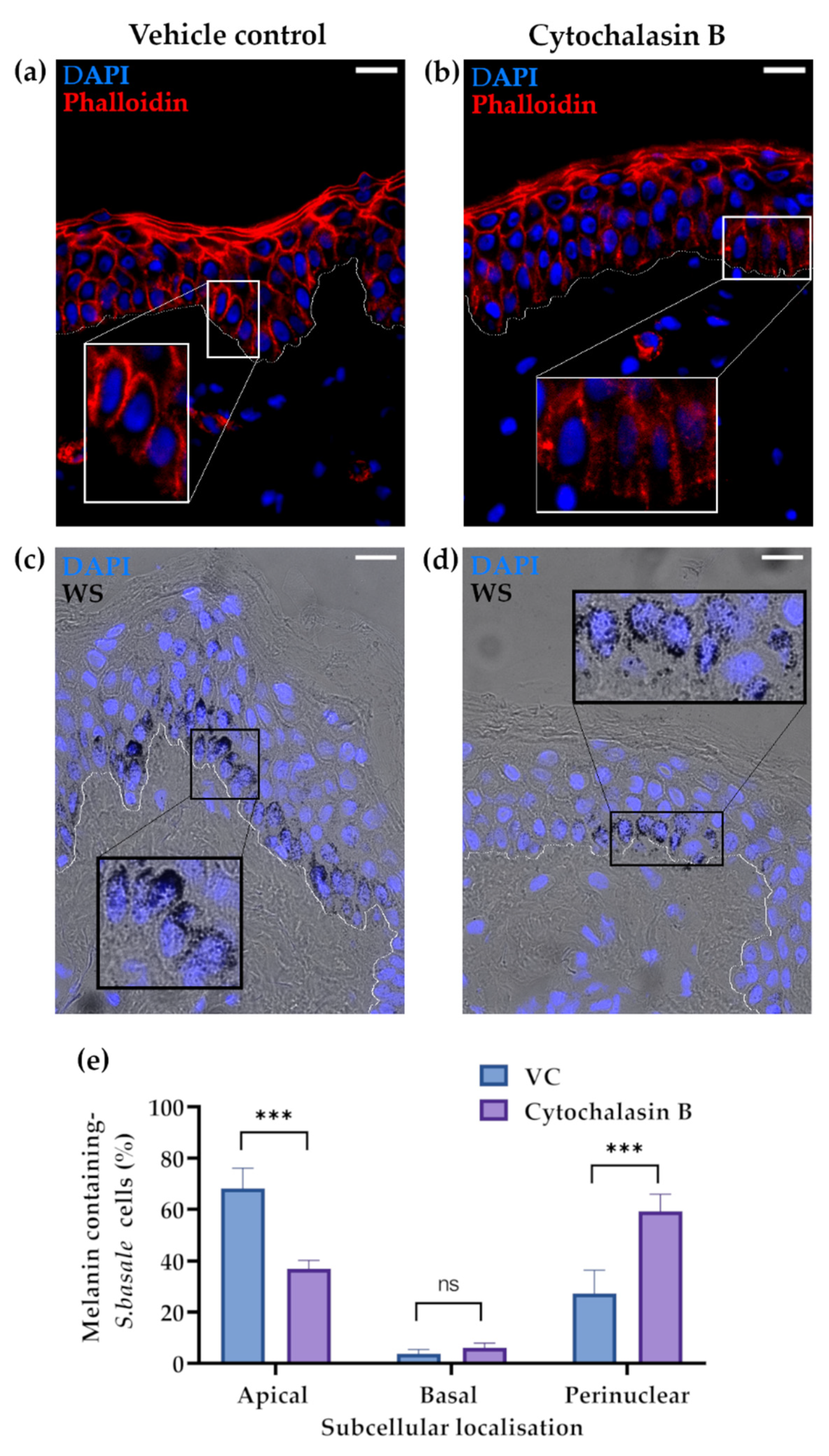
2.2. The Actin Cytoskeleton Is Involved in Melanin Granule Aggregation in S. basale KCs
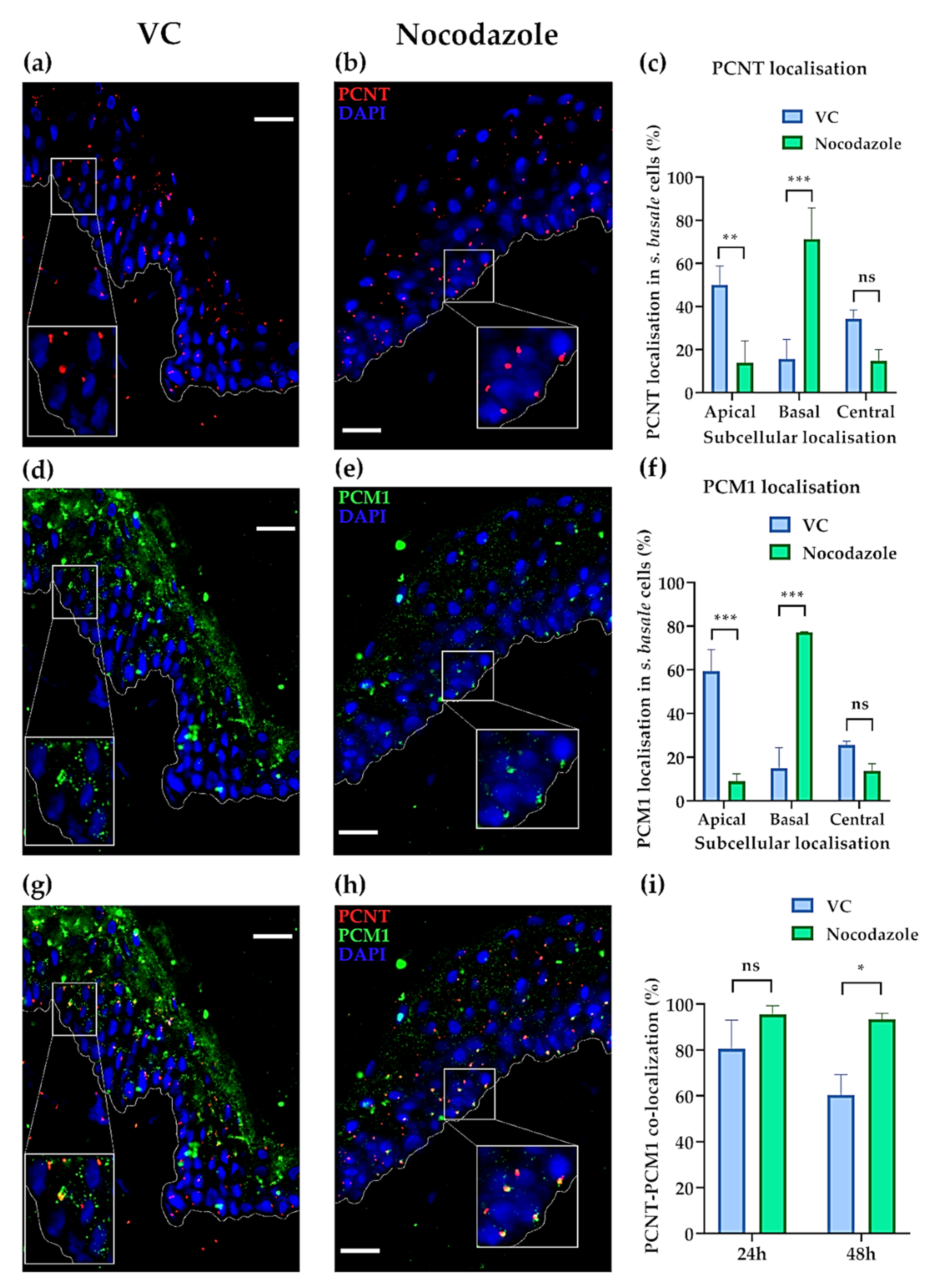
2.3. The Centrosome, Centriolar Satellites and Melanin Granules Show a Coordinated Movement
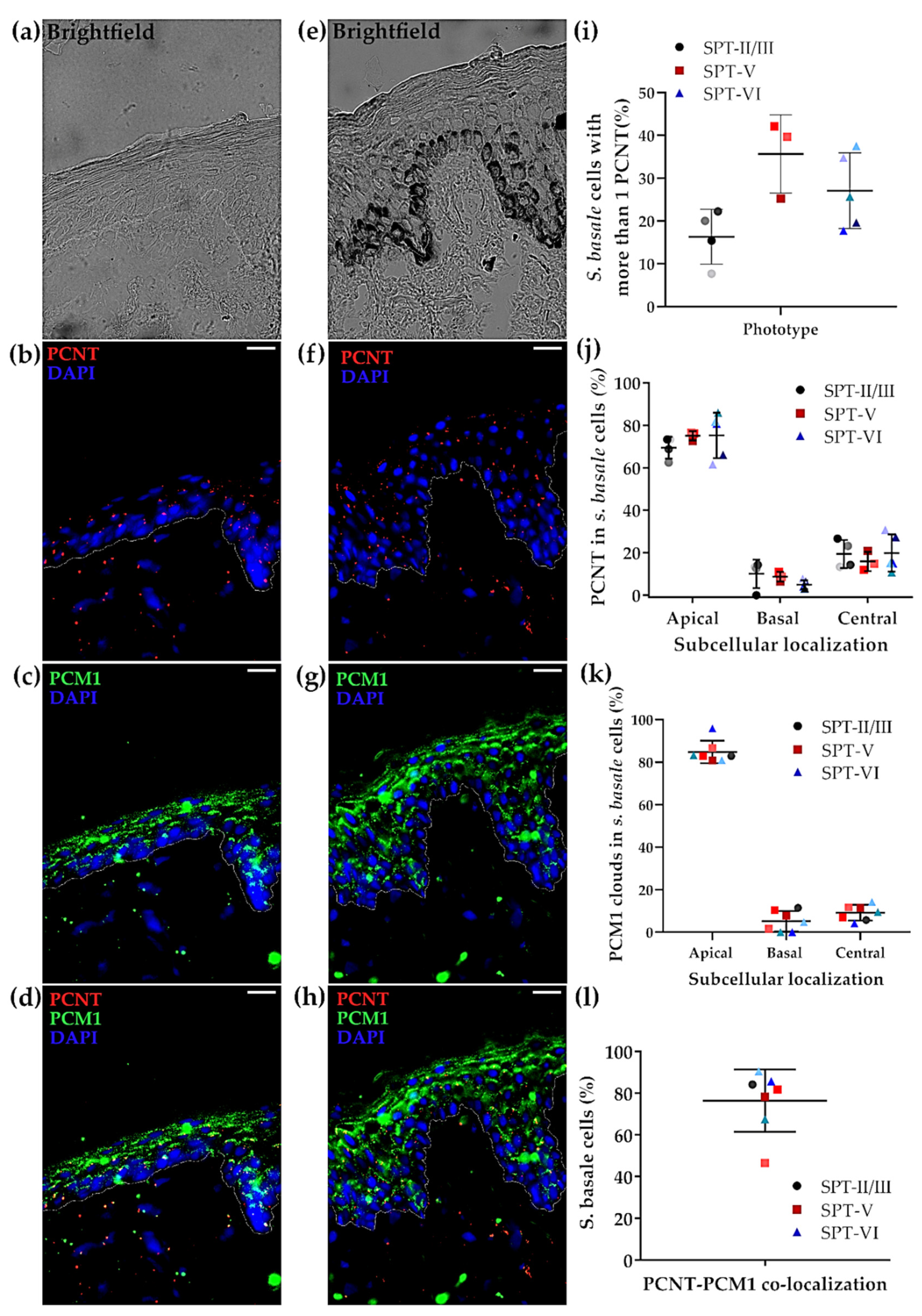
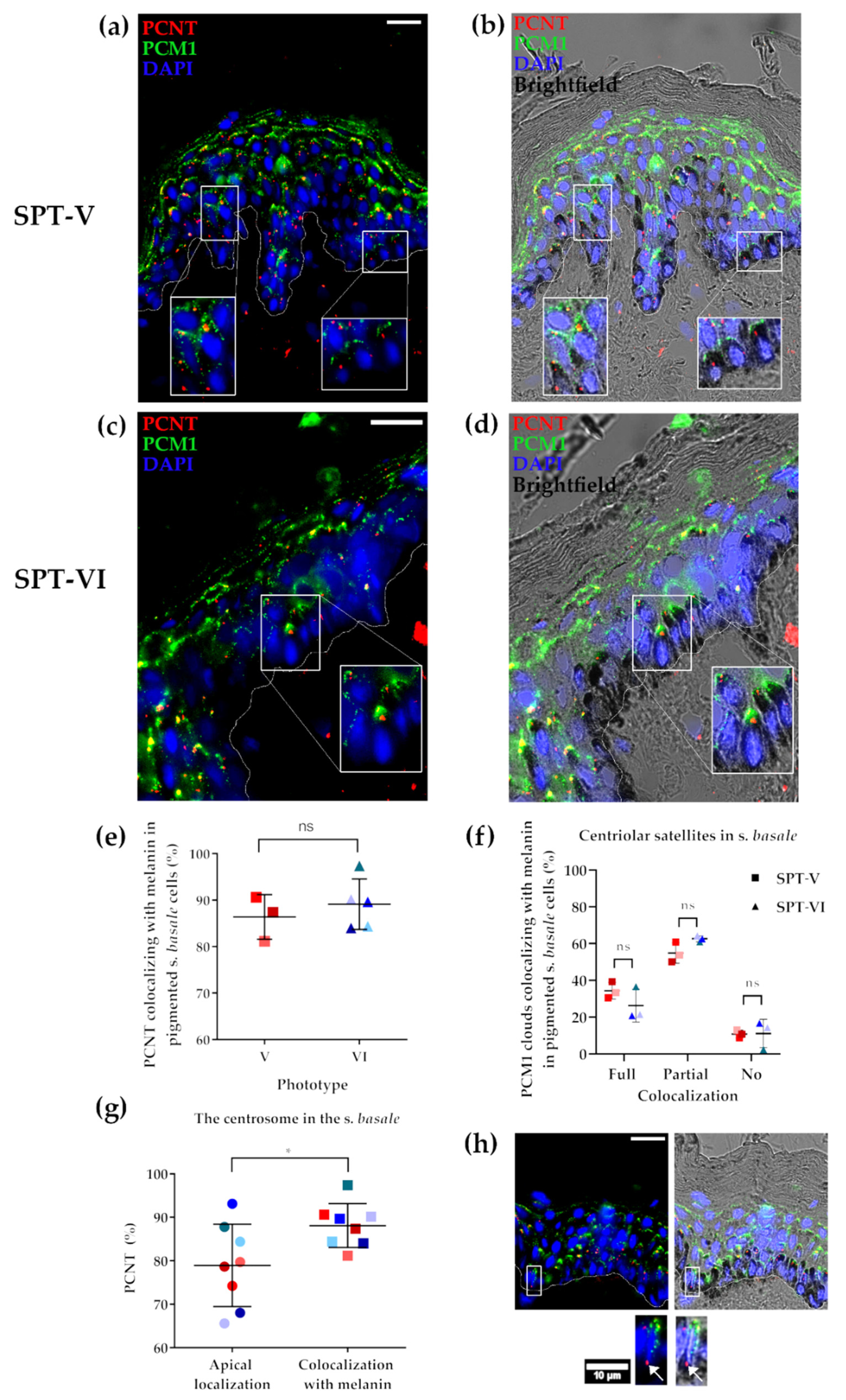
2.4. The Status of the Centrosome/Microtubule Machinery in Low versus High SPT Human Skin In Situ
2.5. Melanin Co-Localises with the Centrosome (PCNT) in Both Light and Dark Skin Phototypes In Vivo
3. Discussion
4. Materials and Methods
4.1. Human Skin Samples
4.2. Histoculture of Full-Thickness Human Skin Ex Vivo
4.3. Immunohistochemistry/Immunofluorescence
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAPI | 4′,6-diamidino-2-phenylindole |
| DNCT1 | Dynactin 1 |
| F | Female |
| KC | Keratinocyte |
| KRT10 | Keratin 10 |
| M | Male |
| MC | Melanocyte |
| MT | Microtubule |
| MTOC | MT-organising centre |
| NINL | Ninein-like |
| PCM1 | Pericentriolar Material 1 |
| PCNT | Pericentrin |
| RPE | Retinal pigmented epithelium |
| S. | stratum |
| SPT | Skin phototype |
| UVR | Ultraviolet radiation |
| VC | Vehicle control |
| WS | Warthin–Starry |
References
- Kobayashi, N.; Nakagawa, A.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Hashimoto, M.W.; Ishigaki, Y.; Ohnishi, T.; Mori, T. Supranuclear Melanin Caps Reduce Ultraviolet Induced DNA Photoproducts in Human Epidermis. J. Investig. Dermatol. 1998, 110, 806–810. [Google Scholar] [CrossRef]
- Fajuyigbe, D.; Lwin, S.M.; Diffey, B.L.; Baker, R.; Tobin, D.J.; Sarkany, R.P.E.; Young, A.R. Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J. 2018, 32, 3700–3706. [Google Scholar] [CrossRef]
- Byers, H.R.; Maheshwary, S.; Amodeo, D.M.; Dykstra, S.G. Role of cytoplasmic dynein in perinuclear aggregation of phagocytosed melanosomes and supranuclear melanin cap formation in human keratinocytes. J. Investig. Dermatol. 2003, 121, 813–820. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D.J. An explanation for the mysterious distribution of melanin in human skin—A rare example of asymmetric (melanin) organelle distribution during mitosis of basal layer progenitor keratinocytes. Br. J. Dermatol. 2018, 179. [Google Scholar] [CrossRef]
- Correia, M.S.; Moreiras, H.; Pereira, F.J.C.; Neto, M.V.; Festas, T.C.; Tarafder, A.K.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Melanin Transferred to Keratinocytes Resides in Nondegradative Endocytic Compartments. J. Investig. Derm. 2018, 138, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Hurbain, I.; Romao, M.; Sextius, P.; Bourreau, E.; Marchal, C.; Bernerd, F.; Duval, C.; Raposo, G. Melanosome Distribution in Keratinocytes in Different Skin Types: Melanosome Clusters Are Not Degradative Organelles. J. Investig. Dermatol. 2018, 138, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Borovanský, J.; Elleder, M. Melanosome Degradation: Fact or Fiction. Pigment. Cell Res. 2003, 16, 280–286. [Google Scholar] [CrossRef]
- Borovanský, J.; Hach, P.; Smetana, K., Jr.; Elleder, M.; Matouš-Malbohan, I. Attempts to induce melanosome degradation in vivo. Folia Biol. 1999, 45, 47–52. [Google Scholar] [CrossRef]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M.C. Melanosomes at a glance. J. Cell Sci. 2008, 121, 3995. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.A., 3rd; Sellers, J.R. Walking to work: Roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 2011, 13, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Langford, G.M. Actin- and microtubule-dependent organelle motors: Interrelationships between the two motility systems. Curr. Opin. Cell Biol. 1995, 7, 82–88. [Google Scholar] [CrossRef]
- Wu, X.; Bowers, B.; Rao, K.; Wei, Q.; Hammer, J.A., 3rd. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J. Cell Biol. 1998, 143, 1899–1918. [Google Scholar] [CrossRef]
- Alzahofi, N.; Welz, T.; Robinson, C.L.; Page, E.L.; Briggs, D.A.; Stainthorp, A.K.; Reekes, J.; Elbe, D.A.; Straub, F.; Kallemeijn, W.W.; et al. Rab27a co-ordinates actin-dependent transport by controlling organelle-associated motors and track assembly proteins. Nat. Commun. 2020, 11, 3495. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.L.; Evans, R.D.; Briggs, D.A.; Ramalho, J.S.; Hume, A.N. Inefficient recruitment of kinesin-1 to melanosomes precludes it from facilitating their transport. J. Cell Sci. 2017, 130, 2056–2065. [Google Scholar] [CrossRef]
- Evans, R.D.; Robinson, C.; Briggs, D.A.; Tooth, D.J.; Ramalho, J.S.; Cantero, M.; Montoliu, L.; Patel, S.; Sviderskaya, E.V.; Hume, A.N. Myosin-Va and Dynamic Actin Oppose Microtubules to Drive Long-Range Organelle Transport. Curr. Biol. 2014, 24, 1743–1750. [Google Scholar] [CrossRef]
- Wu, X.; Rao, K.; Bowers, M.B.; Copeland, N.G.; Jenkins, N.A.; Hammer, J.A., 3rd. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 2001, 114, 1091–1100. [Google Scholar]
- Hume, A.N.; Collinson, L.M.; Hopkins, C.R.; Strom, M.; Barral, D.C.; Bossi, G.; Griffiths, G.M.; Seabra, M.C. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic 2002, 3, 193–202. [Google Scholar] [CrossRef]
- Wu, X.S.; Rao, K.; Zhang, H.; Wang, F.; Sellers, J.R.; Matesic, L.E.; Copeland, N.G.; Jenkins, N.A.; Hammer, J.A. Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 2002, 4, 271–278. [Google Scholar] [CrossRef]
- Hirokawa, N. Kinesin and Dynein Superfamily Proteins and the Mechanism of Organelle Transport. Science 1998, 279, 519. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, N.; Maruta, Y.; Ishida, M.; Fukuda, M. Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes. J. Cell Sci. 2012, 125, 1508–1518. [Google Scholar] [CrossRef]
- Matsui, T.; Ohbayashi, N.; Fukuda, M. The Rab interacting lysosomal protein (RILP) homology domain functions as a novel effector domain for small GTPase Rab36: Rab36 regulates retrograde melanosome transport in melanocytes. J. Biol. Chem. 2012, 287, 28619–28631. [Google Scholar] [CrossRef]
- Ishida, M.; Ohbayashi, N.; Fukuda, M. Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP. Sci. Rep. 2015, 5, 8238. [Google Scholar] [CrossRef]
- Hara, M.; Yaar, M.; Byers, H.R.; Goukassian, D.; Fine, R.E.; Gonsalves, J.; Gilchrest, B.A. Kinesin participates in melanosomal movement along melanocyte dendrites. J. Investig. Dermatol. 2000, 114, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Vancoillie, G.; Lambert, J.; Naeyaert, J.M.; Mulder, A.; Koerten, H.K.; Mommaas, A.M.; Van Oostveldt, P. Kinesin and Kinectin Can Associate with the Melanosomal Surface and Form a Link with Microtubules in Normal Human Melanocytes1. J. Investig. Dermatol. 2000, 114, 421–429. [Google Scholar] [CrossRef]
- Yin, L.; Coelho, S.G.; Ebsen, D.; Smuda, C.; Mahns, A.; Miller, S.A.; Beer, J.Z.; Kolbe, L.; Hearing, V.J. Epidermal gene expression and ethnic pigmentation variations among individuals of Asian, European and African ancestry. Exp. Dermatol. 2014, 23, 731–735. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Jiang, M.; Paniagua, A.E.; Volland, S.; Wang, H.; Balaji, A.; Li, D.G.; Lopes, V.S.; Burgess, B.L.; Williams, D.S. Microtubule motor transport in the delivery of melanosomes to the actin-rich apical domain of the retinal pigment epithelium. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Lechler, T.; Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005, 437, 275–280. [Google Scholar] [CrossRef]
- Tellkamp, F.; Vorhagen, S.; Niessen, C.M. Epidermal Polarity Genes in Health and Disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015255. [Google Scholar] [CrossRef] [PubMed]
- Meiring, J.C.M.; Shneyer, B.I.; Akhmanova, A. Generation and regulation of microtubule network asymmetry to drive cell polarity. Curr. Opin. Cell Biol. 2020, 62, 86–95. [Google Scholar] [CrossRef]
- Bornens, M. The centrosome in cells and organisms. Science 2012, 335, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Delaval, B.; Doxsey, S.J. Pericentrin in cellular function and disease. J. Cell Biol. 2010, 188, 181. [Google Scholar] [CrossRef] [PubMed]
- Prosser, S.L.; Pelletier, L. Centriolar satellite biogenesis and function in vertebrate cells. J. Cell Sci. 2020, 133, jcs239566. [Google Scholar] [CrossRef] [PubMed]
- Balczon, R.; Bao, L.; Zimmer, W.E. PCM-1, A 228-kD centrosome autoantigen with a distinct cell cycle distribution. J. Cell Biol. 1994, 124, 783–793. [Google Scholar] [CrossRef]
- Quarantotti, V.; Chen, J.-X.; Tischer, J.; Gonzalez Tejedo, C.; Papachristou, E.K.; D’Santos, C.S.; Kilmartin, J.V.; Miller, M.L.; Gergely, F. Centriolar satellites are acentriolar assemblies of centrosomal proteins. EMBO J. 2019, 38, e101082. [Google Scholar] [CrossRef] [PubMed]
- Dammermann, A.; Merdes, A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 2002, 159, 255–266. [Google Scholar] [CrossRef]
- Teusel, F.; Henschke, L.; Mayer, T.U. Chapter 6—Small molecule tools in mitosis research. In Methods in Cell Biology; Maiato, H., Schuh, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 144, pp. 137–155. [Google Scholar]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. 2018. [Google Scholar] [CrossRef]
- Singh, S.K.; Kurfurst, R.; Nizard, C.; Schnebert, S.; Perrier, E.; Tobin, D.J. Melanin transfer in human skin cells is mediated by filopodia--a model for homotypic and heterotypic lysosome-related organelle transfer. FASEB J. 2010, 24, 3756–3769. [Google Scholar] [CrossRef]
- Ugarte, F.; Porth, K.; Sadekova, S. Histone H3 Phosphorylation in Human Skin Histoculture as a Tool to Evaluate Patient’s Response to Antiproliferative Drugs. Biomark Insights 2015, 10, 73–76. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Wibawa, J.I.; Bell, M.; Tobin, D.J. Melanin fate in the human epidermis: A reassessment of how best to detect and analyse histologically. Exp. Derm. 2016, 25, 501–504. [Google Scholar] [CrossRef]
- Hans, F.; Dimitrov, S. Histone H3 phosphorylation and cell division. Oncogene 2001, 20, 3021–3027. [Google Scholar] [CrossRef] [PubMed]
- Sharlow, E.R.; Paine, C.S.; Babiarz, L.; Eisinger, M.; Shapiro, S.; Seiberg, M. The protease-activated receptor-2 upregulates keratinocyte phagocytosis. J. Cell Sci. 2000, 113, 3093. [Google Scholar] [PubMed]
- McGuire, J.; Moellmann, G. Cytochalasin B: Effects on Microfilaments and Movement of Melanin Granules within Melanocytes. Science 1972, 175, 642. [Google Scholar] [CrossRef]
- Flanagan, A.M.; Stavenschi, E.; Basavaraju, S.; Gaboriau, D.; Hoey, D.A.; Morrison, C.G. Centriole splitting caused by loss of the centrosomal linker protein C-NAP1 reduces centriolar satellite density and impedes centrosome amplification. Mol. Biol. Cell 2017, 28, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.; Ahn, Y.; Lee, E.J.; Hwang, S.; Almurayshid, A.; Park, K.; Chung, H.-J.; Kim, H.J.; Lee, S.-H.; et al. Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes. Pigment. Cell Melanoma Res. 2020, 33, 403–415. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, B.; Pan, Y.; Huang, P.; Wang, C. Autophagy participates in isoliquiritigenin–induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed. Pharmacother. 2018, 97, 248–254. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, M.O.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy Has a Significant Role in Determining Skin Color by Regulating Melanosome Degradation in Keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef]
- Zhang, C.-F.; Gruber, F.; Ni, C.; Mildner, M.; Koenig, U.; Karner, S.; Barresi, C.; Rossiter, H.; Narzt, M.-S.; Nagelreiter, I.M.; et al. Suppression of Autophagy Dysregulates the Antioxidant Response and Causes Premature Senescence of Melanocytes. J. Investig. Dermatol. 2015, 135, 1348–1357. [Google Scholar] [CrossRef]
- Koster, M.I.; Roop, D.R. Mechanisms Regulating Epithelial Stratification. Annu. Rev. Cell Dev. Biol. 2007, 23, 93–113. [Google Scholar] [CrossRef]
- Katajisto, P.; Döhla, J.; Chaffer, C.L.; Pentinmikko, N.; Marjanovic, N.; Iqbal, S.; Zoncu, R.; Chen, W.; Weinberg, R.A.; Sabatini, D.M. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 2015, 348, 340. [Google Scholar] [CrossRef] [PubMed]
- Lång, E.; Połeć, A.; Lång, A.; Valk, M.; Blicher, P.; Rowe, A.D.; Tønseth, K.A.; Jackson, C.J.; Utheim, T.P.; Janssen, L.M.C.; et al. Coordinated collective migration and asymmetric cell division in confluent human keratinocytes without wounding. Nat. Commun. 2018, 9, 3665. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, A.K.; Bolasco, G.; Correia, M.S.; Pereira, F.J.; Iannone, L.; Hume, A.N.; Kirkpatrick, N.; Picardo, M.; Torrisi, M.R.; Rodrigues, I.P.; et al. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Investig. Dermatol. 2014, 134, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Masedunskas, A.; Weigert, R.; Copeland, N.G.; Jenkins, N.A.; Hammer, J.A. Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc. Natl. Acad. Sci. USA 2012, 109, E2101–E2109. [Google Scholar] [CrossRef] [PubMed]
- Bruder, J.M.; Pfeiffer, Z.A.; Ciriello, J.M.; Horrigan, D.M.; Wicks, N.L.; Flaherty, B.; Oancea, E. Melanosomal dynamics assessed with a live-cell fluorescent melanosomal marker. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Kulukian, A.; Holland, A.J.; Vitre, B.; Naik, S.; Cleveland, D.W.; Fuchs, E. Epidermal development, growth control, and homeostasis in the face of centrosome amplification. Proc. Natl. Acad. Sci. USA 2015, 112, E6311–E6320. [Google Scholar] [CrossRef]
- Devi, R.; Pelletier, L.; Prosser, S.L. Charting the complex composite nature of centrosomes, primary cilia and centriolar satellites. Curr. Opin. Struct. Biol. 2021, 66, 32–40. [Google Scholar] [CrossRef]
- Martello, A.; Lauriola, A.; Mellis, D.; Parish, E.; Dawson, J.C.; Imrie, L.; Vidmar, M.; Gammoh, N.; Mitić, T.; Brittan, M.; et al. Trichoplein binds PCM1 and controls endothelial cell function by regulating autophagy. EMBO Rep. 2020, 21. [Google Scholar] [CrossRef]
- Khouj, E.M.; Prosser, S.L.; Tada, H.; Chong, W.M.; Liao, J.-C.; Sugasawa, K.; Morrison, C.G. Differential requirements for the EF-hand domains of human centrin 2 in primary ciliogenesis and nucleotide excision repair. J. Cell Sci. 2019, 132, jcs228486. [Google Scholar] [CrossRef]
- Hori, A.; Toda, T. Regulation of centriolar satellite integrity and its physiology. Cell Mol. Life Sci. 2017, 74, 213–229. [Google Scholar] [CrossRef]
- Hlavaty, D.; Lechler, T. Roles for microtubules in the proliferative and differentiated cells of stratified epithelia. Curr. Opin. Cell Biol. 2021, 68, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Vasioukhin, V.; Bauer, C.; Degenstein, L.; Wise, B.; Fuchs, E. Hyperproliferation and Defects in Epithelial Polarity upon Conditional Ablation of α-Catenin in Skin. Cell 2001, 104, 605–617. [Google Scholar] [CrossRef]
- Dias Gomes, M.; Letzian, S.; Saynisch, M.; Iden, S. Polarity signaling ensures epidermal homeostasis by coupling cellular mechanics and genomic integrity. Nat. Commun. 2019, 10, 3362. [Google Scholar] [CrossRef]
- Langton, A.K.; Alessi, S.; Hann, M.; Chien, A.L.; Kang, S.; Griffiths, C.E.M.; Watson, R.E.B. Aging in Skin of Color: Disruption to Elastic Fiber Organization Is Detrimental to Skin’s Biomechanical Function. J. Investig. Dermatol. 2019, 139, 779–788. [Google Scholar] [CrossRef]
| SPT | Sex | Age (Years) | Histoculture Samples | In Situ |
|---|---|---|---|---|
| VI | F | 25 | x | |
| VI | F | 18 | x | |
| VI | F | 77 | x | |
| VI | F | 19 | x | |
| VI | M | 25 | x | |
| V | F | 21 | x | |
| V | F | 27 | x | |
| V | F | 22 | x | |
| II/III | F | 46 | x | |
| II/III | F | 46 | x | x |
| II/III | F | 40 | x | |
| II/III | F | 43 | x | x |
| II/III | F | 43 | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellano-Pellicena, I.; Morrison, C.G.; Bell, M.; O’Connor, C.; Tobin, D.J. Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes. Int. J. Mol. Sci. 2021, 22, 3143. https://doi.org/10.3390/ijms22063143
Castellano-Pellicena I, Morrison CG, Bell M, O’Connor C, Tobin DJ. Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes. International Journal of Molecular Sciences. 2021; 22(6):3143. https://doi.org/10.3390/ijms22063143
Chicago/Turabian StyleCastellano-Pellicena, Irene, Ciaran G. Morrison, Mike Bell, Clare O’Connor, and Desmond J. Tobin. 2021. "Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes" International Journal of Molecular Sciences 22, no. 6: 3143. https://doi.org/10.3390/ijms22063143
APA StyleCastellano-Pellicena, I., Morrison, C. G., Bell, M., O’Connor, C., & Tobin, D. J. (2021). Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes. International Journal of Molecular Sciences, 22(6), 3143. https://doi.org/10.3390/ijms22063143







