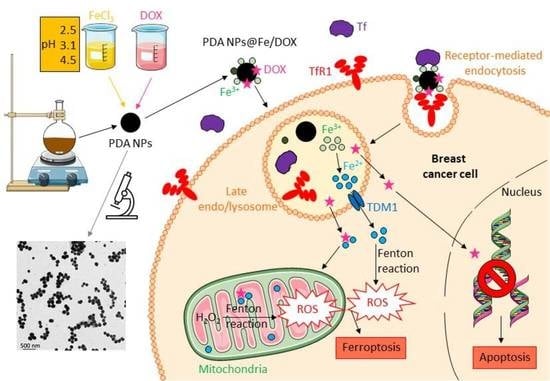
Tailored-Made Polydopamine Nanoparticles to Induce Ferroptosis in Breast Cancer Cells in Combination with Chemotherapy
Abstract
:1. Introduction
2. Results
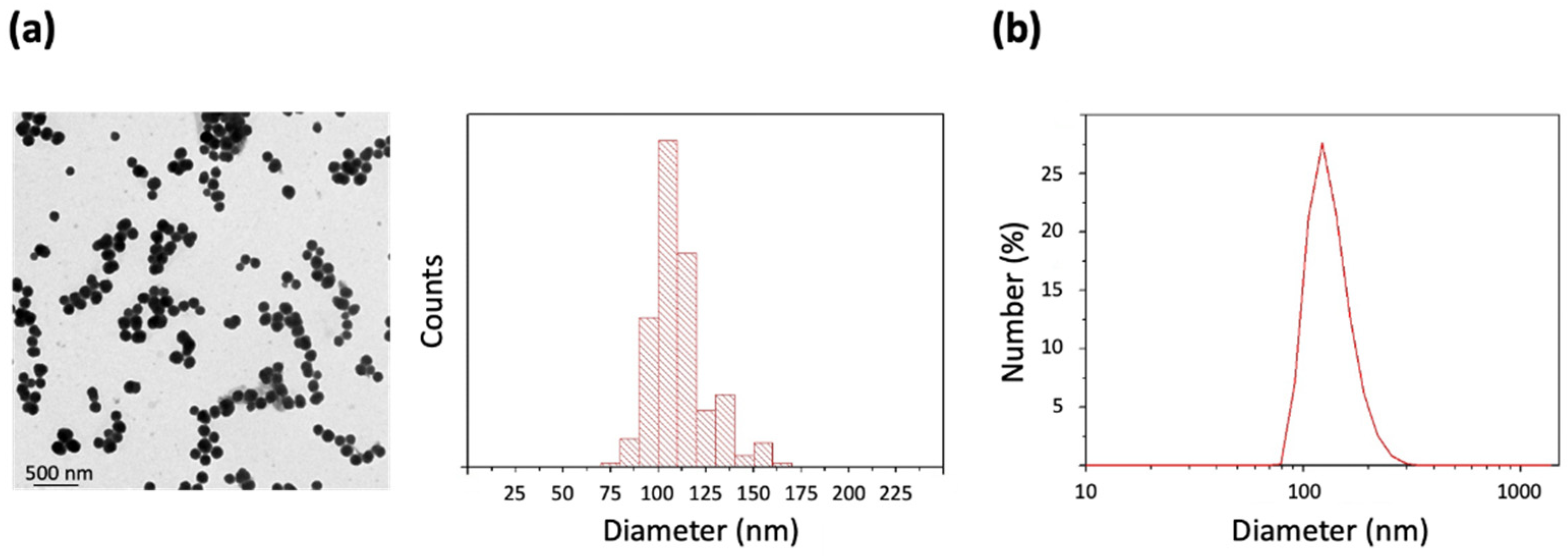
2.1. Preparation and Characterization of PDA NPs
2.2. pH Effect on the Fe3+-Adsorption Capacity of PDA NPs
2.3. Fe3+-Release and Ca2+-Loading from/to the Different PDA NPs@Fe
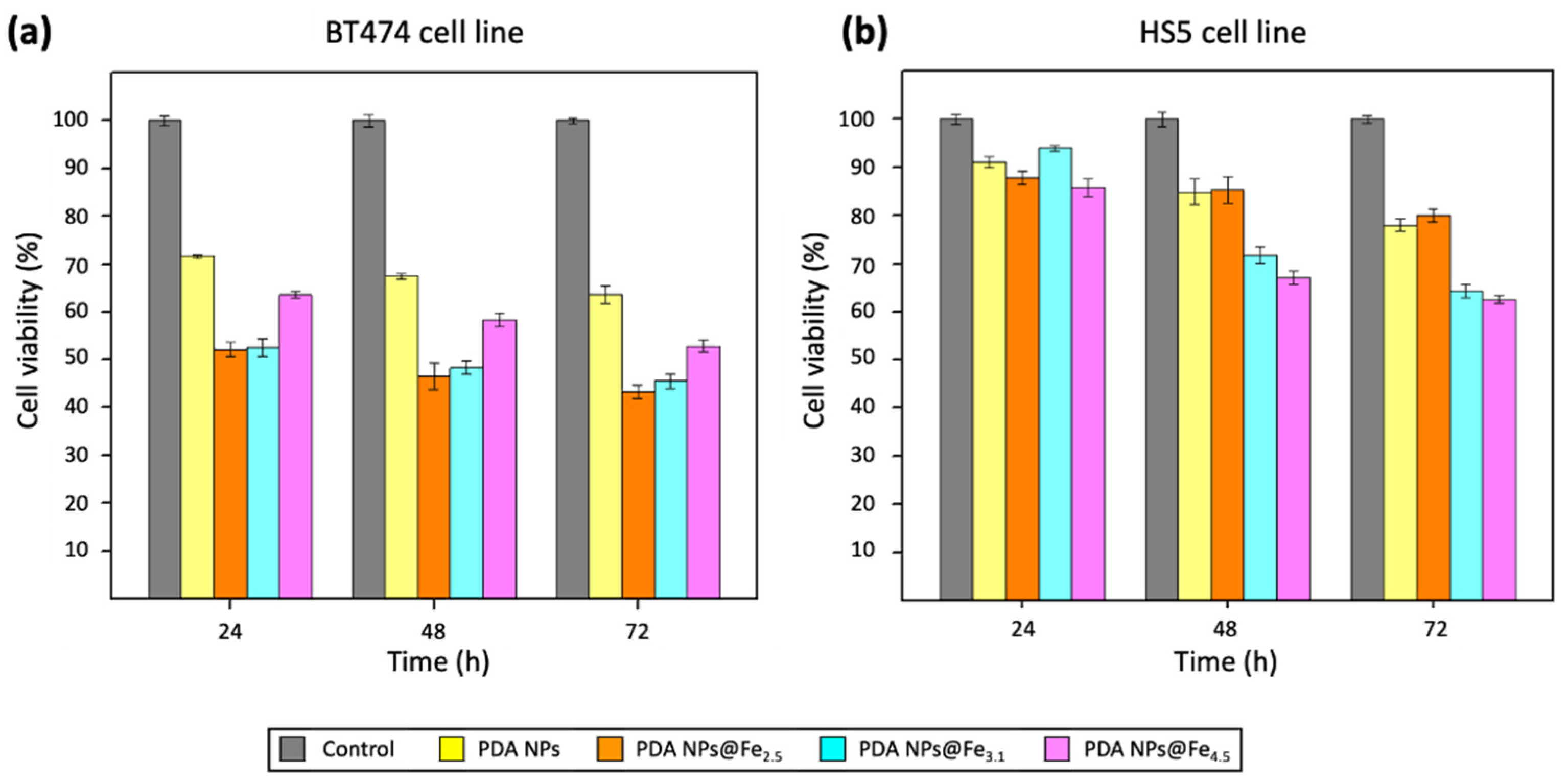
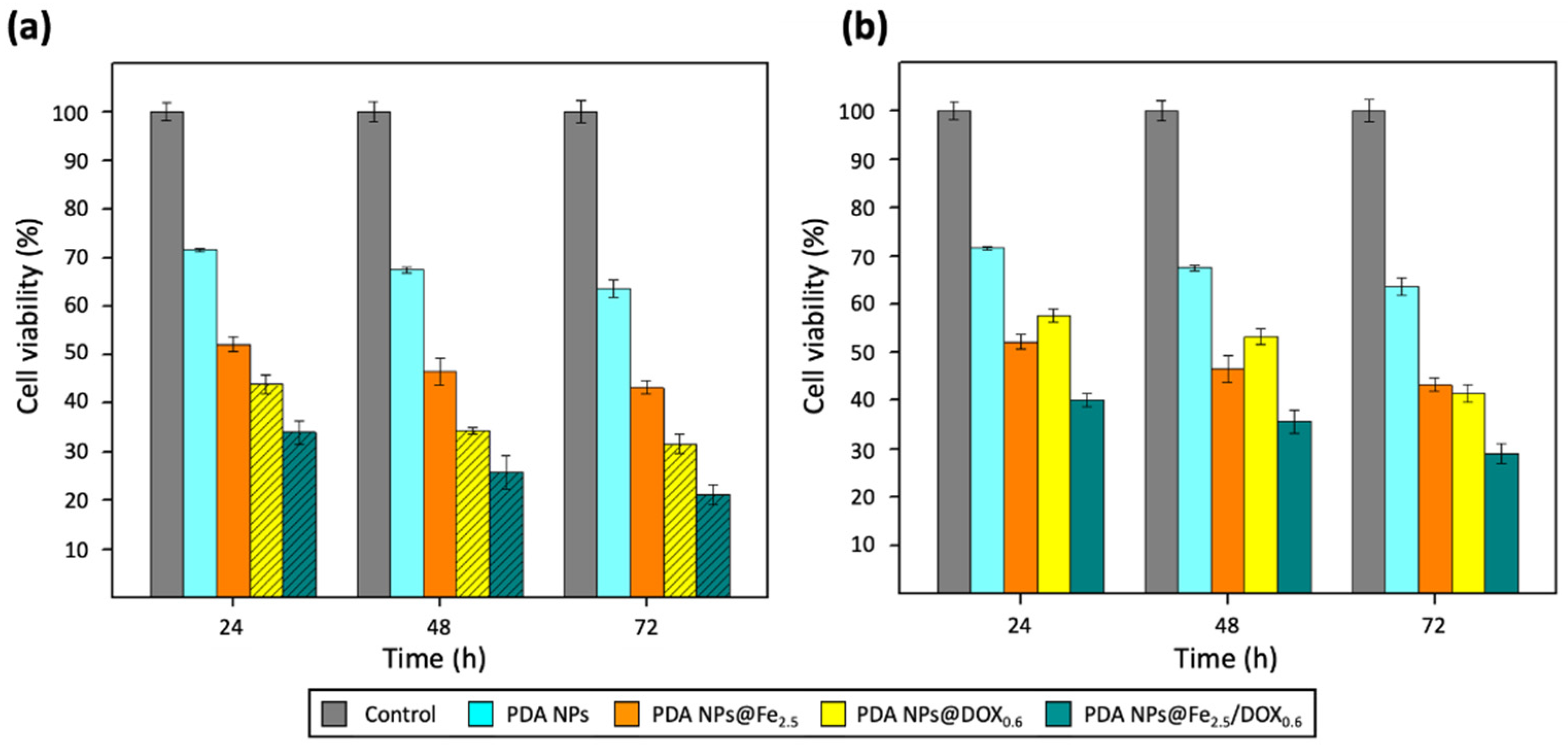
2.4. Therapeutic Activity of PDA NPs@Fe as Function of the Fe3+-Loading pH
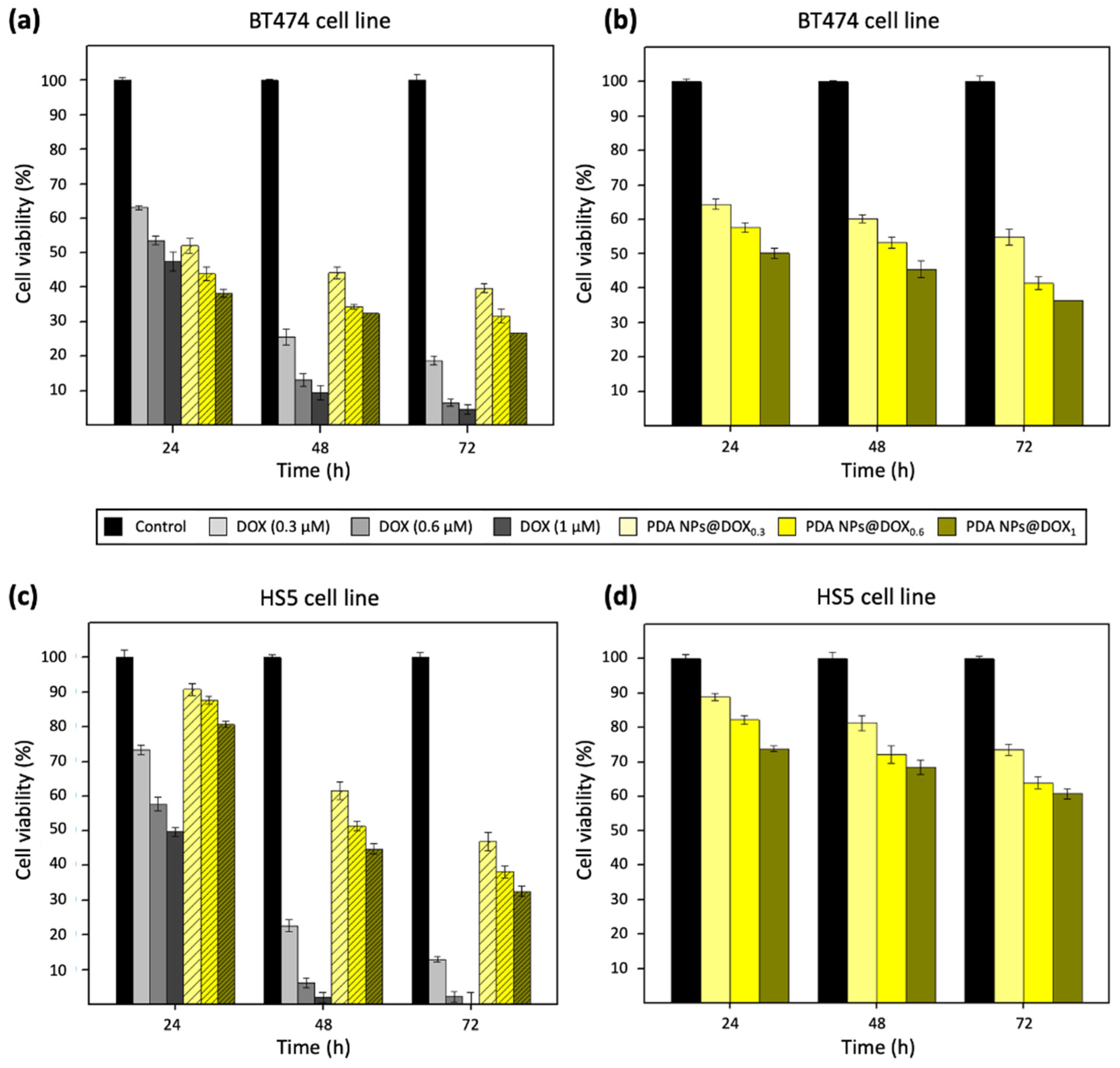
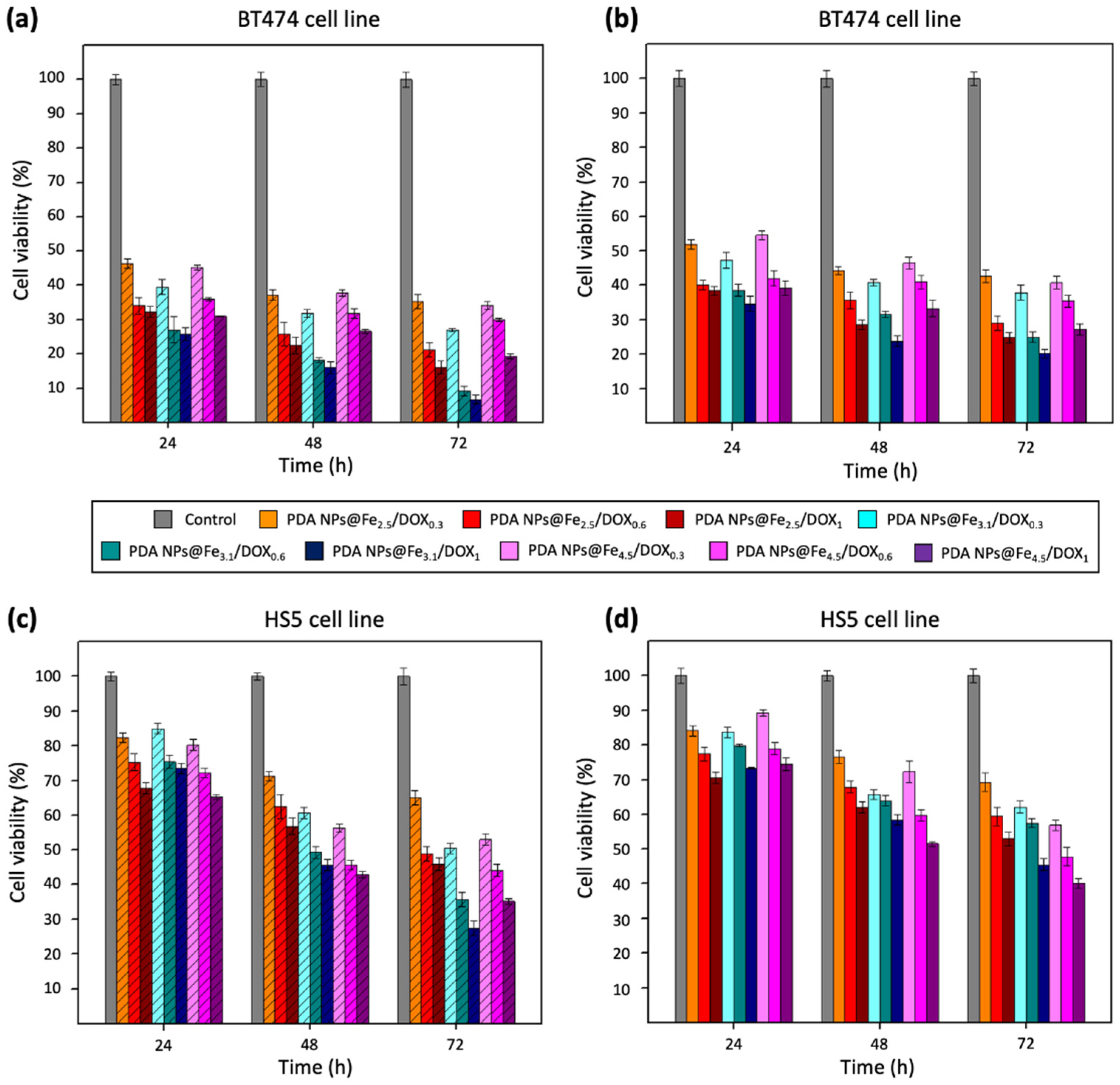
2.5. Therapeutic Activity of PDA NPs@Fe/DOX
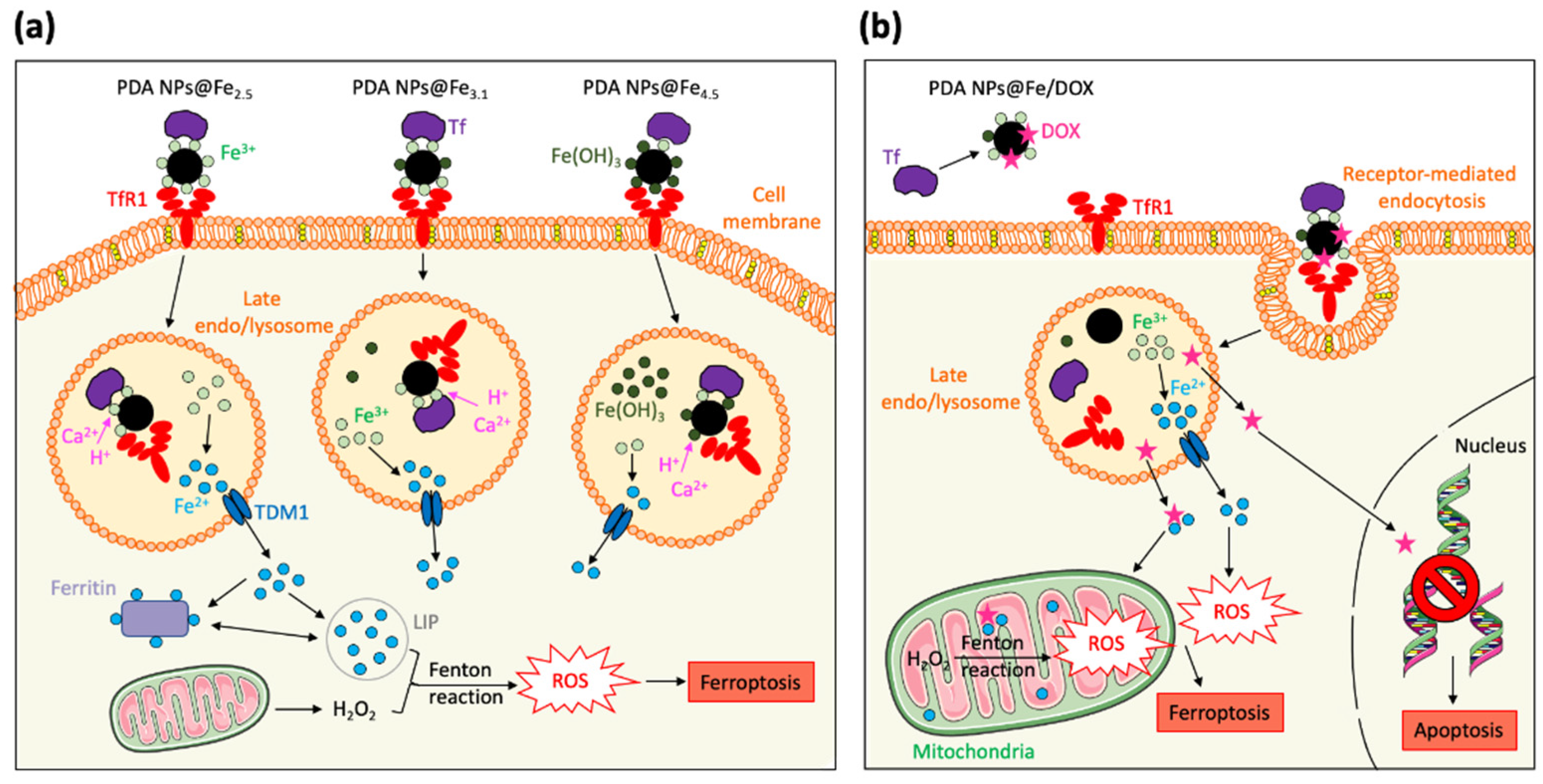
2.6. In Vitro ROS Production after Treatment with PDA NPs and PDA NPs@Fe
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of PDA NPs
4.2. Fe3+-Loading to PDA NPs at Different pH Values
4.3. Fe3+-Release and Ca2+-Loading from/to the Different PDA NPs@Fe
4.4. DOX-Loading to PDA NPs and the Different PDA NPs@Fe
4.5. Cell Culture
4.6. In Vitro Cytotoxicity
4.7. In Vitro ROS Detection
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOX | Doxorubicin |
| LIP | Labile iron pool |
| MDR | Multidrug resistance |
| PDA NPs | Polydopamine nanoparticles |
| PDA NPs@Fe2.5 | Polydopamine nanoparticles loaded with Fe3+ at pH 2.5 |
| PDA NPs@Fe3.1 | Polydopamine nanoparticles loaded with Fe3+ at pH 3.1 |
| PDA NPs@Fe4.5 | Polydopamine nanoparticles loaded with Fe3+ at pH 4.5 |
| PDA NPs@DOX0.3 | Polydopamine nanoparticles loaded with a 0.3 µM DOX solution |
| PDA NPs@DOX0.6 | Polydopamine nanoparticles loaded with a 0.6 µM DOX solution |
| PDA NPs@DOX1 | Polydopamine nanoparticles loaded with a 1 µM DOX solution |
| PDA NPs@DOXA | Isolated DOX-loaded polydopamine nanoparticles |
| PDA NPs@DOXW | Whole suspension of DOX-loaded polydopamine nanoparticles plus unloaded DOX |
| PDA NPs@Fe/DOX | Polydopamine nanoparticles loaded with Fe3+ and DOX |
| ROS | Reactive Oxygen Species |
| Tf | Transferrin |
| TfR1 | Transferrin receptor-1 |
References
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for reactive oxygen species mediated approach: An emerging paradigm for cancer treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef]
- Trachoatham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.; Zhang, M.; Yu, W.; Gao, F.; Li, C.; Wang, S.-B.; Feng, J.; Zhang, X.-Z. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano 2018, 12, 12181–12192. [Google Scholar] [CrossRef] [PubMed]
- Hartleben, G.; Schorpp, K.; Kwon, Y.; Betz, B.; Tsokanos, F.-F.; Dantes, Z.; Schäfer, A.; Rothenaigner, I.; Kuhn, J.M.M.; Morigny, P.; et al. Combination therapies induce cancer cell death through the integrated stress response and disturbed pyrimidine metabolism. EMBO Mol. Med. 2021, Mar 5, e12461. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural. Regen. Res. 2017, 12, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Forciniti, S.; Greco, L.; Grizzi, F.; Malesci, A.; Laghi, L. Iron metabolism in cancer progression. Int. J. Mol. Sci. 2020, 21, 2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a central player and promising target in cancer progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, K.K.P.; Sandoval, S.; Cortes-Mateos, M.J.; Alfaro, J.G.; Kummel, A.C.; Trogler, W.C. Self-assembled targeting of cancer cells by iron(III)-doped, silica nanoparticles. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 8017–8025. [Google Scholar] [CrossRef]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Constanzo, F.; Biamonte, F. Ferroptosis and cancer: Mitochondria meet the “iron maiden” cell death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Bebber, C.M.; Müller, F.; Clement, L.P.; Weber, J.; von Karstedt, S. Ferroptosis in cancer cell biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of non-apoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Luo, J.; Zhang, Z.; Dong, D.; Shen, Y.; Fang, Y.; Hu, L.; Liu, M.; Dai, C.; Peng, S.; et al. Iron and magnetic: New research direction of the ferroptosis-based cancer therapy. Ann. J. Cancer Res. 2018, 8, 1933–1946. [Google Scholar]
- Vega, M.A.; Nieto, C.; Marcelo, G.; Martín del Valle, E.M. Cytotoxicity of paramagnetic cations-loaded polydopamine nanoparticles. Colloids Surf. B Biointerfaces 2018, 167, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, Z.; Liu, L.; Zhang, X.; Shi, W.; Ge, D.; Sun, Y. Fe2+/Fe3+ ions chelated with ultrasmall polydopamine nanoparticles induce ferroptosis for cancer therapy. ACS Biomater. Sci. Eng. 2019, 5, 4861–4869. [Google Scholar] [CrossRef]
- Dong, L.; Wang, C.; Zhen, W.; Jia, X.; An, S.; Xu, Z.; Zhang, W.; Jiang, X. Biodegradable iron-coordinated hollow polydopamine nanospheres for dihydroartemisin delivery and selective enhanced therapy in tumor cells. J. Mater. Chem. B 2019, 7, 6172. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-containing polydopamine nanomaterials: Catalysis, energy and theranostics. Small 2020, 16, e1907042. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Martín del Valle, E.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201. [Google Scholar] [CrossRef] [Green Version]
- Nieto, C.; Vega, M.A.; Enrique, J.; Marcelo, G.; Martín del Valle, E.M. Size matters in the cytotoxicity of polydopamine nanoparticles in different types of tumors. Cancers 2019, 11, 1679. [Google Scholar] [CrossRef] [Green Version]
- Nieto, C.; Marcelo, G.; Vega, M.A.; Martín del Valle, E.M. Antineoplastic behaviour of polydopamine nanoparticles prepared in different water/alcohol media. Colloids Surf. B Biointerfaces 2021, 199, 111506. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefor, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-J.; Li, W.-T.; Li, Z.-H.-R.; Zhang, L.P.; Gai, C.C.; Zhang, W.F.; Ding, D.J. Iron-chelated polydopamine decorated doxorubicin-loaded nanodevices for reactive oxygen species enhanced cancer combination therapy. Therapy. Front. Pharmacol. 2019, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Lizunova, A.A.; Loshkarev, A.A.; Tokunov, Y.M.; Ivanov, U.V. Comparison of the results of measurements of the sizes of nanoparticles in stable colloidal solutions by the methods of acoustic spectroscopy, dynamic light scattering, and transmission electron microscopy. Meas. Teach. 2017, 59, 1151–1155. [Google Scholar] [CrossRef]
- Buriel Marti, F.; Lucena Conde, F.; Hernández Méndez, J.; Arribas Jimeno, S. Química Analítica Cuantitativa, 18th ed.; Paraninfo: Madrid, Spain, 2006; p. 1050. [Google Scholar]
- Dong, Y.-D.; Boyd, B.J. Applications of X-ray scattering in pharmaceutical science. Int. J. Pharm. 2011, 417, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Raffaelo, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the center of cell signalling: Interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klosterman, L.; Bettinger, C.J. Calcium-mediated control of polydopamine film oxidation and iron chelation. Int. J. Mol. Sci. 2017, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Carreira, A.C.; de Almeida, R.F.M.; Silva, L.C. Development of lysosome-mimicking vesicles to study the effect of abnormal accumulation of sphingosine on membrane properties. Sci. Rep. 2017, 7, 3949. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, P.; Lu, L.X. Iron promotes breast cancer cell migration via IL-6/JAK2/STAT3 signalling pathways in a paracrine or autocrine IL-6-rich inflammatory environment. J. Inorg. Biochem. 2020, 210, 111159. [Google Scholar] [CrossRef]
- Lamy, P.-J.; Durigova, A.; Jacot, W. Iron homeostasis and anemia markers in early breast cancer. Clin. Chim. Acta 2014, 434, 34–40. [Google Scholar] [CrossRef]
- Gong, Y.; Gallis, B.M.; Goodlett, D.R.; Yang, Y.; Lu, H.; Lacoste, E.; Lai, H.; Sasaki, T. Effects of transferrin conjugates of artemisin and artemisin dimer on breast cancer cell lines. Anticancer Res. 2013, 33, 123–132. [Google Scholar]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernández-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440. [Google Scholar] [CrossRef]
- Wen, S.-H.; Su, S.-C.; Liou, B.-H.; Lin, C.-H.; Lee, K.-R. Sulbactam-enhanced cytotoxicity of doxorubicin in breast cancer cells. Cancer Cell Int. 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loibl, S.; Weber, K.E.; Timms, K.M.; Elkin, E.P.; Hahnen, E.; Fasching, P.A.; Lederer, B.; Denkert, C.; Schneeweiss, A.; Braun, S.; et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann. Oncol. 2018, 29, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Wagner, U.; Aavall-Lundquist, E.; Gebski, V.; Heywood, M.; Vasey, P.A.; Volgger, B.; Vergote, I.; Pignata, S.; Ferrero, A.; et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol. 2010, 28, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Makhson, A.N.; Semiglazov, V.F.; Tjulandin, S.A.; Balashova, O.I.; Bondarenko, I.N.; Bogdanova, N.V.; Manikhas, G.M.; Oliynychenko, G.P.; Chatikhine, V.A.; et al. Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: Results from a randomized phase III study. J. Clin. Oncol. 2009, 27, 4522–4529. [Google Scholar]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Anticoli, S.; Amatore, D.; Matarrese, P.; De Angelis, M.; Palamara, A.T.; Nencioni, L.; Ruggieri, A. Counteraction of HCV-induced oxidative stress concurs to establish chronic infection in liver cell cultures. Oxid. Med. Cell Longev. 2019, 2019, 6452390. [Google Scholar] [PubMed]

| pH | Fe3+ Loaded (q) | Fe3+ Released (q) | Fe3+ Released (%) | Ca2+ Loaded (q) | Ca2+ Loaded (%) |
|---|---|---|---|---|---|
| 2.5 | 16 mg/g | 6 mg/g | 38 | 21 mg/g | 13 |
| 3.1 | 15 mg/g | 4 mg/g | 30 | 20 mg/g | 13 |
| 4.5 | 100 mg/g | 75 mg/g | 75 | 16 mg/g | 10 |
| Initial [DOX] | PDA NPs | PDA NPs@Fe2.5 | PDA NPs@Fe3.1 | PDA NPs@Fe4.5 |
|---|---|---|---|---|
| 0.3 µM | 21.4 ng/mg | 16.8 ng/mg | 19.0 ng/mg | 18.8 ng/mg |
| 0.6 µM | 42.1 ng/mg | 33.2 ng/mg | 37.3 ng/mg | 35.7 ng/mg |
| 1 µM | 57.9 ng/mg | 51.7 ng/mg | 55.8 ng/mg | 54.6 ng/mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, C.; Vega, M.A.; Martín del Valle, E.M. Tailored-Made Polydopamine Nanoparticles to Induce Ferroptosis in Breast Cancer Cells in Combination with Chemotherapy. Int. J. Mol. Sci. 2021, 22, 3161. https://doi.org/10.3390/ijms22063161
Nieto C, Vega MA, Martín del Valle EM. Tailored-Made Polydopamine Nanoparticles to Induce Ferroptosis in Breast Cancer Cells in Combination with Chemotherapy. International Journal of Molecular Sciences. 2021; 22(6):3161. https://doi.org/10.3390/ijms22063161
Chicago/Turabian StyleNieto, Celia, Milena A. Vega, and Eva M. Martín del Valle. 2021. "Tailored-Made Polydopamine Nanoparticles to Induce Ferroptosis in Breast Cancer Cells in Combination with Chemotherapy" International Journal of Molecular Sciences 22, no. 6: 3161. https://doi.org/10.3390/ijms22063161