A Bioinformatics Systems Biology Analysis of the Current Oral Proteomic Biomarkers and Implications for Diagnosis and Treatment of External Root Resorption
Abstract
:1. Introduction
2. Etiology and Pathogenesis
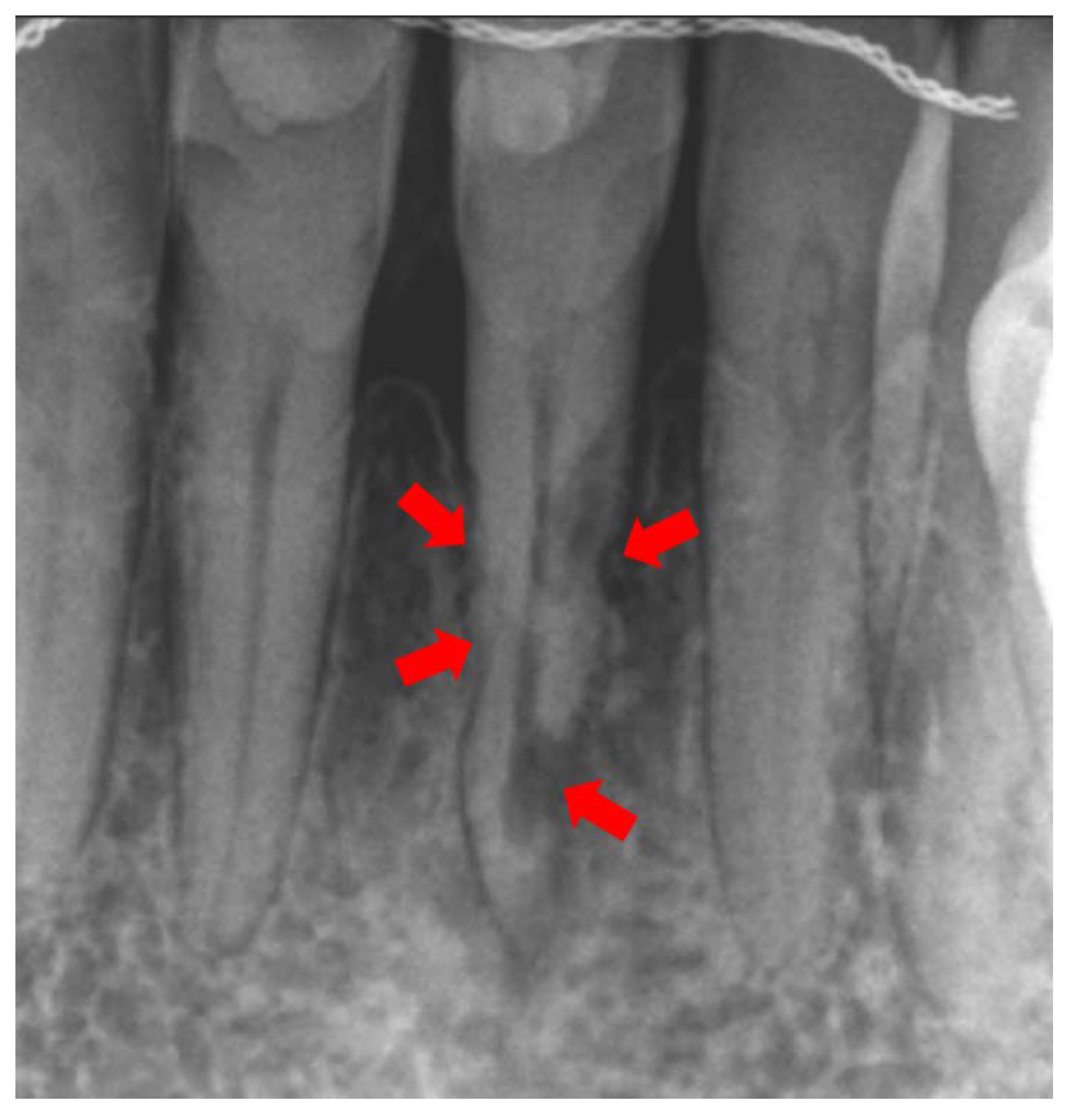
3. Diagnosis
4. Biomarkers Associated with ERR
5. Treatment Options for ERR
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakland, L.K. Root resorption. Dent. Clin. N. Am. 1992, 36, 491–507. [Google Scholar] [PubMed]
- Consolaro, A. The four mechanisms of dental resorption initiation. Dent. Press J. Orthod. 2013, 18, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Mavridou, A.M.; Pyka, G.; Kerckhofs, G.; Wevers, M.; Bergmans, L.; Gunst, V.; Huybrechts, B.; Schepers, E.; Hauben, E.; Lambrechts, P. A novel multimodular methodology to investigate external cervical tooth resorption. Int. Endod. J. 2016, 49, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Espona, J.; Roig, E.; Durán-Sindreu, F.; Abella, F.; Machado, M.; Roig, M. Invasive Cervical Resorption: Clinical Management in the Anterior Zone. J. Endod. 2018, 44, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Foschi, F.; Mannocci, F.; Patel, K. External cervical resorption: A three-dimensional classification. Int. Endod. J. 2018, 51, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Kanagasingam, S.; Pitt Ford, T. External cervical resorption: A review. J. Endod. 2009, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Ferner, R.E. Biomarkers-A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.; Lagarrigue, S.; Liaubet, L.; Robert-Granié, C.; Sancristobal, M.; Tosser-Klopp, G. Pathway results from the chicken data set using GOTM, Pathway Studio and Ingenuity softwares. BMC Proc. 2009, 3 (Suppl. 4), S11. [Google Scholar] [CrossRef] [Green Version]
- Yuryev, A.; Kotelnikova, E.; Daraselia, N. Ariadne’s ChemEffect and Pathway Studio knowledge base. Expert. Opin. Drug Discov. 2009, 4, 1307–1318. [Google Scholar] [CrossRef]
- Weltman, B.; Vig, K.W.; Fields, H.W.; Shanker, S.; Kaizar, E.E. Root resorption associated with orthodontic tooth movement: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, G.; Licata, M.E.; Guiglia, R.; Giuliana, G. Root resorption and orthodontic treatment. Review of the literature. Minerva Stomatol. 2007, 56, 31–44. [Google Scholar]
- Kapoor, P.; Kharbanda, O.P.; Monga, N.; Miglani, R.; Kapila, S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: A systematic review. Prog. Orthod. 2014, 15, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Aihara, N.; Kojima, T.; Kasai, K. RANKL increase in compressed periodontal ligament cells from root resorption. J. Dent. Res. 2006, 85, 751–756. [Google Scholar] [CrossRef]
- George, D.I.; Miller, R.L. Idiopathic resorption of teeth. A report of three cases. Am. J. Orthod. 1986, 89, 13–20. [Google Scholar] [CrossRef]
- Bergmans, L.; Van Cleynenbreugel, J.; Verbeken, E.; Wevers, M.; Van Meerbeek, B.; Lambrechts, P. Cervical external root resorption in vital teeth. J. Clin. Periodontol. 2002, 29, 580–585. [Google Scholar] [CrossRef]
- Neely, A.L.; Thumbigere-Math, V.; Somerman, M.J.; Foster, B.L. A Familial Pattern of Multiple Idiopathic Cervical Root Resorption With a 30-Year Follow-Up. J. Periodontol. 2016, 87, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Neely, A.L.; Gordon, S.C. A familial pattern of multiple idiopathic cervical root resorption in a father and son: A 22-year follow-up. J. Periodontol. 2007, 78, 367–371. [Google Scholar] [CrossRef]
- Macdonald-Jankowski, D. Multiple idiopathic cervical root resorption most frequently seen in younger females. Evid. Based Dent. 2005, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, S.; Siddiqui, F.; Khurshid, Z.; Zohaib, S.; Zafar, M.S.; Ansari, S.A. Effect of bisphosphonates on root resorption after tooth replantation—A systematic review. Dent. Traumatol. 2017, 33, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Iwamatsu-Kobayashi, Y.; Satoh-Kuriwada, S.; Yamamoto, T.; Hirata, M.; Toyoda, J.; Endo, H.; Kindaichi, K.; Komatsu, M. A case of multiple idiopathic external root resorption: A 6-year follow-up study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2005, 100, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Llavayol, M.; Pons, M.; Ballester, M.L.; Berástegui, E. Multiple Cervical Root Resorption in a Young Adult Female Previously Treated with Chemotherapy: A Case Report. J. Endod. 2019, 45, 349–353. [Google Scholar] [CrossRef]
- Kumar, V.; Chawla, A.; Kaur, A. Multiple Idiopathic Cervical Root Resorptions in Patients with Hepatitis B Virus Infection. J. Endod. 2018, 44, 1575–1577. [Google Scholar] [CrossRef]
- Shafi, I.; Welbury, R. Idiopathic Radiographic Apical Root Resorption in Wind Instrument Players. Dent. Update 2015, 42, 972–976. [Google Scholar] [CrossRef]
- Talebzadeh, B.; Rahimi, S.; Abdollahi, A.A.; Nouroloyuni, A.; Asghari, V. Varicella Zoster Virus and Internal Root Resorption: A Case Report. J. Endod. 2015, 41, 1375–1381. [Google Scholar] [CrossRef]
- Kjær, I.; Strøm, C.; Worsaae, N. Regional aggressive root resorption caused by neuronal virus infection. Case. Rep. Dent. 2012, 2012, 693240. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, T.; Schawalder, P.; Ackermann, M.; Bosshardt, D.D. Human and feline invasive cervical resorptions: The missing link?—Presentation of four cases. J. Endod. 2009, 35, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Samara, E.; Kelly, E.; Walker, R.; Borumandi, F. Multiple idiopathic cervical root resorption: Case report of an unusual presentation. Spec. Care Dent. 2021, 41, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, X.; Yan, K.; Liu, S.; Sun, Z.; Li, S. Multiple idiopathic cervical root resorption involving all permanent teeth. Aust. Endod. J. 2020, 46, 263–271. [Google Scholar] [CrossRef]
- Yu, V.S.; Messer, H.H.; Tan, K.B. Multiple idiopathic cervical resorption: Case report and discussion of management options. Int. Endod. J. 2011, 44, 77–85. [Google Scholar] [CrossRef]
- Warnsinck, C.J.; Shemesh, H. External cervical root resorption. Ned. Tijdschr. Tandheelkd. 2018, 125, 109–115. [Google Scholar] [CrossRef]
- Vieira, G.M. Protein biomarkers of external root resorption: A new protein extraction protocol. Are we going in the right direction? Dent. Press J. Orthod. 2014, 19, 62–69. [Google Scholar] [CrossRef] [Green Version]
- George, A.; Evans, C.A. Detection of root resorption using dentin and bone markers. Orthod. Craniofac. Res. 2009, 12, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, G.; Wang, Y.; Gluhak-Heinrich, J.; Yang, G.; Chen, L.; Li, T.; Wu, L.A.; Chen, Z.; MacDougall, M.; Chen, S. Tissue-specific expression of dentin sialophosphoprotein (DSPP) and its polymorphisms in mouse tissues. Cell Biol. Int. 2009, 33, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Mah, J.; Prasad, N. Dentine phosphoproteins in gingival crevicular fluid during root resorption. Eur. J. Orthod. 2004, 26, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Logani, A.; Shah, N. Dentine sialoprotein expression in gingival crevicular fluid during trauma-induced root resorption. Int. Endod. J. 2013, 46, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H. The functional significance of dentin sialoprotein-phosphophoryn and dentin sialoprotein. Int. J. Oral Sci. 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Alyahya, L.; Myers, G.L. Denosumab Use as a Predictor Variable for External Cervical Resorption: A Case-Control Study. J. Endod. 2020. [Google Scholar]
- Fukushima, H.; Kajiya, H.; Takada, K.; Okamoto, F.; Okabe, K. Expression and role of RANKL in periodontal ligament cells during physiological root-resorption in human deciduous teeth. Eur. J. Oral Sci. 2003, 111, 346–352. [Google Scholar] [CrossRef]
- Low, E.; Zoellner, H.; Kharbanda, O.P.; Darendeliler, M.A. Expression of mRNA for osteoprotegerin and receptor activator of nuclear factor kappa beta ligand (RANKL) during root resorption induced by the application of heavy orthodontic forces on rat molars. Am. J. Orthod. Dentofacial. Orthop. 2005, 128, 497–503. [Google Scholar] [CrossRef]
- Tyrovola, J.B.; Spyropoulos, M.N.; Makou, M.; Perrea, D. Root resorption and the OPG/RANKL/RANK system: A mini review. J. Oral Sci. 2008, 50, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Bletsa, A.; Berggreen, E.; Brudvik, P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur. J. Oral Sci. 2006, 114, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Bakker, A.D.; Everts, V.; Klein-Nulend, J. Mechanical loading prevents the stimulating effect of IL-1β on osteocyte-modulated osteoclastogenesis. Biochem. Biophys. Res. Commun. 2012, 420, 11–16. [Google Scholar] [CrossRef]
- Yao, Z.; Xing, L.; Qin, C.; Schwarz, E.M.; Boyce, B.F. Osteoclast precursor interaction with bone matrix induces osteoclast formation directly by an interleukin-1-mediated autocrine mechanism. J. Biol. Chem. 2008, 283, 9917–9924. [Google Scholar] [CrossRef] [Green Version]
- Kereshanan, S.; Stephenson, P.; Waddington, R. Identification of dentine sialoprotein in gingival crevicular fluid during physiological root resorption and orthodontic tooth movement. Eur. J. Orthod. 2008, 30, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, L.; Carinci, F.; Martini, M.; Gemmati, D.; Nardone, M.; Siciliani, G. Quantitive evaluation of dentin sialoprotein (DSP) using microbeads—A potential early marker of root resorption. Oral Implantol. 2016, 9, 132–142. [Google Scholar]
- Silva, T.A.; Lara, V.S.; Silva, J.S.; Oliveira, S.H.; Butler, W.T.; Cunha, F.Q. Macrophages and mast cells control the neutrophil migration induced by dentin proteins. J. Dent. Res. 2005, 84, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Atsawasuwan, P.; Lazari, P.; Chen, Y.; Zhou, X.; Viana, G.; Evans, C.A. Secretory microRNA-29 expression in gingival crevicular fluid during orthodontic tooth movement. PLoS ONE 2018, 13, e0194238. [Google Scholar] [CrossRef] [Green Version]
- Balducci, L.; Ramachandran, A.; Hao, J.; Narayanan, K.; Evans, C.; George, A. Biological markers for evaluation of root resorption. Arch. Oral Biol. 2007, 52, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jacox, L.A.; Little, S.H.; Ko, C.C. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J. Med. Sci. 2018, 34, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagiya, T.; Nakamura, S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J. Periodontal. Res. 2013, 48, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Azuma, J.; Tsao, P.S. MicroRNA-29b regulation of abdominal aortic aneurysm development. Trends Cardiovasc. Med. 2014, 24, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Long term effect of low intensity pulsed ultrasound on a human tooth slice organ culture. Arch. Oral Biol. 2012, 57, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Yang, G.; Song, G.; Chen, Z.; Chen, S. Immunohistochemical localization of the NH(2)-terminal and COOH-terminal fragments of dentin sialoprotein in mouse teeth. Cell Tissue Res. 2012, 349, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Suto, M.; Nemoto, E.; Kanaya, S.; Suzuki, R.; Tsuchiya, M.; Shimauchi, H. Nanohydroxyapatite increases BMP-2 expression via a p38 MAP kinase dependent pathway in periodontal ligament cells. Arch. Oral Biol. 2013, 58, 1021–1028. [Google Scholar] [CrossRef]
- Angelova, A.; Takagi, Y.; Okiji, T.; Kaneko, T.; Yamashita, Y. Immunocompetent cells in the pulp of human deciduous teeth. Arch. Oral Biol. 2004, 49, 29–36. [Google Scholar] [CrossRef]
- Yagi, Y.; Suda, N.; Yamakoshi, Y.; Baba, O.; Moriyama, K. In vivo application of amelogenin suppresses root resorption. J. Dent. Res. 2009, 88, 176–181. [Google Scholar] [CrossRef]
- Noda, K.; Seshima, F.; Okubo, N.; Ishii, Y.; Ota, M.; Yamada, S.; Saito, A. Effect of platelet-derived grwth factor-BB on root resorption after reimplantation of partially denuded tooth in dog. Dent. Traumatol. 2012, 28, 217–225. [Google Scholar] [CrossRef]
- Ishii, Y.; Fujita, T.; Okubo, N.; Ota, M.; Yamada, S.; Saito, A. Effect of basic fibroblast growth factor (FGF-2) in combination with beta tricalcium phosphate on root coverage in dog. Acta. Odontol. Scand. 2013, 71, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiratani, S.; Ota, M.; Fujita, T.; Seshima, F.; Yamada, S.; Saito, A. Effect of basic fibroblast growth factor on root resorption after delayed autotransplantation of tooth in dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e14–e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, K.; Cohen, S.; Berman, L. Cohen’s Pathways of the Pulp; Mosby Elsevier: Maryland Heights, MO, USA, 2011. [Google Scholar]
- Sharma, S.; Kumar, P.; Jain, V.; Logani, A. Multiple idiopathic cervical root resorption: Diagnosis, clinical/radiographical/histological presentation, and rehabilitation—A 7-year follow-up case report. J. Conserv. Dent. 2019, 22, 313–317. [Google Scholar] [PubMed]
- Wu, J.; Lin, L.Y.; Yang, J.; Chen, X.F.; Ge, J.Y.; Wu, J.R.; Sun, W.B. Multiple idiopathic cervical root resorption: A case report. Int. Endod. J. 2016, 49, 189–202. [Google Scholar] [CrossRef] [PubMed]
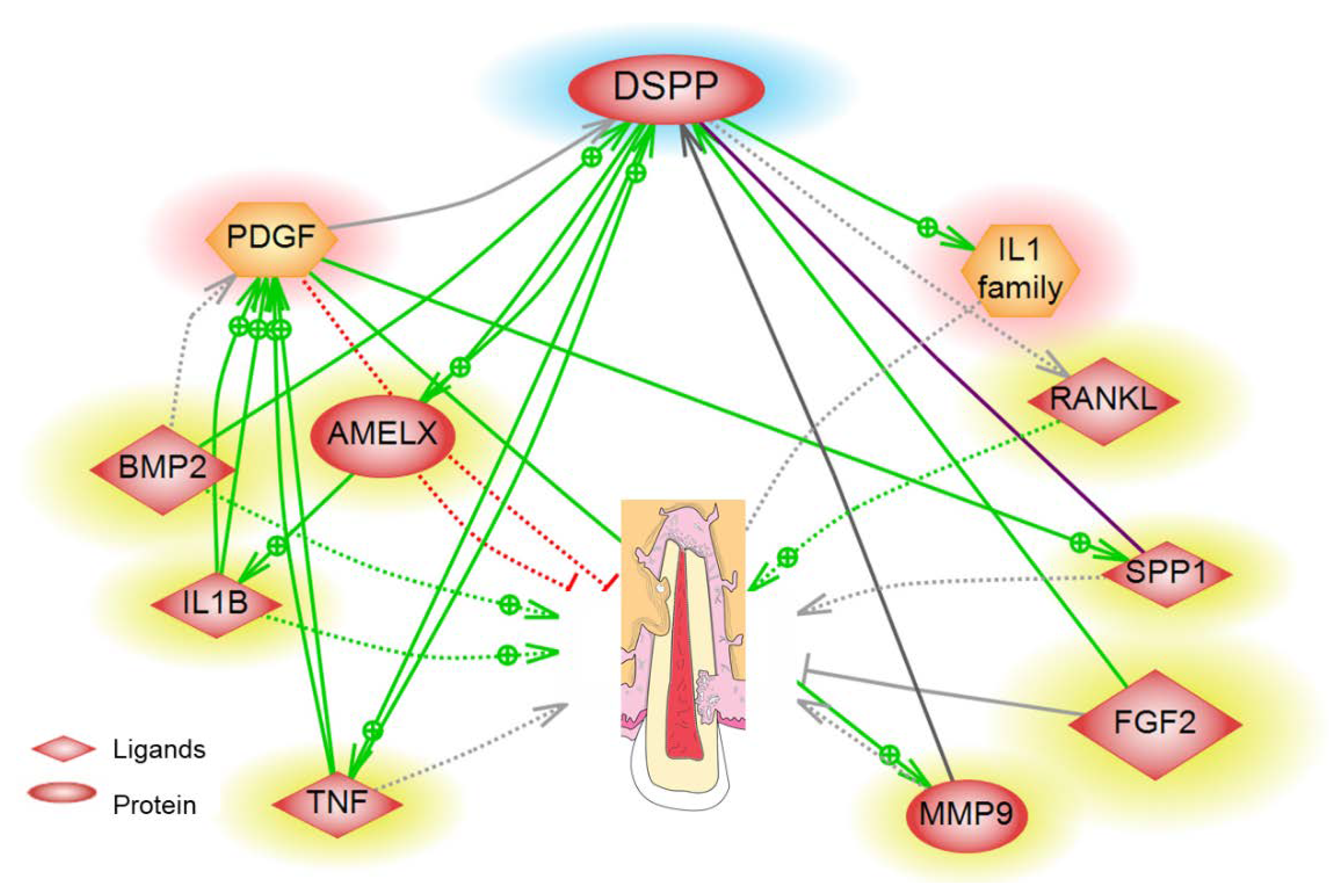
| Biomarker | Pathway/Function | Detection Site | References |
|---|---|---|---|
| RANKL/OPG | RANKL/RANK/OPG Signaling Pathway | GCF | Alyahya et al. [37], Fukushima et al. [38], Low et al. [39], Tyrovola et al. [40], Yamaguchi et al. [13] |
| Interleukin-1B | Inflammatory Pathway | GCF | Bletsa et al. [41], Kulkarni et al. [42], Yao et al. [43] |
| TNF-Alpha | Inflammatory Pathway | GCF | Kapoor et al. [12], Bletsa et al. [41] |
| Dentin Sialoprotein (DSP) | BMP/Smad, JNK, ERK, MAPK, and NF-κB signalling | GCF | Ritchie et al. [36], Kumar et al. [35], Kereshanan et al. [44], Lombardo et al. [45] |
| Dentin phosphoprotein (DPP) | AKT and mTOR | GCF | Mah et al. [34], Silva et al. [46], Yuan et al. [33] |
| Interleukin-6 | Notch Signaling | GCF/PDL | Yamaguchi et al. [13] |
| MicroRNA-29 | Osteoclast regulation | GCF | Atsawasuwan et al. [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mona, M.; Abbasi, Z.; Kobeissy, F.; Chahbandar, A.; Pileggi, R. A Bioinformatics Systems Biology Analysis of the Current Oral Proteomic Biomarkers and Implications for Diagnosis and Treatment of External Root Resorption. Int. J. Mol. Sci. 2021, 22, 3181. https://doi.org/10.3390/ijms22063181
Mona M, Abbasi Z, Kobeissy F, Chahbandar A, Pileggi R. A Bioinformatics Systems Biology Analysis of the Current Oral Proteomic Biomarkers and Implications for Diagnosis and Treatment of External Root Resorption. International Journal of Molecular Sciences. 2021; 22(6):3181. https://doi.org/10.3390/ijms22063181
Chicago/Turabian StyleMona, Mahmoud, Zunnaira Abbasi, Firas Kobeissy, Abdulrahman Chahbandar, and Roberta Pileggi. 2021. "A Bioinformatics Systems Biology Analysis of the Current Oral Proteomic Biomarkers and Implications for Diagnosis and Treatment of External Root Resorption" International Journal of Molecular Sciences 22, no. 6: 3181. https://doi.org/10.3390/ijms22063181
APA StyleMona, M., Abbasi, Z., Kobeissy, F., Chahbandar, A., & Pileggi, R. (2021). A Bioinformatics Systems Biology Analysis of the Current Oral Proteomic Biomarkers and Implications for Diagnosis and Treatment of External Root Resorption. International Journal of Molecular Sciences, 22(6), 3181. https://doi.org/10.3390/ijms22063181







