DHA and Its Metabolites Have a Protective Role against Methylmercury-Induced Neurotoxicity in Mouse Primary Neuron and SH-SY5Y Cells
Abstract
1. Introduction
2. Results
2.1. Protective Effect of DHA against MeHg-Induced Cytotoxicity in SH-SY5Y Cells and Mouse Primary Neuronal Cells
2.2. DHA Treatment Alleviated MeHg-Induced Cytotoxicity via the Activation of RXR
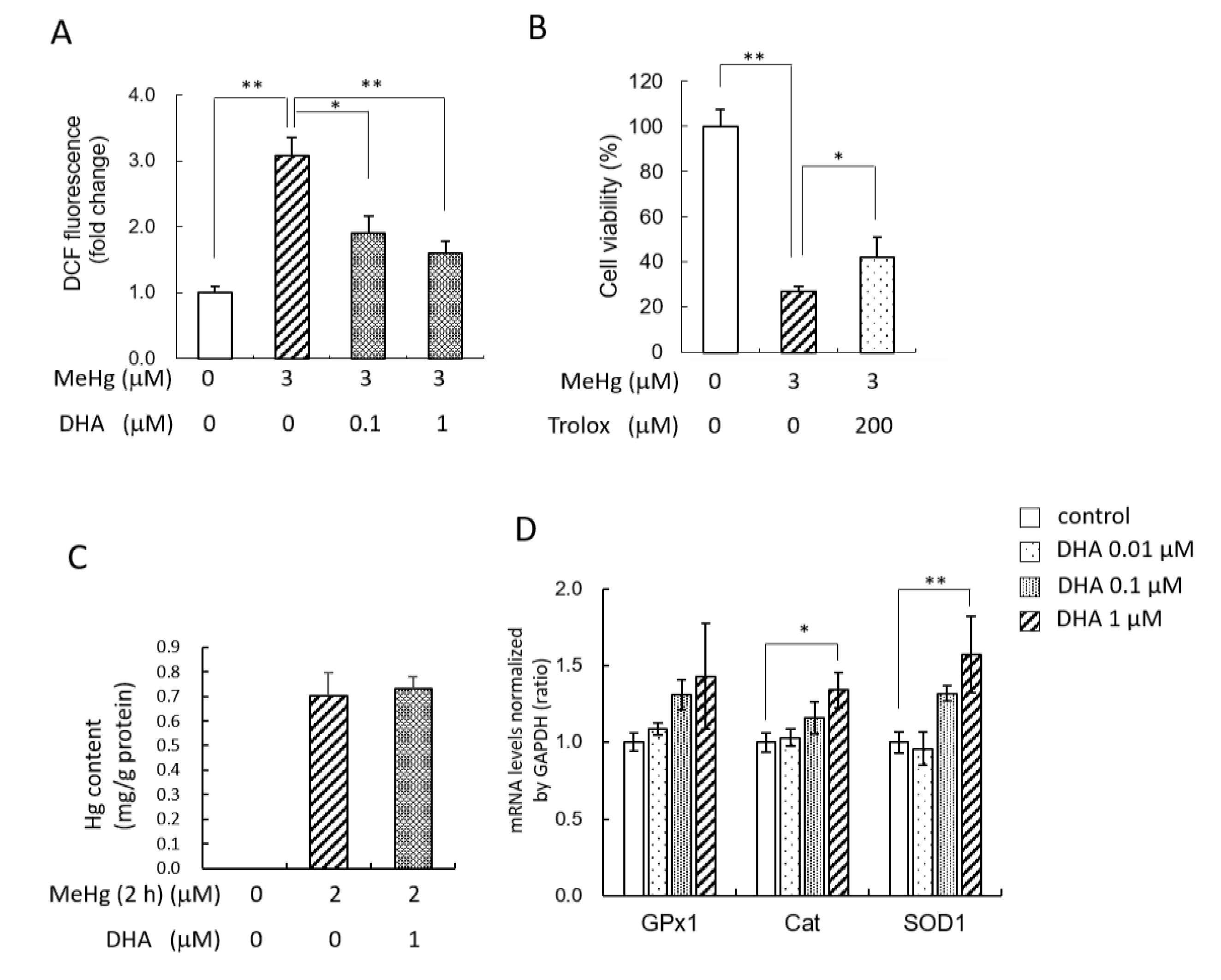
2.3. Pretreatment with DHA Reduced MeHg-Induced ROS
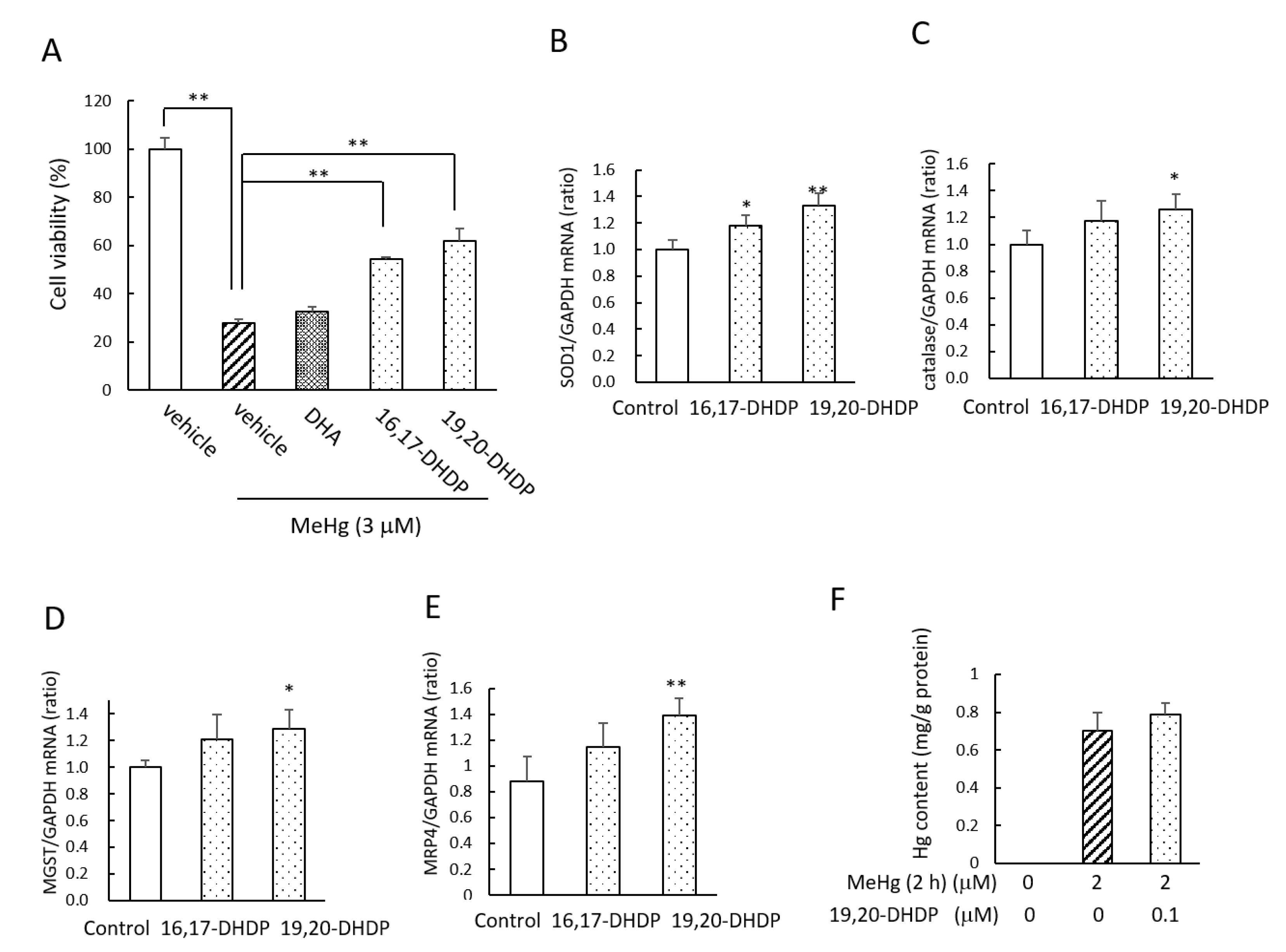
2.4. The DHA Metabolites DHDPs Also Have Neuroprotective Effects against MeHg
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation of Murine Cortical Primary Neuron
4.3. Culture of SH-SY5Y Cells
4.4. Measurement of Cell Viability
4.5. Measurement of ROS Levels
4.6. MeHg Treatment and Determination of Total Hg
4.7. Isolation of RNA and Reverse-Transcription RNA
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MeHg | methylmercury |
| PUFA | polyunsaturated fatty acids |
| DHA | docosahexaenoic acid |
| RXR | retinoid X receptor |
| ROS | reactive oxygen species |
| DHDP | dihydroxydocosapentaenoic acids |
References
- Harding, G.; Dalziel, J.; Vass, P. Bioaccumulation of methylmercury within the marine food web of the outer Bay of Fundy, Gulf of Maine. PLoS ONE 2018, 13, e0197220. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T. Pathology of Minamata disease. With special reference to its pathogenesis. Acta Pathol. Jpn. 1982, 32 (Suppl. 1), 73–99. [Google Scholar]
- Eto, K. Pathology of Minamata disease. Toxicol. Pathol. 1997, 25, 614–623. [Google Scholar] [CrossRef]
- Innis, S.M. Essential fatty acid transfer and fetal development. Placenta 2005, 26 (Suppl. A), S70–S75. [Google Scholar] [CrossRef]
- Strain, J.J.; Yeates, A.J.; van Wijngaarden, E.; Thurston, S.W.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Love, T.M.; Smith, T.H.; Yost, K.; et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: Associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am. J. Clin. Nutr. 2015, 101, 530–537. [Google Scholar] [CrossRef]
- Choi, A.L.; Mogensen, U.B.; Bjerve, K.S.; Debes, F.; Weihe, P.; Grandjean, P.; Budtz-Jorgensen, E. Negative confounding by essential fatty acids in methylmercury neurotoxicity associations. Neurotoxicol. Teratol. 2014, 42, 85–92. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Lengqvist, J.; Mata De Urquiza, A.; Bergman, A.C.; Willson, T.M.; Sjovall, J.; Perlmann, T.; Griffiths, W.J. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol. Cell Proteom. 2004, 3, 692–703. [Google Scholar] [CrossRef]
- Itoh, T.; Yamamoto, K. Peroxisome proliferator activated receptor gamma and oxidized docosahexaenoic acids as new class of ligand. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Oguro, A.; Ishihara, Y.; Siswanto, F.M.; Yamazaki, T.; Ishida, A.; Imaishi, H.; Imaoka, S. Contribution of DHA diols (19,20-DHDP) produced by cytochrome P450s and soluble epoxide hydrolase to the beneficial effects of DHA supplementation in the brains of rotenone-induced rat models of Parkinson’s disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158858. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System. Antioxidants 2020, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.F.; LeBel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar]
- Ishihara, Y.; Tsuji, M.; Kawamoto, T.; Yamazaki, T. Involvement of reactive oxygen species derived from mitochondria in neuronal injury elicited by methylmercury. J. Clin. Biochem. Nutr. 2016, 59, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Van Dao, C.; Miyamoto, A.; Shiraishi, M. Comparative analysis of in vitro neurotoxicity of methylmercury, mercury, cadmium, and hydrogen peroxide on SH-SY5Y cells. J. Vet. Med. Sci. 2019, 81, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Itoh, K.; Tanaka, M.; Tsuji, M.; Kawamoto, T.; Kawato, S.; Vogel, C.F.A.; Yamazaki, T. Potentiation of 17beta-estradiol synthesis in the brain and elongation of seizure latency through dietary supplementation with docosahexaenoic acid. Sci. Rep. 2017, 7, 6268. [Google Scholar] [CrossRef]
- Girardi, C.S.; Rostirolla, D.C.; Lini, F.J.M.; Brum, P.O.; Delgado, J.; Ribeiro, C.T.; Teixeira, A.A.; Peixoto, D.O.; Heimfarth, L.; Kunzler, A.; et al. Nuclear RXRalpha and RXRbeta receptors exert distinct and opposite effects on RA-mediated neuroblastoma differentiation. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 317–328. [Google Scholar] [CrossRef]
- Sarafian, T.A. Methylmercury-induced generation of free radicals: Biological implications. Met. Ions Biol. Syst. 1999, 36, 415–444. [Google Scholar]
- Takanezawa, Y.; Nakamura, R.; Hamaguchi, M.; Yamamoto, K.; Sone, Y.; Uraguchi, S.; Kiyono, M. Docosahexaenoic acid enhances methylmercury-induced endoplasmic reticulum stress and cell death and eicosapentaenoic acid potentially attenuates these effects in mouse embryonic fibroblasts. Toxicol. Lett. 2019, 306, 35–42. [Google Scholar] [CrossRef]
- Kaur, P.; Schulz, K.; Aschner, M.; Syversen, T. Role of docosahexaenoic acid in modulating methylmercury-induced neurotoxicity. Toxicol. Sci. 2007, 100, 423–432. [Google Scholar] [CrossRef]
- Kaur, P.; Heggland, I.; Aschner, M.; Syversen, T. Docosahexaenoic acid may act as a neuroprotector for methylmercury-induced neurotoxicity in primary neural cell cultures. Neurotoxicology 2008, 29, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Demar, J.C., Jr.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 2005, 94, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; DeMar, J.C., Jr.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J. Lipid Res. 2007, 48, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Impellizzeri, D.; Di Paola, R.; Esposito, E.; Gladman, S.; Yip, P.; Priestley, J.V.; Michael-Titus, A.T.; Cuzzocrea, S. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: In-vivo and in-vitro studies. J. Neuroinflamm. 2014, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Teter, B.; Ubeda, O.J.; Morihara, T.; Dhoot, D.; Nyby, M.D.; Tuck, M.L.; Frautschy, S.A.; Cole, G.M. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer’s disease (AD): Relevance to AD prevention. J. Neurosci. 2007, 27, 14299–14307. [Google Scholar] [CrossRef]
- de Urquiza, A.M.; Liu, S.; Sjoberg, M.; Zetterstrom, R.H.; Griffiths, W.; Sjovall, J.; Perlmann, T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 2000, 290, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Wietrzych-Schindler, M.; Szyszka-Niagolov, M.; Ohta, K.; Endo, Y.; Perez, E.; de Lera, A.R.; Chambon, P.; Krezel, W. Retinoid x receptor gamma is implicated in docosahexaenoic acid modulation of despair behaviors and working memory in mice. Biol. Psychiatry 2011, 69, 788–794. [Google Scholar] [CrossRef]
- Wallen-Mackenzie, A.; Mata de Urquiza, A.; Petersson, S.; Rodriguez, F.J.; Friling, S.; Wagner, J.; Ordentlich, P.; Lengqvist, J.; Heyman, R.A.; Arenas, E.; et al. Nurr1-RXR heterodimers mediate RXR ligand-induced signaling in neuronal cells. Genes Dev. 2003, 17, 3036–3047. [Google Scholar] [CrossRef]
- Krezel, W.; Ruhl, R.; de Lera, A.R. Alternative retinoid X receptor (RXR) ligands. Mol. Cell Endocrinol. 2019, 491, 110436. [Google Scholar] [CrossRef]
- de Vera, I.M.; Giri, P.K.; Munoz-Tello, P.; Brust, R.; Fuhrmann, J.; Matta-Camacho, E.; Shang, J.; Campbell, S.; Wilson, H.D.; Granados, J.; et al. Identification of a Binding Site for Unsaturated Fatty Acids in the Orphan Nuclear Receptor Nurr1. ACS Chem. Biol. 2016, 11, 1795–1799. [Google Scholar] [CrossRef]
- Schulman, I.G.; Li, C.; Schwabe, J.W.; Evans, R.M. The phantom ligand effect: Allosteric control of transcription by the retinoid X receptor. Genes Dev. 1997, 11, 299–308. [Google Scholar] [CrossRef]
- Paerregaard, S.I.; Agerholm, M.; Serup, A.K.; Ma, T.; Kiens, B.; Madsen, L.; Kristiansen, K.; Jensen, B.A. FFAR4 (GPR120) Signaling Is Not Required for Anti-Inflammatory and Insulin-Sensitizing Effects of Omega-3 Fatty Acids. Mediat. Inflamm. 2016, 2016, 1536047. [Google Scholar] [CrossRef]
- Ishihara, Y.; Sakurai, H.; Oguro, A.; Tsuji, M.; Vogel, C.F.A.; Yamazaki, T. Retinoid X receptor-mediated neuroprotection via CYP19 upregulation and subsequent increases in estradiol synthesis. J. Steroid Biochem. Mol. Biol. 2019, 193, 105421. [Google Scholar] [CrossRef]
- Jayashankar, S.; Glover, C.N.; Folven, K.I.; Brattelid, T.; Hogstrand, C.; Lundebye, A.K. Cerebral gene expression and neurobehavioural responses in mice pups exposed to methylmercury and docosahexaenoic acid through the maternal diet. Environ. Toxicol. Pharmacol. 2012, 33, 26–38. [Google Scholar] [CrossRef]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A. n-3 fatty acid-derived lipid mediators in the brain: New weapons against oxidative stress and inflammation. Curr. Med. Chem. 2012, 19, 532–543. [Google Scholar] [CrossRef]
- Arnold, C.; Markovic, M.; Blossey, K.; Wallukat, G.; Fischer, R.; Dechend, R.; Konkel, A.; von Schacky, C.; Luft, F.C.; Muller, D.N.; et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J. Biol. Chem. 2010, 285, 32720–32733. [Google Scholar] [CrossRef] [PubMed]
- Simmons-Willis, T.A.; Koh, A.S.; Clarkson, T.W.; Ballatori, N. Transport of a neurotoxicant by molecular mimicry: The methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem. J. 2002, 367, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Jiang, H.; Syversen, T.; Rocha, J.B.; Farina, M.; Aschner, M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J. Neurochem. 2008, 107, 1083–1090. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Slitt, A.L.; Maher, J.M.; Augustine, L.M.; Goedken, M.J.; Chan, J.Y.; Cherrington, N.J.; Klaassen, C.D.; Manautou, J.E. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol. Appl. Pharmacol. 2008, 226, 74–83. [Google Scholar] [CrossRef]
- Usuki, F.; Fujimura, M.; Yamashita, A. Endoplasmic reticulum stress preconditioning modifies intracellular mercury content by upregulating membrane transporters. Sci. Rep. 2017, 7, 12390. [Google Scholar] [CrossRef]
- Kishi, R.; Sata, F.; Katakura, Y.; Wang, R.S.; Nakajima, T. Effects of pregnancy, age and sex in the metabolism of styrene in rat liver in relation to the regulation of cytochrome P450 enzymes. J. Occup. Health 2005, 47, 49–55. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, K.I.; Lin, H.C.; Lee, T.S. Genetic Deletion of Soluble Epoxide Hydroxylase Causes Anxiety-Like Behaviors in Mice. Mol. Neurobiol. 2019, 56, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Takemoto, T.; Itoh, K.; Ishida, A.; Yamazaki, T. Dual role of superoxide dismutase 2 induced in activated microglia: Oxidative stress tolerance and convergence of inflammatory responses. J. Biol. Chem. 2015, 290, 22805–22817. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Yanagisawa, R.; Motomura, E.; Nakamura, M.; Sakamoto, M.; Takeya, M.; Eto, K. Increased methylmercury toxicity related to obesity in diabetic KK-Ay mice. J. Appl. Toxicol. 2014, 34, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Yanagisawa, R.; Sakai, A.; Mogi, M.; Shuto, S.; Shudo, M.; Kashiwagi, H.; Kudo, M.; Nakamura, M.; Sakamoto, M. Toxicokinetics of methylmercury in diabetic KK-Ay mice and C57BL/6 mice. J. Appl. Toxicol. 2020. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oguro, A.; Fujita, K.; Ishihara, Y.; Yamamoto, M.; Yamazaki, T. DHA and Its Metabolites Have a Protective Role against Methylmercury-Induced Neurotoxicity in Mouse Primary Neuron and SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 3213. https://doi.org/10.3390/ijms22063213
Oguro A, Fujita K, Ishihara Y, Yamamoto M, Yamazaki T. DHA and Its Metabolites Have a Protective Role against Methylmercury-Induced Neurotoxicity in Mouse Primary Neuron and SH-SY5Y Cells. International Journal of Molecular Sciences. 2021; 22(6):3213. https://doi.org/10.3390/ijms22063213
Chicago/Turabian StyleOguro, Ami, Kenta Fujita, Yasuhiro Ishihara, Megumi Yamamoto, and Takeshi Yamazaki. 2021. "DHA and Its Metabolites Have a Protective Role against Methylmercury-Induced Neurotoxicity in Mouse Primary Neuron and SH-SY5Y Cells" International Journal of Molecular Sciences 22, no. 6: 3213. https://doi.org/10.3390/ijms22063213
APA StyleOguro, A., Fujita, K., Ishihara, Y., Yamamoto, M., & Yamazaki, T. (2021). DHA and Its Metabolites Have a Protective Role against Methylmercury-Induced Neurotoxicity in Mouse Primary Neuron and SH-SY5Y Cells. International Journal of Molecular Sciences, 22(6), 3213. https://doi.org/10.3390/ijms22063213




