PET/CT Imaging and Physiology of Mice on High Protein Diet
Abstract
:1. Introduction
2. Results
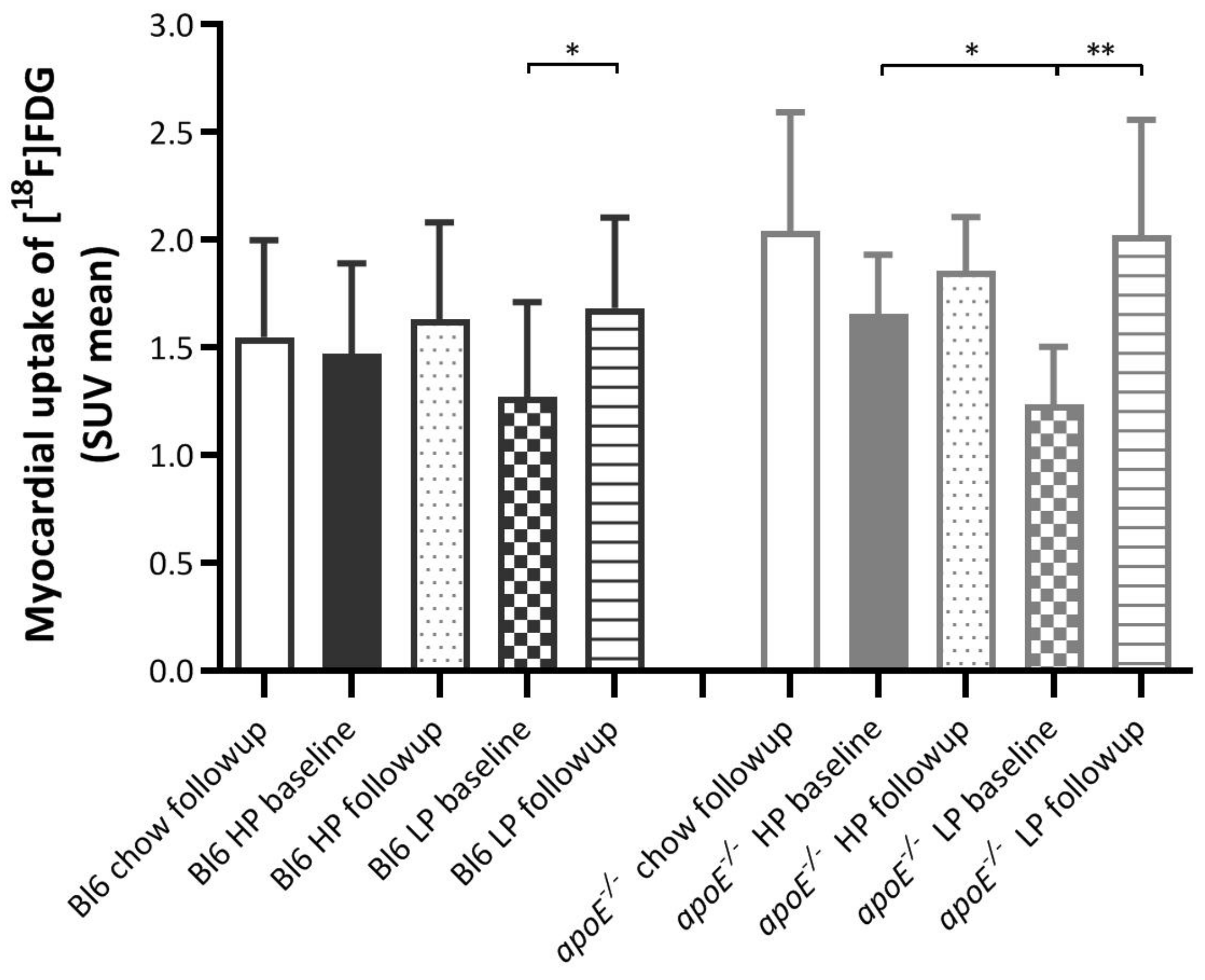
2.1. Effects of Diets on Myocardial [18F]FDG Uptake
2.2. Effects of Diets on Blood Glucose Levels
2.3. Effects of Diets on Myocardial Volume
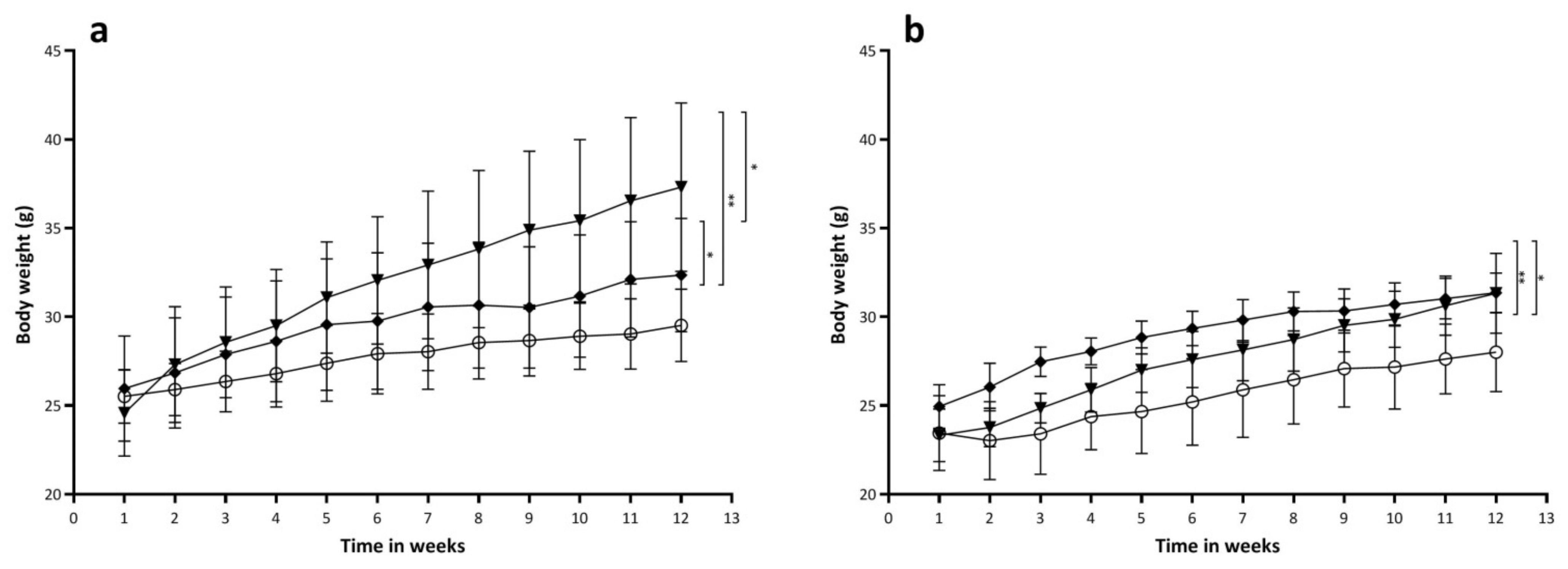
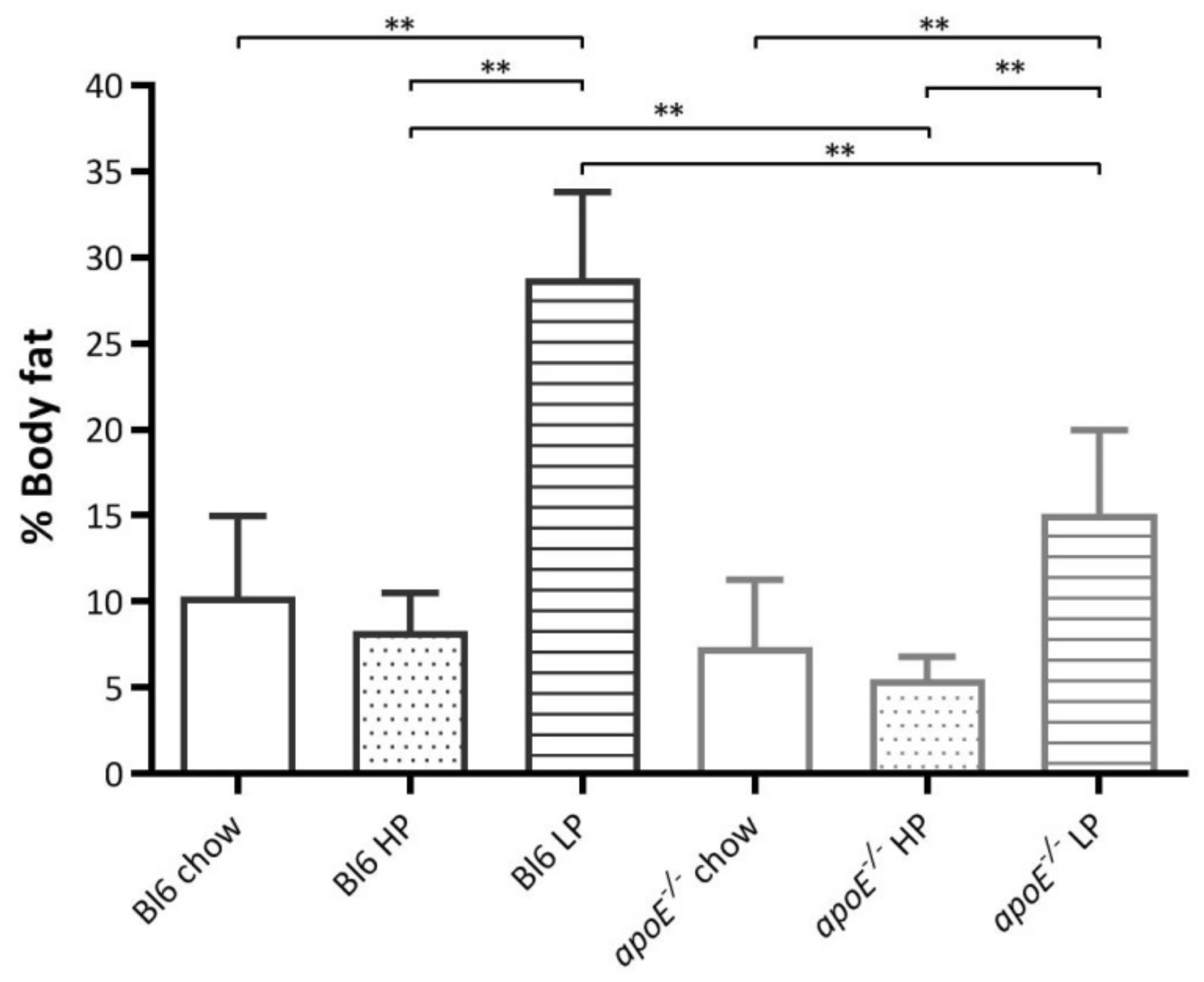
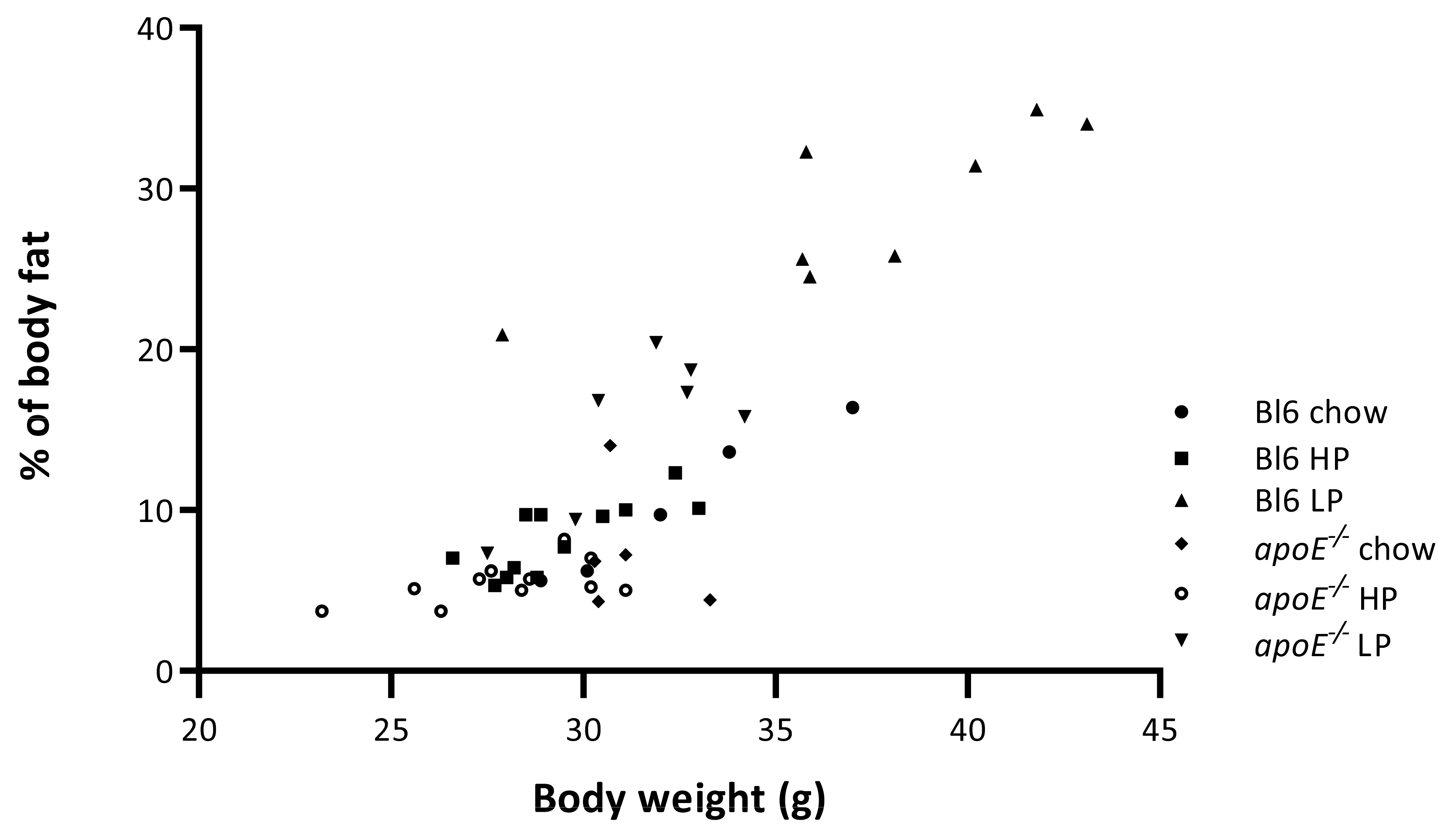
2.4. Effects of Diets on Body Weight and Body Composition
3. Discussion
4. Methods
4.1. Animals
4.2. PET/CT Procedure
4.3. Diet
4.4. PET Analysis
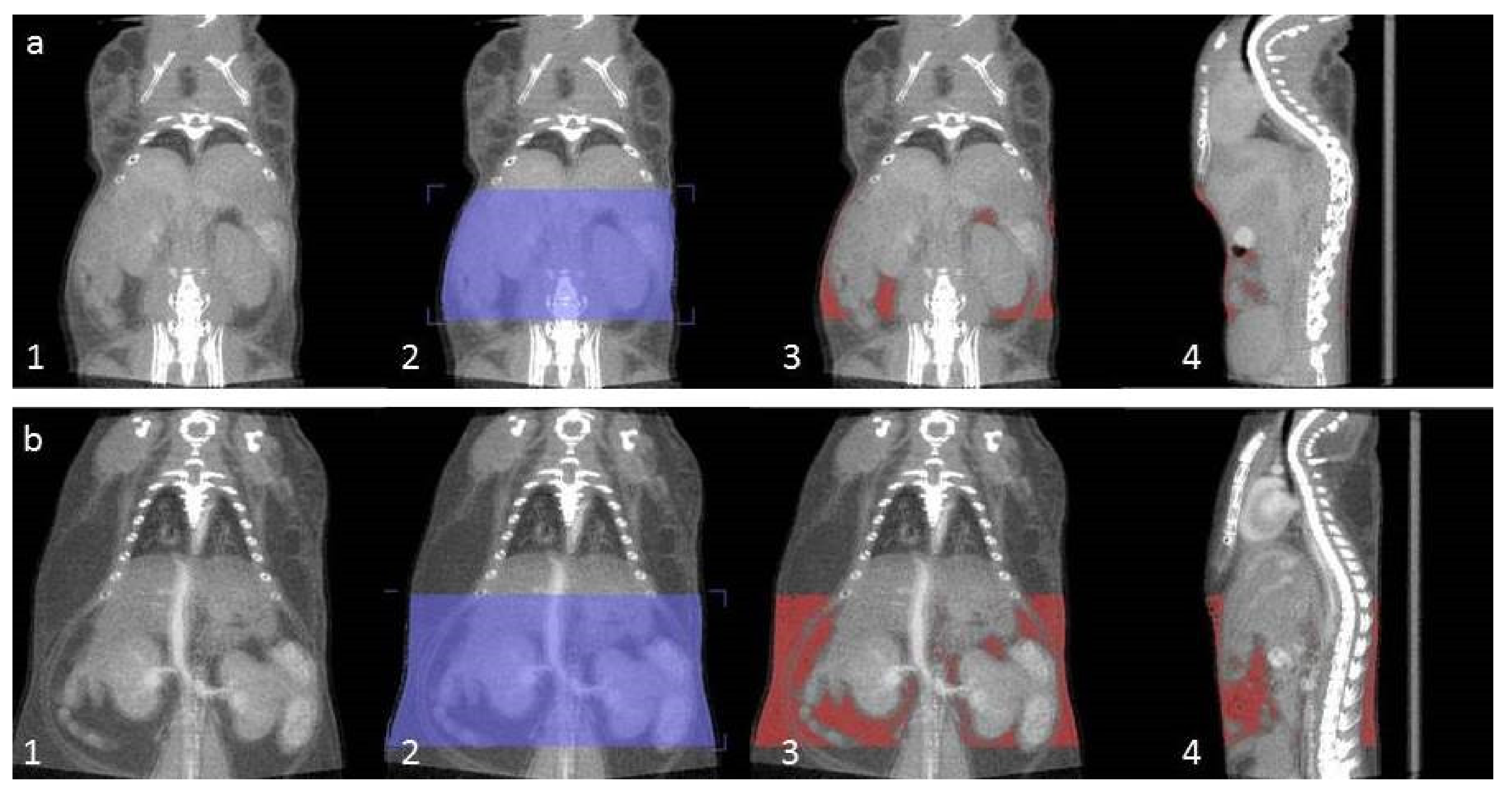
4.5. CT Analysis
4.6. Statistical Analysis
5. New Knowledge Gained
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, J.; Craig, B.A.; Leidy, H.J.; Amankwaah, A.F.; Anguah, K.O.B.; Jacobs, A.; Jones, B.L.; Jones, J.B.; Keeler, C.L.; Keller, C.E.; et al. The effects of increased protein intake on fullness: A meta-analysis and its limitations. J. Acad. Nutr. Diet. 2016, 68, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 1, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, A.M.; Verberne, H.J.; Budde, R.P.; Lam, M.G. Additional Heparin preadministration improves cardiac glucose metabolism suppression over low-carbohydrate diet alone in 18F-FDG PET imaging. J. Nucl. Med. 2016, 57, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Tupper, T.; Wang, Y.; McDonagh, E.A.; Yap, J.; Kung, A.L. Utilizing ketogenic diet as an alternative to fasting in preclinical 18F-FDG PET. In World Mol Imaging Congress (WMIC) 2011 Program Book; World Molecular Imaging Society: San Diego, CA, USA, 2011. [Google Scholar]
- Slart, R.H.; Bax, J.J.; Van Veldhuisen, D.J.; Van der Wall, E.E.; Dierckx, R.A.; Jager, P.L. Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int. J. Cardiovasc. Imaging 2006, 63, 80–81. [Google Scholar] [CrossRef]
- Soares, J.; Rodrigues Filho, F.; Izaki, M.; Giorgi, M.C.P.; Catapirra, R.M.; Abe, R.; Vinagre, C.G.; Cerri, G.G.; Meneghetti, J.C. Low-carbohydrate diet versus euglycemic hyperinsulinemic clamp for the assessment of myocardial viability with 18F-fluorodeoxyglucose-PET: A pilot study. Int. J. Cardiovasc. Imaging 2014, 15, 423–430. [Google Scholar] [CrossRef]
- Knuuti, M.J.; Yki-Järvinen, H.; Voipio-Pulkki, L.M.; Mäki, M.; Ruotsalainen, U.; Härkönen, R.; Teräs, M.; Haaparanta, M.; Bergman, J.; Hartiala, J.; et al. Enhancement of myocardial [fluorine-18]fluorodeoxyglucose uptake by a nicotinic acid derivative. J. Nucl. Med. 1994, 35, 989–998. [Google Scholar] [PubMed]
- Brooks, G.A. Amino acid and protein metabolism during exercise and recovery. Med. Sci. Sports Exerc. 1987, 19 (Suppl. 5), S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro, M.B.T.; Cunha, D.B.; Araujo, M.C.; Bezerra, I.N.; Adegboye, A.R.A.; Kac, G.; Sichieri, R. High protein diet promotes body weight loss among Brazilian postpartum women. Matern. Child Nutr. 2019, 15, e12746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallotto, M.R.; De Godoy, M.R.C.; Holscher, H.D.; Buff, P.R.; Swanson, K.S. Effects of weight loss with a moderate-protein, high-fiber diet on body composition, voluntary physical activity, and fecal microbiota of obese cats. Am. J. Vet. Res. 2018, 79, 181–190. [Google Scholar] [CrossRef] [PubMed]
- André, A.; Leriche, I.; Chaix, G.; Thorin, C.; Burger, M.; Nguyen, P. Recovery of insulin sensitivity and optimal body composition after rapid weight loss in obese dogs fed a high-protein medium-carbohydrate diet. J. Anim. Physiol. Anim. Nutr. 2017, 101 (Suppl. 1), 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shai, S.Y.; Sukhanov, S.; Higashi, Y.; Vaughn, C.; Rosen, C.J.; Delafontaine, P. Low circulating insulin-like growth factor I increases atherosclerosis in ApoE-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1898–H1906. [Google Scholar] [CrossRef] [Green Version]
- Thackeray, J.T.; Bankstahl, J.P.; Wang, Y.; Wollert, K.C.; Bengel, F.M. Clinically relevant strategies for lowering cardiomyocyte glucose uptake for 18F-FDG imaging of myocardial inflammation in mice. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 771–780. [Google Scholar] [CrossRef]
- Foo, S.Y.; Heller, E.R.; Wykrzykowska, J.; Sullivan, C.J.; Manning-Tobin, J.J.; Moore, K.J.; Gerszten, R.E.; Rosenzweig, A. Vascular effects of a low-carbohydrate high-protein diet. Proc. Natl. Acad. Sci. USA 2009, 106, 15418–15423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostogrys, R.B.; Franczyk-Żarów, M.; Maślak, E.; Gajda, M.; Mateuszuk, L.; Jackson, C.L.; Chłopicki, S. Low carbohydrate, high protein diet promotes atherosclerosis in apolipoprotein E/low-density lipoprotein receptor double knockout mice (apoE/LDLR(-/-)). Atherosclerosis 2012, 223, 327–331. [Google Scholar] [CrossRef]
- Kostogrys, R.B.; Johann, C.; Czyżyńska, I.; Franczyk-Żarów, M.; Drahun, A.; Maślak, E.; Jasztal, A.; Gajda, M.; Mateuszuk, Ł.; Wrobel, T.P.; et al. Characterisation of Atherogenic Effects of Low Carbohydrate, High Protein Diet (LCHP) in ApoE/LDLR-/-Mice. J. Nutr. Health Aging 2015, 19, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.R.; Morgan, H.E. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu. Rev. Physiol. 1974, 36, 413–459. [Google Scholar] [CrossRef]
- Neely, J.R.; Rovetto, M.J.; Oram, J.F. Myocardial utilization of carbohydrate and lipids. Prog. Cardiovasc. Dis. 1972, 15, 289–329. [Google Scholar] [CrossRef]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Van Loon, L.J. Dietary protein for athletes: From requirements to optimum adaptation. J. Sports Sci. 2011, 29 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Sánchez, M.; Navas-Carrillo, D.; Orenes-Piñero, E. Controversies surrounding high-protein diet intake: Satiating effect and kidney and bone health. Adv. Nutr. 2015, 6, 260–266. [Google Scholar] [CrossRef]
- Zutphen, L.F.M.; Baumans, V.; Beynen, A.C. Principles of Laboratory Animal Science; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Stegger, L.; Hoffmeier, A.N.; Schäfers, K.P.; Hermann, S.; Schober, O.; Schäfers, M.A.; Theilmeier, G. Accurate noninvasive measurement of infarct size in mice with high-resolution PET. J. Nucl. Med. 2006, 47, 1837–1844. [Google Scholar] [PubMed]
- Stegger, L.; Heijman, E.; Schäfers, K.P.; Nicolay, K.; Schäfers, M.A.; Strijkers, G.J. Quantification of left ventricular volumes and ejection fraction in mice using PET, compared with MRI. J. Nucl. Med. 2009, 50, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Sasser, T.A.; Chapman, S.E.; Li, S.; Hudson, C.; Orton, S.P.; Diener, J.M.; Gammon, S.T.; Correcher, C.; Leevy, W.M. Segmentation and Measurement of Fat Volumes in Murine Obesity Models Using X-ray Computed Tomography. J. Vis. Exp. 2012, 1, 5–62. [Google Scholar] [CrossRef] [PubMed]

| Baseline | Follow-Up | |
|---|---|---|
| Groups | mmol/L | mmol/L |
| Bl6 chow | 8.8 ± 0.8 | |
| apoE−/− chow | 9.3 ± 1.4 | |
| Bl6 HP | 8.0 ± 1.5 | 7.8 ± 1.34 |
| apoE−/− HP | 10.4 ± 1.4 | 8.5 ± 1.8 |
| Bl6 LP | 7.3 ± 0.8 | 7.8 ± 1.4 |
| apoE−/− LP | 8.5 ± 1.5 | 10.1 ± 2.3 |
| Chow | HP | LP | |
|---|---|---|---|
| Gross energy (kcal/kg) | 3845.4 | 4719.4 * | 4693.3 * |
| Ingredients | % | % | % |
| (Crude) protein | 23.5 | 58.0 | 27.7 |
| (Crude) fat | 5.0 | 21.0 ** | 21.0 ** |
| (Crude) fiber | 4.3 | 5.0 | 5.0 |
| Starch | 38.3 | 1.8 | 11.4 |
| Sugar | 4.0 | 6.35 | 27.4 |
| Nutrients | g/kg | g/kg | g/kg |
| (Crude) protein | - | 497.9 | 239.7 |
| (Crude) fat | - | 215.8 | 212.8 |
| (Crude) fiber | - | 49.9 | 50.9 |
| Sugar + starch | - | 85.3 | 376.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sijbesma, J.W.A.; van Waarde, A.; Stegger, L.; Dierckx, R.A.J.O.; Boersma, H.H.; Slart, R.H.J.A. PET/CT Imaging and Physiology of Mice on High Protein Diet. Int. J. Mol. Sci. 2021, 22, 3236. https://doi.org/10.3390/ijms22063236
Sijbesma JWA, van Waarde A, Stegger L, Dierckx RAJO, Boersma HH, Slart RHJA. PET/CT Imaging and Physiology of Mice on High Protein Diet. International Journal of Molecular Sciences. 2021; 22(6):3236. https://doi.org/10.3390/ijms22063236
Chicago/Turabian StyleSijbesma, Jürgen W. A., Aren van Waarde, Lars Stegger, Rudi A. J. O. Dierckx, Hendrikus H. Boersma, and Riemer H. J. A. Slart. 2021. "PET/CT Imaging and Physiology of Mice on High Protein Diet" International Journal of Molecular Sciences 22, no. 6: 3236. https://doi.org/10.3390/ijms22063236
APA StyleSijbesma, J. W. A., van Waarde, A., Stegger, L., Dierckx, R. A. J. O., Boersma, H. H., & Slart, R. H. J. A. (2021). PET/CT Imaging and Physiology of Mice on High Protein Diet. International Journal of Molecular Sciences, 22(6), 3236. https://doi.org/10.3390/ijms22063236








