Asthma and Chronic Rhinosinusitis: How Similar Are They in Pathogenesis and Treatment Responses?
Abstract
:1. Introduction
2. Pathogenic Mechanisms of Upper and Lower Airway Inflammation in Asthma and CRS
3. Biological Agents Targeting Type 2 Inflammation
4. Clinical Efficacy of Biological Agents in Asthma and CRS
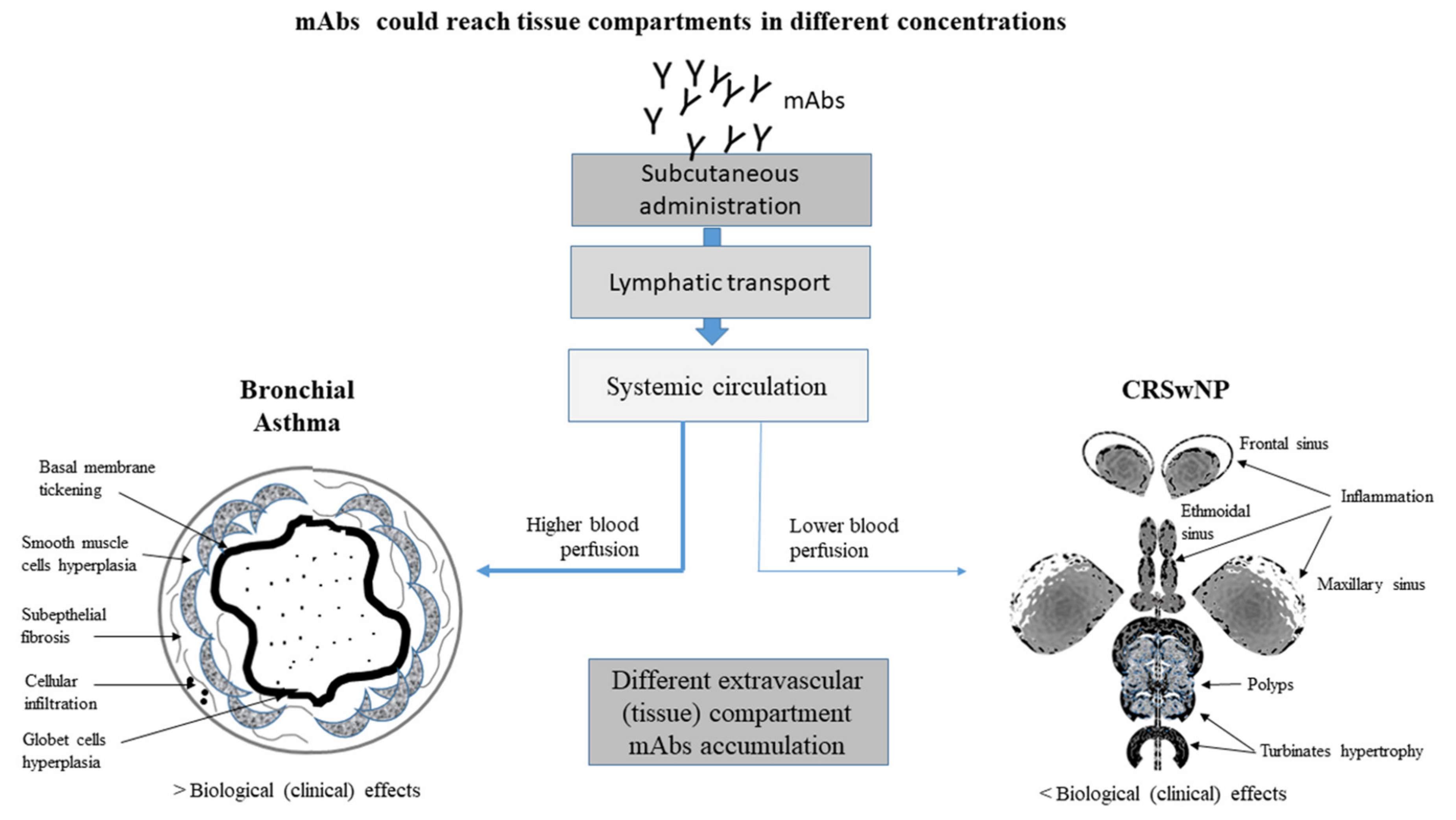
5. Asthma and CRS May Display Different Clinical Outcomes in Response to Biological Treatment
6. Conclusions
Funding
Conflicts of Interest
References
- Siddiqui, S.; Shikotra, A.; Richardson, M.; Doran, E.; Choy, D.; Bell, A.; Austin, C.D.; Eastham-Anderson, J.; Hargadon, B.; Arron, J.R.; et al. Airway pathological heterogeneity in asthma: Visualization of disease microclusters using topological data analysis. J. Allergy Clin. Immunol. 2018, 142, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, N.; Li, J.; Liu, X.; Xiong, Q.; Hu, C.; Chen, D.; Guan, L.; Chang, K.; Li, D.; et al. Cross-reactive antibodies against dust mite-derived enolase induce neutrophilic airway inflammation. Eur. Respir. J. 2021, 57, 1902375. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Schwartz, L.B.; Langmack, E.L.; Halliday, J.L.; Trudeau, J.B.; Gibbs, R.L.; Chu, H.W. Evidence That Severe Asthma Can be Divided Pathologically into Two Inflammatory Subtypes with Distinct Physiologic and Clinical Characteristics. Am. J. Respir. Crit. Care Med. 1999, 160, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F.; Zeki, A.A.; Kraft, M. Eosinophilic and non eosinophilic asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 22–37. [Google Scholar] [CrossRef]
- Takhar, P.; Corrigan, C.J.; Smurthwaite, L.; O’Connor, B.J.; Durham, S.R.; Lee, T.H.; Gould, H.J. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007, 119, 213–218. [Google Scholar] [CrossRef]
- Froidure, A.; Mouthuy, J.; Durham, S.R.; Chanez, P.; Sibille, Y.; Pilette, C. Asthma phenotypes and IgE responses. Eur. Respir. J. 2015, 47, 304–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bel, E.H.; Brinke, A.T. New Anti-Eosinophil Drugs for Asthma and COPD. Chest 2017, 152, 1276–1282. [Google Scholar] [CrossRef]
- Wangberg, H.; Woessner, K. Choice of biologics in asthma endotypes. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Severe Adult Asthmas: Integrating Clinical Features, Biology and Therapeutics to Improve Outcomes. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Shaw, D.E.; Sousa, A.R.; Fowler, S.J.; Fleming, L.J.; Roberts, G.; Corfield, J. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur. Respir. J. 2015, 46, 1308–1321. [Google Scholar] [CrossRef] [Green Version]
- Brinke, A.T.; Grootendorst, D.C.; Schmidt, J.T.; De Bruïne, F.T.; Van Buchem, M.A.; Sterk, P.J.; Rabe, K.H.; Bel, E.H. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J. Allergy Clin. Immunol. 2002, 109, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, T.R.; Radhakrishna, N.; Hore-Lacy, F.; Smith, C.; Hoy, R.; Dabscheck, E.; Hew, M. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016, 21, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, L.C.; Phillips, B.R.; Ramratnam, S.; Ross, K.; Bhakta, N.R.; Cardet, J.C.; Castro, M.; Peters, S.P.; Phipatanakul, W.; Aujla, S.; et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am. J. Respir. Crit. Care Med. 2017, 195, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J. Executive summary of EPOS 2020 including integrated care pathways. Rhinology 2020, 58, 82–111. [Google Scholar] [CrossRef]
- Benjamin, M.R.; Stevens, W.W.; Li, N.; Bose, S.; Grammer, L.C.; Kern, R.C.; Tan, B.K.; Conley, D.B.; Smith, S.S.; Welch, K.C.; et al. Clinical Characteristics of Patients with Chronic Rhinosinusitis without Nasal Polyps in an Academic Setting. J. Allergy Clin. Immunol. Pr. 2019, 7, 1010–1016. [Google Scholar] [CrossRef]
- Delemarre, T.; Holtappels, G.; De Ruyck Zhang, N.; Nauwynck, H.; Bachert, C.; Gevaert, E. Type 2 inflammation in chronic rhinusinusitis without nasal polyps: Another relevant endotype. J. Allergy Clin. Immunol. 2020, 146, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, D.W.; Gevaert, P. Chronic Rhinosinusitis without Nasal Polyps. J. Allergy Clin. Immunol. Pr. 2016, 4, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.-O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G.; et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456. [Google Scholar] [CrossRef] [Green Version]
- Mortuaire, G.; Leroy, X.; Gengler, I.; Chevalier, D.; Prin, L.; Picry, A. Histopathological classification of refractory chronic rhinosinusitis with nasal polyps. Histol. Histopathol. 2015, 30, 1447–1454. [Google Scholar]
- Peters, M.C.E.; Wenzel, S. Intersection of biology and therapeutics: Type 2 targeted therapeutics for adult asthma. Lancet 2020, 395, 371–383. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.E.; Lee, S.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [Green Version]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.; Sousa, A.; Smith, S.; Martin, N.; Mayer, B.; et al. Late Breaking Abstract-Add-on mepolizumab for chronic rhinosinusitis with nasal polyps: Synapse study. Airw. Pharmacol. Treat. 2020, 56. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; D’Ancona, G.; Elstad, M.; Green, L.; Fernandes, M.; Thomson, L.; Roxas, C.; Dhariwal, J.; Nanzer, A.M.; Kent, B.D.; et al. Real-World Effectiveness and the Characteristics of a “Super-Responder” to Mepolizumab in Severe Eosinophilic Asthma. Chest 2020, 158, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Eger, K.; Kroes, J.A.; Brinke, A.T.; Bel, E.H. Long-Term Therapy Response to Anti-IL-5 Biologics in Severe Asthma—A Real-Life Evaluation. J. Allergy Clin. Immunol. Pr. 2021, 9, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Brusselle, G.; Louis, R.; Vandenplas, O.; Michils, A.; Pilette, C.; Peche, R.; Manise, M.; Joos, G. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir. Med. 2014, 108, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Boonpiyathada, T.; Sözenera, Z.C.; Satitsuksanoaa, P.; Akdis, C.A. Immunologic mechanisms in asthma. Sem. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef]
- Maggi, L.; Montaini, G.; Mazzoni, A.; Rossettini, B.; Capone, M.; Rossi, M.C.; Santarlasci, V.; Liotta, F.; Rossi, O.; Gallo, O.; et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J. Allergy Clin. Immunol. 2017, 139, 964–976. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.-I.; Shim, J.-U. Association between Sputum Natural Killer T Cells and Eosinophilic Airway Inflammation in Human Asthma. Int. Arch. Allergy Immunol. 2010, 153, 239–248. [Google Scholar] [CrossRef]
- Acharya, K.R.; Ackerman, S.J. Eosinophil Granule Proteins: Form and Function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, A.J.; Kon, O.M.; Smith, S.J.; Zeibecoglou, K.; Khan, L.; Barata, L.T.; McEuen, A.R.; Buckley, M.G.; Walls, A.F.; Meng, Q.; et al. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J. Allergy Clin. Immunol. 2000, 105, 99–107. [Google Scholar] [CrossRef]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.E.; Wenzel, S.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Matucci, A.; Vultaggio, A.; Maggi, E.; Kasujee, I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir. Res. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckrich, J.; Hinkel, J.; Fischl, A.; Herrmann, E.; Holtappels, G.; Bachert, C.; Zielen, S. Nasal IgE in subjects with allergic and non-allergic rhinitis. World Allergy Organ. J. 2020, 13, 100129. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.N.; Guo, Y.B.; Li, X.; Li, C.L.; Tan, W.P.; Fan, X.L.; Qin, Z.L.; Chen, D.; Wen, W.P.; Zheng, S.G.; et al. ILC2 frequency and activity are inhibited by glucocorticoid treatment via STAT pathway in patients with asthma. Allergy 2018, 73, 1860–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Smith, S.G.; Salter, B.; El-Gammal, A.; Oliveria, J.P.; Obminski, C.; Watson, R.; O’Byrne, P.M.; Gauvreau, G.M.; Sehmi, R. Allergen-induced Increases in Sputum Levels of Group 2 Innate Lymphoid Cells in Subjects with Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Walford, H.H.; Lund, S.J.; Baum, R.E.; White, A.A.; Bergeron, C.M.; Husseman, J.; Bethel, K.J.; Scott, D.R.; Khorram, N.; Miller, M.; et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin. Immunol. 2014, 155, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Muroi, M.; Horiguchi, K.; Tanamoto, K.; Nagase, T.; Yamashita, N. Activation through toll-like receptor 2 on group 2 innate lymphoid cells can induce asthmatic characteristics. Clin. Exp. Allergy 2019, 49, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; Menegatti, S.; Bustamante, J.; Le Bourhis, L.; Allez, M.; Rogge, L.; Casanova, J.-L.; Yssel, H.; Di Santo, J.P. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J. Exp. Med. 2016, 213, 569–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joubert, P.; Hamid, Q. Role of airway smooth muscle in airway remodeling. J. Allergy Clin. Immunol. 2005, 116, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Lu, B.; Wu, H.-K.; Zhang, H.; Yao, F.-F. Apigenin inhibits TGF-β1-induced proliferation and migration of airway smooth muscle cells. Int. J. Clin. Exp. Pathol. 2015, 8, 12557–12563. [Google Scholar]
- Snidvongs, K.; Lam, M.; Sacks, R.; Earls, P.; Kalish, L.; Phillips, P.S.; Pratt, E.; Harvey, R.J. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int. Forum Allergy Rhinol. 2012, 2, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, H.N.; Tajudeen, B.A.; Mahdavinia, M.; Gattuso, P.; Ghai, R.; Batra, P.S. Inflammatory infiltrate and mucosal remodeling in chronic rhinosinusitis with and without polyps: Structured histopathologic analysis. Int. Forum Allergy Rhinol. 2017, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, H.H.; Aizen, M.; Barkans, J.; Robinson, U.S.; Kay, A.B. Remodeling and Airway Hyperresponsiveness but not Cellular Inflammation Persist after Allergen Challenge in Asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Payne, D.N.R.; Rogers, A.V.; Ädelroth, E.; Bandi, V.; Guntupalli, K.K.; Bush, A.; Jeffery, P.K. Early Thickening of the Reticular Basement Membrane in Children with Difficult Asthma. Am. J. Respir. Crit. Care Med. 2003, 167, 78–82. [Google Scholar] [CrossRef]
- Cokugras, H.; Akcakaya, N.; Seckin Camcioglu, Y.; Sarimurat, N.; Aksoy, F. Ultrastructural examination of bronchial biopsy specimens from children with moderate asthma. Thorax 2001, 56, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samitas, K.; Poulos, N.; Semitekolou, M.; Morianos, I.; Tousa, S.; Economidou, E.; Robinson, D.S.; Kariyawasam, H.H.; Zervas, E.; Corrigan, C.J.; et al. Activin-A is overexpressed in severe asthma and is implicated in angiogenic processes. Eur. Respir. J. 2016, 47, 769–782. [Google Scholar] [CrossRef] [Green Version]
- Kariyawasam, H.H.; Pegorier, S.; Barkans, J.; Xanthou, G.; Aizen, M.; Ying, S.; Kay, A.B.; Lloyd, C.M.; Robinson, U.S. Activin and transforming growth factor-beta signaling pathways are activated after allergen challenge in mild asthma. J. Allergy Clin. Immunol. 2009, 124, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Samitas, K.; Zervas, E.; Vittorakis, S.; Semitekolou, M.; Alissafi, T.; Bossios, A.; Gogos, H.; Economidou, E.; Lotvall, J.; Xanthou, G.; et al. Osteopontin expression and relation to disease severity in human asthma. Eur. Respir. J. 2010, 37, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Akdis, C.A. Vascular Endothelial Growth Factor as a Key Inducer of Angiogenesis in the Asthmatic Airways. Curr. Allergy Asthma Rep. 2012, 13, 1–9. [Google Scholar] [CrossRef]
- Holgate, S.T.; Davies, D.E.; Rorke, S.; Cakebread, J.; Murphy, G.; Powell, R.M.; Holloway, J.W. ADAM 33 and its Association with Airway Remodeling and Hyperresponsiveness in Asthma. Clin. Rev. Allergy Immunol. 2004, 27, 23–34. [Google Scholar] [CrossRef]
- Holgate, S.T. Mechanisms of Asthma and Implications for its Prevention and Treatment: A Personal Journey. Allergy Asthma Immunol. Res. 2013, 5, 343–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holgate, S.T.; Davies, D.E.; Lackie, P.M.; Wilson, S.J.; Puddicombe, S.M.; Lordan, J.L. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2000, 105, 193–204. [Google Scholar] [CrossRef]
- Bhimrao, S.K.; Wilson, S.; Howarth, P. Airway Inflammation in Atopic Patients: Comparison of Upper & Lower Airway. Otolaryngol. Neck Surg. 2009, 141, P33. [Google Scholar] [CrossRef]
- Lim, M.C.; Taylor, R.M.; Naclerio, R.M. The histology of allergic rhinitis and its comparison to cellular changes in nasal lavage. Am. J. Respir. Crit. Care Med. 1995, 151, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Eifan, A.O.; Orban, N.T.; Jacobson, M.R.; Durham, S.R. Severe Persistent Allergic Rhinitis. Inflammation but no Histologic Features of Structural Upper Airway Remodeling. Am. J. Respir. Crit. Care Med. 2015, 192, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- De Borja, C.F.; Picado, C.; Martinez-Anton, A.; Alobid, I.; Pujols, L.; Valero, A.; Roca-Ferrer, J.; Mullol, J. Differential expression of remodeling markers by tissue structure in nasal polyposis. Am. J. Rhinol. Allergy 2013, 27, e69–e74. [Google Scholar]
- Wang, L.-F.; Chien, C.-Y.; Chiang, F.-Y.; Chai, C.-Y.; Tai, C.-F. Corelationship between Matrix Metalloproteinase 2 and 9 Expression and Severity of Chronic Rhinosinusitis with Nasal Polyposis. Am. J. Rhinol. Allergy 2012, 26, e1–e4. [Google Scholar] [CrossRef]
- Barham, H.P.; Osborn, J.L.; Snidvongs, K.; Mrad, N.; Sacks, R.; Harvey, R.J. Remodeling changes of the upper airway with chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 5, 565–572. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Zhang, L.; Bachert, C. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Meng, J.; Qiao, X.; Liu, Y.; Liu, F.; Zhang, N.; Zhang, J.; Holtappels, G.; Luo, B.; Zhou, P.; et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J. Allergy Clin. Immunol. 2010, 125, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Latella, G.; Viscido, A. Controversial Contribution of Th17/IL-17 Toward the Immune Response in Intestinal Fibrosis. Dig. Dis. Sci. 2020, 65, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in Persistent Asthma with Elevated Eosinophil Levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkstrom, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.; Barker, P.; Mathe, S.S.; et al. Calima study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Humbert, M.; Beasley, R.; Ayres, J.; Slavin, R.; Hébert, J.; Bousquet, J.; Beeh, K.-M.; Ramos, S.; Canonica, G.W.; Hedgecock, S.; et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): Innovate. Allergy 2005, 60, 309–316. [Google Scholar] [CrossRef]
- Castro, M.; Zangrilli, J.E.; Wechsler, M.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Corren, J.; Weinstein, S.; Janka, L.; Zangrilli, J.; Garin, M. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest 2016, 150, 799–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjermer, L.; Lemiere, C.; Maspero, J.; Weiss, S.; Zangrilli, J.; Germinaro, M. Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase 3 Study. Chest 2016, 150, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacGlashan, D.W., Jr.; Bochner, B.S.; Adelman, D.C.; Jardieu, P.M.; Togias, A.; McKenzie-White, A.; Sterbinsky, S.A.; Hamilton, R.G.; Lichtenstein, L.M. Down-regulation of Fc (epsilon) RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. Immunology 1997, 158, 1438–1445. [Google Scholar]
- Schroeder, J.T.; Bieneman, A.P.; Chichester, K.L.; Hamilton, R.G.; Xiao, H.; Saini, S. Decreases in human dendritic cell-dependent T(H)2-like responses after acute in vivo IgE neutralization. J. Allergy Clin. Immunol. 2010, 125, 896–901. [Google Scholar] [CrossRef]
- Clutterbuck, E.J.; Hirst, E.M.; Sanderson, C.J. Human Interleukin-5 (IL-5) Regulates the Production of Eosinophils in Human Bone Marrow Cultures: Comparison and Interaction with IL-1, IL-3, IL-6 and GMCSF. Blood 1989, 73, 1504–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busse, W.W.; Katial, R.; Gossage, D.; Sari, S.; Wang, B.; Kolbeck, R.; Coyle, A.J.; Koike, M.; Spitalny, G.L.; Kiener, P.A.; et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti–IL-5 receptor α antibody, in a phase I study of subjects with mild asthma. J. Allergy Clin. Immunol. 2010, 125, 1237–1244. [Google Scholar] [CrossRef]
- Corren, J. Role of Interleukin-13 in Asthma. Curr. Allergy Asthma Rep. 2013, 13, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Van Crombruggen, K.; Zhang, N.; Gevaert, P.; Tomassen, P.; Bachert, C. Pathogenesis of chronic rhinosinusitis: Inflammation. J. Allergy Clin. Immunol. 2011, 128, 728–732. [Google Scholar] [CrossRef]
- Fahy, J.V.; Fleming, H.E.; Wong, H.H.; Liu, J.T.; Su, J.Q.; Reimann, J. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1997, 155, 1828–1834. [Google Scholar] [CrossRef] [Green Version]
- Casale, T.B.; Luskin, A.T.; Busse, W.; Zeiger, R.S.; Trzaskoma, B.; Yang, M.; Griffin, N.M.; Chipps, B.E. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence from Prospero, A Prospective Real-World Study. J. Allergy Clin. Immunol. Pr. 2019, 7, 156–164. [Google Scholar] [CrossRef]
- Hanania, N.A.; Wenzel, S.; Rosén, K.; Hsieh, H.-J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef]
- Busse, W.; Spector, S.; Rosén, K.; Wang, Y.; Alpan, O. High eosinophil count: A potential biomarker for assessing successful omalizumab treatment effects. J. Allergy Clin. Immunol. 2013, 132, 485–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipps, B.E.; Lanier, B.; Milgrom, H.; Deschildre, A.; Hedlin, G.; Szefler, S.J.; Kattan, M.; Kianifard, F.; Ortiz, B.; Haselkorn, T.; et al. Omalizumab in children with uncontrolled allergic asthma: Review of clinical trial and real-world experience. J. Allergy Clin. Immunol. 2017, 139, 1431–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gevaert, P.; Calus, L.; Van Zele, T.; Blomme, K.; De Ruyck, N.; Bauters, W.; Hellings, P.; Brusselle, G.; De Bacquer, D.; Van Cauwenberge, P.; et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J. Allergy Clin. Immunol. 2013, 131, 110–116. [Google Scholar] [CrossRef]
- Tiotiu, A.; Oster, J.P.; Roux, P.R.; Thi, P.L.N.; Peiffer, G.; Bonniaud, P.; Dalphin, J.C.; De Blay, F. Effectiveness of Omalizumab in Severe Allergic Asthma and Nasal Polyposis: A Real-Life Study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral Glucocorticoid-Sparing Effect of Mepolizumab in Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; Fitzgerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, H.G.; Yancey, S.W.; Mayer, B.; Gunsoy, N.B.; Keene, O.N.; Bleecker, E.R.E.; Brightling, C.; Pavord, I.D. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: A secondary analysis of the Dream and Mensa studies. Lancet Respir. Med. 2016, 4, 549–556. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Busse, W.; Maspero, J.F.; Katelaris, C.H.; Saralaya, D.; Guillonneau, S.; Zhang, B.; Taniou, C.; Staudinger, H.; Chao, J.; Amin, N.; et al. Dupilumab improves SNOT-22 scores in asthma patients with chronic rhinosinusitis or nasal polypsosis (CRS/NP) in Liberty Asthma Quest. Allergy Immunol. 2018, 52, PA1125. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Maspero, J.F.; Rabe, K.F.; Papi, A.; Wenzel, S.E.; Ford, L.B.; Pavord, I.D.; Zhang, B.; Staudinger, H.; Pirozzi, G.; et al. Liberty Asthma Quest: Phase 3 Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate Dupilumab Efficacy/Safety in Patients with Uncontrolled, Moderate-to-Severe Asthma. Adv. Ther. 2018, 35, 737–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipwort, B.; Chan, R.; Kuo, R.R.W. Eosinophil paradox with mepolizumab in chronic rhinosinusitis with nasal polyposis. J. Clin. Immunol. 2020, 146, 683. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T.M.; Prussin, C.; Panettieri, R.A.; Lee, S.; Ferguson, B.J.; Adappa, N.D.; Lane, A.P.; Ba, M.L.P.; Sullivan, M.; Ba, M.S.; et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope 2019, 129, E61–E66. [Google Scholar] [CrossRef]
- Gevaert, P.; Van Bruaene, N.; Cattaert, T.; Van Steen, K.; Van Zele, T.; Acke, F.; De Ruyck, N.; Blomme, K.; Sousa, A.R.; Marshall, R.P.; et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J. Allergy Clin. Immunol. 2011, 128, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J.; et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, M.; Paramo, F.A.; Kjarsgaard, M.; Salter, B.; Nair, G.; Lavigne, N.; Radford, K.; Sehmi, R.; Nair, P. Weight-adjusted Intravenous Reslizumab in Severe Asthma with Inadequate Response to Fixed-Dose Subcutaneous Mepolizumab. Am. J. Respir. Crit. Care Med. 2018, 197, 38–46. [Google Scholar] [CrossRef]
- Vultaggio, A.; Nencini, F.; Bormioli, S.; Vivarelli, E.; Dies, L.; Rossi, O.; Parronchi, P.; Maggi, E.; Matucci, A. Low-Dose Mepolizumab Effectiveness in Patients Suffering from Eosinophilic Granulomatosis with Polyangiitis. Allergy Asthma Immunol. Res. 2020, 12, 885–893. [Google Scholar] [CrossRef]
- Kroes, J.A.; Zielhuis, S.W.; Van Roon, E.N.; Brinke, A. Prediction of response to biological treatment with monoclonal antibodies in severe asthma. Biochem. Pharmacol. 2020, 179, 113978. [Google Scholar] [CrossRef]
- Matucci, A.; Vultaggio, A.; Danesi, R. The use of intravenous versus subcutaneous monoclonal antibodies in the treatment of severe asthma: A review. Respir. Res. 2018, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Braunstahl, G.-J.; Overbeek, S.E.; Kleinjan, A.; Prins, J.-B.; Hoogsteden, H.C.; Fokkens, W.J. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J. Allergy Clin. Immunol. 2001, 107, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Braunstahl, G.-J.; Kleinjan, A.; Overbeek, S.E.; Prins, J.-B.; Hoogsteden, H.C.; Fokkens, W.J. Segmental Bronchial Provocation Induces Nasal Inflammation in Allergic Rhinitis Patients. Am. J. Respir. Crit. Care Med. 2000, 161, 2051–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Asthma | CRSwNP | Biomarkers | ||||||
|---|---|---|---|---|---|---|---|---|
| FEV1 | Symp | Exac | OCS Sparing | Symp | Bl Eos | FeNO | IgE | |
| Anti IgE | + | + | + | NA | + | ↓ | ↓↓ | ↓* |
| Anti IL-5 | + | ++ | ++ | + | + | ↓↓ | ↔ | ↔ |
| Anti IL-5Rα | + | ++ | ++ | ++ | NA | ↓↓ | ↔ | ↔ |
| Anti IL-4/IL-13 | ++ | ++ | ++ | ++ | ++ | ↑/→ | ↓↓ | ↓↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matucci, A.; Bormioli, S.; Nencini, F.; Chiccoli, F.; Vivarelli, E.; Maggi, E.; Vultaggio, A. Asthma and Chronic Rhinosinusitis: How Similar Are They in Pathogenesis and Treatment Responses? Int. J. Mol. Sci. 2021, 22, 3340. https://doi.org/10.3390/ijms22073340
Matucci A, Bormioli S, Nencini F, Chiccoli F, Vivarelli E, Maggi E, Vultaggio A. Asthma and Chronic Rhinosinusitis: How Similar Are They in Pathogenesis and Treatment Responses? International Journal of Molecular Sciences. 2021; 22(7):3340. https://doi.org/10.3390/ijms22073340
Chicago/Turabian StyleMatucci, Andrea, Susanna Bormioli, Francesca Nencini, Fabio Chiccoli, Emanuele Vivarelli, Enrico Maggi, and Alessandra Vultaggio. 2021. "Asthma and Chronic Rhinosinusitis: How Similar Are They in Pathogenesis and Treatment Responses?" International Journal of Molecular Sciences 22, no. 7: 3340. https://doi.org/10.3390/ijms22073340






