Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1
Abstract
:1. Introduction
2. TRPA1 and TRPV1: Key Chemosensory Ion Channels in Chemesthesis
2.1. TRPA1
2.1.1. TRPA1 Structure, Expression and Function
2.1.2. Plant-Derived TRPA1 Agonists
2.1.3. TRPA1 Activators in Korean Indigenous Plants
2.2. TRPV1
2.2.1. TRPV1 Structure, Expression and Function
2.2.2. TRPV1 and Primary Taste Qualities
3. Potential Role of TRPA1 and TRPV1 as a Bridge between Taste and Chemesthesis
3.1. Cross Talk between TRPA1 and TRPV1
3.2. TRPA1 and TRPV1 as a Bridge between Taste and Chemesthesis
3.3. Chemesthesis and Sour Taste as Co-Mediators of Acid-Evoked Aversion
3.4. Chemesthesis and Bitter Taste as Co-Mediators of Bitter-Evoked Aversion
3.5. Chemesthesis and Salt Taste
3.6. Effect of Single-Nucleotide Polymorphisms in TRPA1 and TRPV1 on Chemesthesis and Taste
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AITC | Allyl isothiocyanate |
| CALHM | Calcium homeostasis modulator |
| CGRP | Calcitonin generelated peptide |
| ENaC | Epithelial Na+ channel |
| GNAT3 | Guanine nucleotide-binding protein G(t) subunit alpha-3 |
| HEK | Human embryonic kidney |
| IP3 | Inositol triphosphate receptor type 3 |
| KCNJ2 | Potassium inwardly rectifying channel subfamily J member 2 |
| nAChR | Nicotinic acetylcholine receptor |
| PKD2L1 | Polycystin 2 like 1 |
| PLC | Phospholipase C |
| SKN-1a | Transcription factor skinhead-1a |
| T1R | Taste receptor type 1 |
| T2R | Taste receptor type 2 |
| TRP | Transient receptor potential |
| TRPA1 | Transient receptor potential ankyrin member 1 |
| TRPM5 | Transient receptor potential melastatin member 5 |
| TRPV1 | Transient receptor potential vanilloid member 1 |
References
- Sherman, P.W.; Billing, J. Darwinian Gastronomy: Why We Use Spices: Spices taste good because they are good for us. BioScience 1999, 49, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Gottardi, D.; Bukvicki, D.; Prasad, S.; Tyagi, A.K. Beneficial Effects of Spices in Food Preservation and Safety. Front. Microbiol. 2016, 7, 1394. [Google Scholar] [CrossRef] [Green Version]
- Kaefer, C.M.; Milner, J.A. The role of herbs and spices in cancer prevention. J. Nutr. Biochem. 2008, 19, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Green, B.G. Chemesthesis and the Chemical Senses as Components of a “Chemofensor Complex”. Chem. Senses 2011, 37, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Appendino, G. Spices: The Savory and Beneficial Science of Pungency. Rev. Physiol. Biochem. Pharmacol. 2013, 164, 1–76. [Google Scholar] [PubMed]
- Roper, S.D. TRPs in Taste and Chemesthesis. Snake Venoms 2014, 223, 827–871. [Google Scholar]
- Smutzer, G.; Devassy, R.K. Integrating TRPV1 Receptor Function with Capsaicin Psychophysics. Adv. Pharmacol. Sci. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Aroke, E.N.; Powell-Roach, K.L.; Jaime-Lara, R.B.; Tesfaye, M.; Roy, A.; Jackson, P.; Joseph, P.V. Taste the Pain: The Role of TRP Channels in Pain and Taste Perception. Int. J. Mol. Sci. 2020, 21, 5929. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.M.; McAlexander, M.A.; Bíró, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Szallasi, A. Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef]
- Sexton, J.E.; Vernon, J.; Wood, J.N. TRPs and Pain. Snake Venoms 2014, 223, 873–897. [Google Scholar]
- Shimizu, S.; Takahashi, N.; Mori, Y. TRPs as Chemosensors (ROS, RNS, RCS, Gasotransmitters). Snake Venoms 2014, 223, 767–794. [Google Scholar]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Araki, M.; Kanda, N.; Iwata, H.; Sagae, Y.; Masuda, K.; Okuno, Y. Identification of a new class of non-electrophilic TRPA1 agonists by a structure-based virtual screening approach. Bioorganic Med. Chem. Lett. 2020, 30, 127142. [Google Scholar] [CrossRef]
- Chen, J.; Kang, D.; Xu, J.; Lake, M.; Hogan, J.O.; Sun, C.; Walter, K.; Yao, B.; Kim, D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 2013, 4, 2501. [Google Scholar] [CrossRef] [PubMed]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A Gain-of-Function Mutation in TRPA1 Causes Familial Episodic Pain Syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moparthi, L.; Kichko, T.I.; Eberhardt, M.; Högestätt, E.D.; Kjellbom, P.; Johanson, U.; Reeh, P.W.; Leffler, A.; Filipovic, M.R.; Zygmunt, P.M. Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci. Rep. 2016, 6, 28763. [Google Scholar] [CrossRef]
- Sinica, V.; Zimova, L.; Barvikova, K.; Macikova, L.; Barvik, I.; Vlachova, V. Human and Mouse TRPA1 Are Heat and Cold Sensors Differentially Tuned by Voltage. Cells 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Moparthi, L.; Survery, S.; Kreir, M.; Simonsen, C.; Kjellbom, P.; Högestätt, E.D.; Johanson, U.; Zygmunt, P.M. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc. Natl. Acad. Sci. USA 2014, 111, 16901–16906. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nat. Cell Biol. 1997, 389, 816–824. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nat. Cell Biol. 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel that Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Vogt-Eisele, A.K.; Weber, K.; A Sherkheli, M.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Elias, A.; Mrkonjić, S.; Jung, C.; Pardo-Pastor, C.; Vicente, R.; Valverde, M.A. The TRPV4 channel. Handb. Exp. Pharmacol. 2014, 222, 293–319. [Google Scholar]
- Terada, Y.; Narukawa, M.; Watanabe, T. Specific Hydroxy Fatty Acids in Royal Jelly Activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Shintaku, K.; Uchida, K.; Suzuki, Y.; Zhou, Y.; Fushiki, T.; Watanabe, T.; Yazawa, S.; Tominaga, M. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br. J. Pharmacol. 2011, 165, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Iida, T.; Moriyama, T.; Kobata, K.; Morita, A.; Murayama, N.; Hashizume, S.; Fushiki, T.; Yazawa, S.; Watanabe, T.; Tominaga, M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 2003, 44, 958–967. [Google Scholar] [CrossRef]
- Kim, M.J.; Son, H.J.; Kim, Y.; Kweon, H.-J.; Suh, B.-C.; Lyall, V.; Rhyu, M.-R. Selective Activation of hTRPV1 by N-Geranyl Cyclopropylcarboxamide, an Amiloride-Insensitive Salt Taste Enhancer. PLoS ONE 2014, 9, e89062. [Google Scholar] [CrossRef]
- Dewis, M.L.; Phan, T.-H.T.; Ren, Z.; Meng, X.; Cui, M.; Mummalaneni, S.; Rhyu, M.-R.; DeSimone, J.A.; Lyall, V. N-geranyl cyclopropyl-carboximide modulates salty and umami taste in humans and animal models. J. Neurophysiol. 2013, 109, 1078–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhold, K.A.; Bautista, D.M. Molecular and Cellular Mechanisms of Trigeminal Chemosensation. Ann. N. Y. Acad. Sci. 2009, 1170, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Lázaro, S.L.; Simon, S.A.; Rosenbaum, T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J. Physiol. 2013, 591, 3109–3121. [Google Scholar] [CrossRef] [Green Version]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacPherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The Pungency of Garlic: Activation of TRPA1 and TRPV1 in Response to Allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Hinman, A.; Chuang, H.-H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nat. Cell Biol. 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Wang, Z.; Zubcevic, L.; Hsu, A.L.; He, Q.; Borgnia, M.J.; Ji, R.-R.; Lee, S.-Y. Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 2020, 105, 882–894.e5. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.J.; Filipovic, M.R.; Leffler, A.; de la Roche, J.; Kistner, K.; Fischer, M.J.; Fleming, T.; Zimmermann, K.; Ivanovic-Burmazovic, I.; Nawroth, P.P.; et al. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): A possible mechanism of metabolic neuropathies. J. Biol. Chem. 2012, 287, 28291–28306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, Y.; Blair, N.T. Benzoquinone Reveals a Cysteine-Dependent Desensitization Mechanism of TRPA1. Mol. Pharmacol. 2013, 83, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- Moparthi, L.; Kjellström, S.; Kjellbom, P.; Filipovic, M.R.; Zygmunt, P.M.; Johanson, U. Electrophile-Induced Conformational Switch of the Human TRPA1 Ion Channel Detected by Mass Spectrometry. Int. J. Mol. Sci. 2020, 21, 6667. [Google Scholar] [CrossRef]
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortés, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the hTRPA1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Delling, M.; Jun, J.C.; E Clapham, D. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Mukaiyama, M.; Usui, T.; Nagumo, Y. Non-electrophilic TRPA1 agonists, menthol, carvacrol and clotrimazole, open epithelial tight junctions via TRPA1 activation. J. Biochem. 2020, 168, 407–415. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient Receptor Potential Ankyrin 1 Channel Localized to Non-Neuronal Airway Cells Promotes Non-Neurogenic Inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef] [Green Version]
- Purhonen, A.; Louhivuori, L.; Kiehne, K.; Åkerman, K.; Herzig, K. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2007, 582, 229–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Dubin, A.E.; Bursulaya, B.; Viswanath, V.; Jegla, T.J.; Patapoutian, A. Identification of Transmembrane Domain 5 as a Critical Molecular Determinant of Menthol Sensitivity in Mammalian TRPA1 Channels. J. Neurosci. 2008, 28, 9640–9651. [Google Scholar] [CrossRef] [Green Version]
- Ohara, K.; Fukuda, T.; Okada, H.; Kitao, S.; Ishida, Y.; Kato, K.; Takahashi, C.; Katayama, M.; Uchida, K.; Tominaga, M. Identification of Significant Amino Acids in Multiple Transmembrane Domains of Human Transient Receptor Potential Ankyrin 1 (TRPA1) for Activation by Eudesmol, an Oxygenized Sesquiterpene in Hop Essential Oil. J. Biol. Chem. 2015, 290, 3161–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassoli, A.; Borgonovo, G.; Caimi, S.; Scaglioni, L.; Morini, G.; Moriello, A.S.; Di Marzo, V.; De Petrocellis, L. Taste-guided identification of high potency TRPA1 agonists from Perilla frutescens. Bioorganic Med. Chem. 2009, 17, 1636–1639. [Google Scholar] [CrossRef]
- Zhong, J.; Pollastro, F.; Prenen, J.; Zhu, Z.; Appendino, G.; Nilius, B. Ligustilide: A novel TRPA1 modulator. Pflüger’s Arch. 2011, 462, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Kim, Y.; Misaka, T.; Noh, B.S.; Rhyu, M.-R. Activation of the Chemosensory Ion Channels TRPA1 and TRPV1 by Hydroalcohol Extract of Kalopanax pictus Leaves. Biomol. Ther. 2012, 20, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.; Kim, M.J.; Son, H.J.; Kweon, H.-J.; Kim, J.T.; Kim, Y.; Shim, J.; Suh, B.-C.; Rhyu, M.-R. Five hTRPA1 Agonists Found in Indigenous Korean Mint, Agastache rugosa. PLoS ONE 2015, 10, e0127060. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, M.J.; Park, J.-H.; Ishii, S.; Misaka, T.; Rhyu, M.-R. Methyl syringate, a low-molecular-weight phenolic ester, as an activator of the chemosensory ion channel TRPA1. Arch. Pharmacol Res. 2012, 35, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, R.J.; McLatchie, L.M.; Clarke, M.; Winter, J.; Bevan, S.; McIntyre, P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 1998, 250, 177–180. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nat. Cell Biol. 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Gavva, N.R.; Klionsky, L.; Qu, Y.; Shi, L.; Tamir, R.; Edenson, S.; Zhang, T.J.; Viswanadhan, V.N.; Toth, A.; Pearce, L.V.; et al. Molecular Determinants of Vanilloid Sensitivity in TRPV1. J. Biol. Chem. 2004, 279, 20283–20295. [Google Scholar] [CrossRef] [Green Version]
- Jordt, S.E.; Julius, D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Dray, A.; Forbes, C.; Burgess, G. Ruthenium red blocks the capsaicin-induced increase in intracellular calcium and activation of membrane currents in sensory neurones as well as the activation of peripheral nociceptors in vitro. Neurosci. Lett. 1990, 110, 52–59. [Google Scholar] [CrossRef]
- Docherty, R.J.; Yeats, J.C.; Piper, A.S. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 1997, 121, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Gunthorpe, M.; Rami, H.; Jerman, J.; Smart, D.; Gill, C.; Soffin, E.; Hannan, S.L.; Lappin, S.; Egerton, J.; Smith, G.; et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 2004, 46, 133–149. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Q.; Chen, C.; Yang, X.; Xie, R.; Xu, J. The Role of Transient Receptor Potential Vanilloid 1 in Common Diseases of the Digestive Tract and the Cardiovascular and Respiratory System. Front. Physiol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.; Stevens, D.A. Effects of oral chemical irritation on taste. Physiol. Behav. 1984, 32, 995–998. [Google Scholar] [CrossRef]
- Stevens, D.A.; Lawless, H.T. Putting out the fire: Effects of tastants on oral chemical irritation. Percept. Psychophys. 1986, 39, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Kapaun, C.L.; Dando, R. Deconvoluting physical and chemical heat: Temperature and spiciness influence flavor differently. Physiol. Behav. 2017, 170, 54–61. [Google Scholar] [CrossRef]
- Cowart, B.J. Oral chemical irritation: Does it reduce perceived taste intensity? Chem. Senses 1987, 12, 467–479. [Google Scholar] [CrossRef]
- Omelian, J.M.; Samson, K.K.; Sollars, S.I. Chronic Oral Capsaicin Exposure During Development Leads to Adult Rats with Reduced Taste Bud Volumes. Chemosens. Percept. 2016, 9, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omelian, J.M.; Berry, M.J.; Gomez, A.M.; Apa, K.L.; Sollars, S.I. Developmental time course of peripheral cross-modal sensory interaction of the trigeminal and gustatory systems. Dev. Neurobiol. 2015, 76, 626–641. [Google Scholar] [CrossRef] [Green Version]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef] [Green Version]
- Akopian, A.N. Regulation of Nociceptive Transmission at the Periphery via TRPA1-TRPV1 Interactions. Curr. Pharm. Biotechnol. 2011, 12, 89–94. [Google Scholar] [CrossRef]
- Masuoka, T.; Kudo, M.; Yamashita, Y.; Yoshida, J.; Imaizumi, N.; Muramatsu, I.; Nishio, M.; Ishibashi, T. TRPA1 Channels Modify TRPV1-Mediated Current Responses in Dorsal Root Ganglion Neurons. Front. Physiol. 2017, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birder, L.; Nakamura, Y.; Kiss, S.; Nealen, M.; Barrick, S.; Kanai, A.; Wang, E.; Ruiz, G.; De Groat, W.; Apodaca, G.; et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002, 5, 856–860. [Google Scholar] [CrossRef]
- Koizumi, S.; Fujishita, K.; Inoue, K.; Shigemoto-Mogami, Y.; Tsuda, M.; Inoue, K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: The involvement of extracellular ATP and P2Y2 receptor activation. Biochem. J. 2004, 380, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Caterina, M.J. TRPV channels as thermosensory receptors in epithelial cells. Pflügers Arch. 2005, 451, 160–167. [Google Scholar] [CrossRef]
- Lumpkin, E.A.; Caterina, M.J. Mechanisms of sensory transduction in the skin. Nat. Cell Biol. 2007, 445, 858–865. [Google Scholar] [CrossRef]
- Teng, B.; Wilson, C.E.; Tu, Y.-H.; Joshi, N.R.; Kinnamon, S.C.; Liman, E.R. Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1. Curr. Biol. 2019, 29, 3647–3656.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jin, H.; Zhang, W.; Ding, C.; O’Keeffe, S.; Ye, M.; Zuker, C.S. Sour Sensing from the Tongue to the Brain. Cell 2019, 179, 392–402.e15. [Google Scholar] [CrossRef]
- Taruno, A.; Nomura, K.; Kusakizako, T.; Ma, Z.; Nureki, O.; Foskett, J.K. Taste transduction and channel synapses in taste buds. Pflügers Arch. 2021, 473, 3–13. [Google Scholar] [CrossRef]
- Ohmoto, M.; Jyotaki, M.; Foskett, J.K.; Matsumoto, I. Sodium–Taste Cells Require Skn-1a for Generation and Share Molecular Features with Sweet, Umami, and Bitter Taste Cells. Eneuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Nakanishi, M.; Ishidate, F.; Iwata, K.; Taruno, A. All-Electrical Ca2+-Independent Signal Transduction Mediates Attractive Sodium Taste in Taste Buds. Neuron 2020, 106, 816–829.e6. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.Y.; Wu, S.Y. Calcitonin Gene-Related Peptide Reduces Taste-Evoked ATP Secretion from Mouse Taste Buds. J. Neurosci. 2015, 35, 12714–12724. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.J.; Bandell, M.; Patapoutian, A. TRPV1 Is Activated by both Acidic and Basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Maia, A.J.; Stapleton-Kotloski, J.R.; Lyall, V.; Phan, T.H.; Mummalaneni, S.; Melone, P.; Desimone, J.A.; Nicolelis, M.A.; Simon, S.A. Nicotine activates TRPM5-dependent and independent taste pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 1596–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blednov, Y.; Harris, R. Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacol. 2009, 56, 814–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Mummalaneni, S.; Grider, J.R.; Damaj, M.I.; Lyall, V. Nicotinic acetylcholine receptors (nAChRs) are expressed in Trpm5 positive taste receptor cells (TRCs). PLoS ONE 2018, 13, e0190465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Mummalaneni, S.; Larsen, J.; Grider, J.R.; Spielman, A.I.; Özdener, M.H.; Lyall, V. Nicotinic acetylcholine receptor (CHRN) expression and function in cultured human adult fungiform (HBO) taste cells. PLoS ONE 2018, 13, e0194089. [Google Scholar]
- Khan, A.M.; Narayanan, V.S.; Puttabuddi, J.H.; Chengappa, R.; Ambaldhage, V.K.; Naik, P.; Raheel, S.A. Comparison of Taste Threshold in Smokers and Non-Smokers Using Electrogustometry and Fungiform Papillae Count: A Case Control Study. J. Clin. Diagn. Res. 2016, 10, ZC101–ZC105. [Google Scholar] [CrossRef]
- Fan, L.; Balakrishna, S.; Jabba, S.V.; Bonner, P.E.; Taylor, S.R.; Picciotto, M.R.; Jordt, S.-E. Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tob. Control 2016, 25, ii50–ii54. [Google Scholar] [CrossRef]
- Kozlitina, J.; Risso, D.; Lansu, K.; Olsen, R.H.J.; Sainz, E.; Luiselli, D.; Barik, A.; Frigerio-Domingues, C.; Pagani, L.; Wooding, S.; et al. An African-specific haplotype in MRGPRX4 is associated with menthol cigarette smoking. PLoS Genet. 2019, 15, e1007916. [Google Scholar] [CrossRef]
- Uhl, G.R.; Walther, D.; Behm, F.M.; Rose, J.E. Menthol Preference among Smokers: Association with TRPA1 Variants. Nicotine Tob. Res. 2011, 13, 1311–1315. [Google Scholar] [CrossRef]
- Hansen, E.Ø.; Arendt-Nielsen, L.; Boudreau, S.A. A Comparison of Oral Sensory Effects of Three TRPA1 Agonists in Young Adult Smokers and Non-smokers. Front. Physiol. 2017, 8, 663. [Google Scholar] [CrossRef] [Green Version]
- Willis, D.N.; Liu, B.; Ha, M.A.; Jordt, S.; Morris, J.B. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011, 25, 4434–4444. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, M.; Imura, K.; Sato, I. Topographical organization of TRPV1-immunoreactive epithelium and CGRP-immunoreactive nerve terminals in rodent tongue. Eur. J. Histochem. 2012, 56, e21. [Google Scholar] [CrossRef] [PubMed]
- Lyall, V.; Heck, G.L.; Vinnikova, A.K.; Ghosh, S.; Phan, T.-H.T.; Alam, R.I.; Russell, O.F.; Malik, S.A.; Bigbee, J.W.; DeSimone, J.A. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J. Physiol. 2004, 558, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, B.C.; Sukumaran, S.K.; Margolskee, R.F.; Bachmanov, A.A. Amiloride-Insensitive Salt Taste Is Mediated by Two Populations of Type III Taste Cells with Distinct Transduction Mechanisms. J. Neurosci. 2016, 36, 1942–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roebber, J.K.; Roper, S.D.; Chaudhari, N. The Role of the Anion in Salt (NaCl) Detection by Mouse Taste Buds. J. Neurosci. 2019, 39, 6224–6232. [Google Scholar] [CrossRef] [Green Version]
- Rhyu, M.-R.; Lyall, V. Interaction of taste-active nutrients with taste receptors. Curr. Opin. Physiol. 2021, 20, 64–69. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Wei, X.; Liang, Y.; Cui, Y.; Gao, F.; Zhong, J.; Pu, Y.; Zhao, Y.; Yan, Z.; et al. Transient Receptor Potential Vanilloid 1 Activation by Dietary Capsaicin Promotes Urinary Sodium Excretion by Inhibiting Epithelial Sodium Channel α Subunit–Mediated Sodium Reabsorption. Hypertension 2014, 64, 397–404. [Google Scholar] [CrossRef]
- Hao, X.; Chen, J.; Luo, Z.; He, H.; Yu, H.; Ma, L.; Ma, S.; Zhu, T.; Liu, D.; Zhu, Z. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice. Pflüger’s Arch. 2011, 461, 345–353. [Google Scholar] [CrossRef]
- Hochheimer, A.; Krohn, M.; Rudert, K.; Riedel, K.; Becker, S.; Thirion, C.; Zinke, H. Endogenous Gustatory Responses and Gene Expression Profile of Stably Proliferating Human Taste Cells Isolated from Fungiform Papillae. Chem. Senses 2014, 39, 359–377. [Google Scholar] [CrossRef] [Green Version]
- Lyall, V.; Mummalaneni, S.; Mahavadi, S. MHO: Effect of High salt (HS) and Capsaicin (CAP) on ENaC and TRPV1 Expression in Cultured Adult Human Fungiform (HBO) Taste Cells. In Proceedings of the Meeting of the International Symposium of Olfaction and Taste, Portland, OR, USA, 3–7 August 2020; p. 231. [Google Scholar]
- Oka, Y.; Butnaru, M.; von Buchholtz, L.; Ryba, N.J.; Zuker, C.S. High salt recruits aversive taste pathways. Nature 2013, 494, 472–475. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, N.; Okumura, M.; Tadokoro, O.; Sogawa, N.; Tomida, M.; Kondo, E. Effect of single-nucleotide polymorphisms in TRPV1 on burning pain and capsaicin sensitivity in Japanese adults. Mol. Pain 2018, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Tian, W.; Fu, Y.; Oyama, T.T.; Anderson, S.; Cohen, D.M. Functional effects of nonsynonymous polymorphisms in the human TRPV1 gene. Am. J. Physiol. Physiol. 2007, 293, F1865–F1876. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.G.; Rousseau, D.; Duizer, L.; Cockburn, M.; Chiu, W.; Nielsen, D.; El-Sohemy, A. Genetic Variation in Putative Salt Taste Receptors and Salt Taste Perception in Humans. Chem. Senses 2012, 38, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Lee, J.; Kim, M.A.; Back, S.K.; Kil Hong, S.; Na, H.S. Induction of total insensitivity to capsaicin and hypersensitivity to garlic extract in human by decreased expression of TRPV1. Neurosci. Lett. 2007, 411, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Neubert, J.K.; Miguel, A.S.; Xu, K.; Krishnaraju, R.K.; Iadarola, M.J.; Goldman, D.; A Dionne, R. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 2004, 109, 488–496. [Google Scholar] [CrossRef]
- Boukalova, S.; Touska, F.; Marsakova, L.; Hynkova, A.; Sura, L.; Chvojka, S.; Dittert, I.; Vlachova, V. Gain-of-function mutations in the transient receptor potential channels TRPV1 and TRPA1: How painful? Physiol. Res. 2014, 63, S205–S213. [Google Scholar] [CrossRef]
- Riello, M.; Cecchini, M.P.; Zanini, A.; Di Chiappari, M.; Tinazzi, M.; Fiorio, M. Perception of phasic pain is modulated by smell and taste. Eur. J. Pain 2019, 23, 1790–1800. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.L.; McGeary, J.E.; Hayes, J.E. Polymorphisms inTRPV1andTAS2RsAssociate with Sensations from Sampled Ethanol. Alcohol. Clin. Exp. Res. 2014, 38, 2550–2560. [Google Scholar] [CrossRef] [Green Version]
- Schütz, M.; Oertel, B.G.; Heimann, D.; Doehring, A.; Walter, C.; Dimova, V.; Geisslinger, G.; Lötsch, J. Consequences of a Human TRPA1 Genetic Variant on the Perception of Nociceptive and Olfactory Stimuli. PLoS ONE 2014, 9, e95592. [Google Scholar]
- Naert, R.; Talavera, A.; Startek, J.B.; Talavera, K. TRPA1 gene variants hurting our feelings. Pflügers Arch. 2020, 472, 953–960. [Google Scholar] [CrossRef]
- Knaapila, A.; Hwang, L.-D.; Lysenko, A.; Duke, F.F.; Fesi, B.; Khoshnevisan, A.; James, R.S.; Wysocki, C.J.; Rhyu, M.; Tordoff, M.G.; et al. Genetic Analysis of Chemosensory Traits in Human Twins. Chem. Senses 2012, 37, 869–881. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.-W.; Davis, J.C.; Ding, S.-Y.; Nai, Q.; Zhou, F.-M.; Ennis, M. Expression of transient receptor potential (TRP) channel mRNAs in the mouse olfactory bulb. Neurosci. Lett. 2012, 524, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamoun, E.; Carroll, N.A.; Duizer, L.M.; Qi, W.; Feng, Z.; Darlington, G.; Duncan, A.M.; Haines, J.; Ma, D.W.; The Guelph Family Health Study. The Relationship between Single Nucleotide Polymorphisms in Taste Receptor Genes, Taste Function and Dietary Intake in Preschool-Aged Children and Adults in the Guelph Family Health Study. Nutrients 2018, 10, 990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, D.; Baastrup, J.; Nientit, M.R.; Binder, A.; Schünke, M.; Baron, R.; Cascorbi, I. Differential Expression and Functionality of TRPA1 Protein Genetic Variants in Conditions of Thermal Stimulation. J. Biol. Chem. 2012, 287, 27087–27094. [Google Scholar] [CrossRef] [PubMed] [Green Version]


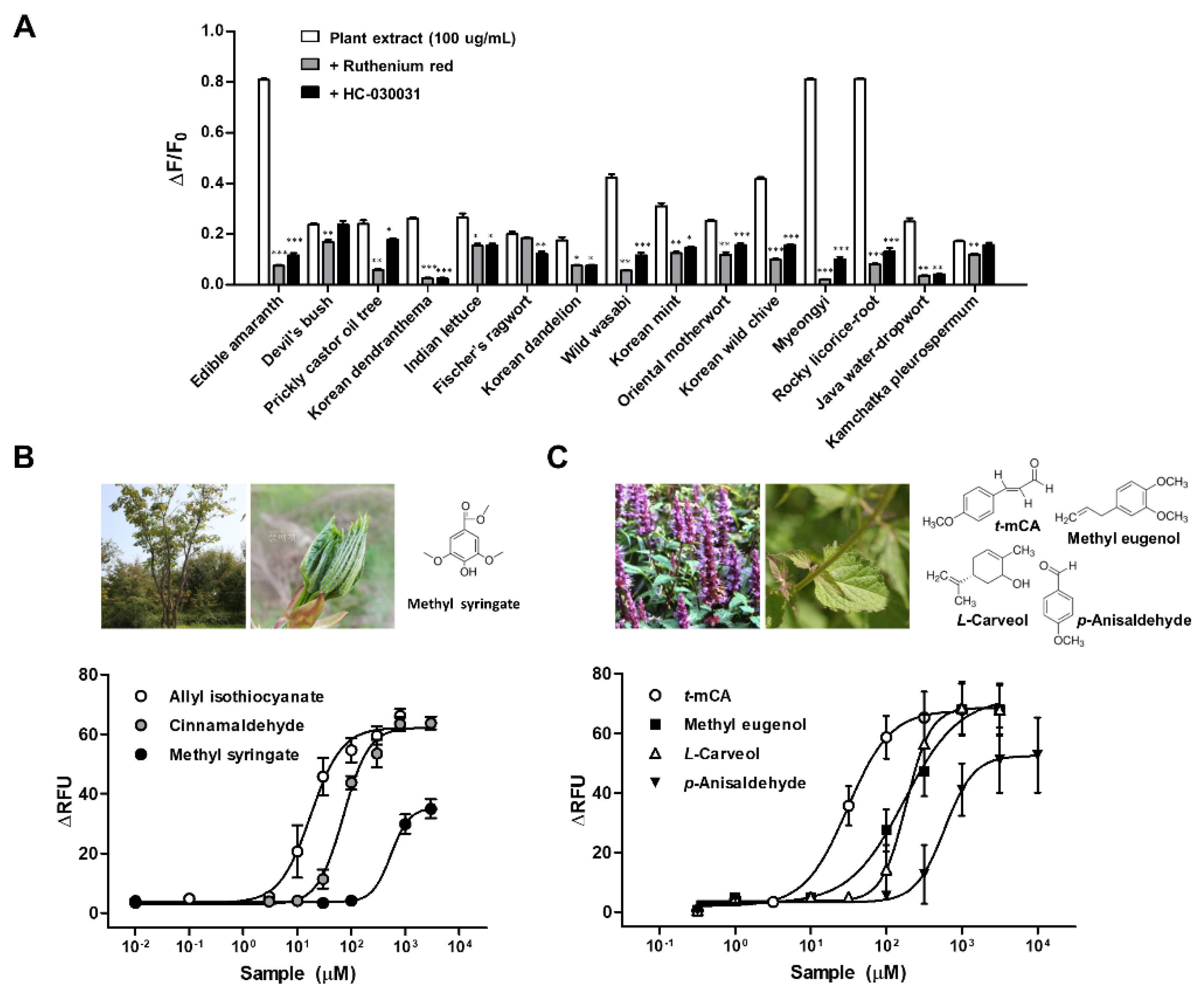

| English Name | Local Name | Used Part | Taxon | Scientific Name |
|---|---|---|---|---|
| Edible amaranth | Bireum | Leaf | Amaranthaceae | Amaranthus mangostanus L. |
| Devil’s bush | Gasiogalpi | Bark | Araliaceae | Eleutherococcus sessiliflorus (Rupr. & Maxim.) Maxim. |
| Prickly castor oil tree | Gaeduleub | Sprout | Araliaceae | Kalopanax septemlobus (Thunb.) Koidz. |
| Korean dendranthema | Sangujeolcho | Whole plant | Compositae | Dendranthema zawadskii (Herb) Tzvelev |
| Indian lettuce | Wanggodeulppaegi | Leaf | Compositae | Lactuca indica L. |
| Fischer’s ragwort | Gomchwi | Leaf | Compositae | Ligularia fischeri (Ledeb.) Turcz. |
| Korean dandelion | Mindeulle | Stem & leaf | Compositae | Taraxacum platycarpum Dahlst. |
| Wild wasabi | Gochunaengi | Leaf | Cruciferae | Eutrema japonicum (Miq.) Koidz |
| Korean mint | Baechohyang | Stem & leaf | Labiatae | Agastache rugosa (Fisch. & Mey.) Kuntze |
| Oriental motherwort | Igmocho | Stem & leaf | Labiatae | Leonurus japonicus Houtt. |
| Korean wild chive | Dallae | Whole plant | Liliaceae | Allium monanthum Maxim. |
| Myeongyi | Ulleungsanmaneul | Leaf | Liliaceae | Allium ochotense Prokh. |
| Rocky licorice-root | Gaehoehyang | Whole plant | Umbelliferae | Ligusticum tachiroei (Franch. & Sav.) M.Hiroe & Constance |
| Java water-dropwort | Dolminari | Stem & leaf | Umbelliferae | Oenanthe javanica (Blume) DC. |
| Kamchatka pleurospermum | Waeusanpul | Stem & leaf | Umbelliferae | Pleurospermum camtschaticum Hoffm. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhyu, M.-R.; Kim, Y.; Lyall, V. Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1. Int. J. Mol. Sci. 2021, 22, 3360. https://doi.org/10.3390/ijms22073360
Rhyu M-R, Kim Y, Lyall V. Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1. International Journal of Molecular Sciences. 2021; 22(7):3360. https://doi.org/10.3390/ijms22073360
Chicago/Turabian StyleRhyu, Mee-Ra, Yiseul Kim, and Vijay Lyall. 2021. "Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1" International Journal of Molecular Sciences 22, no. 7: 3360. https://doi.org/10.3390/ijms22073360
APA StyleRhyu, M.-R., Kim, Y., & Lyall, V. (2021). Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1. International Journal of Molecular Sciences, 22(7), 3360. https://doi.org/10.3390/ijms22073360





