Helicobacter pylori Infection Acts Synergistically with a High-Fat Diet in the Development of a Proinflammatory and Potentially Proatherogenic Endothelial Cell Environment in an Experimental Model
Abstract
:1. Introduction
2. Results
2.1. Validation of H. pylori Infection in an Experimental In Vivo Model of Atherosclerosis
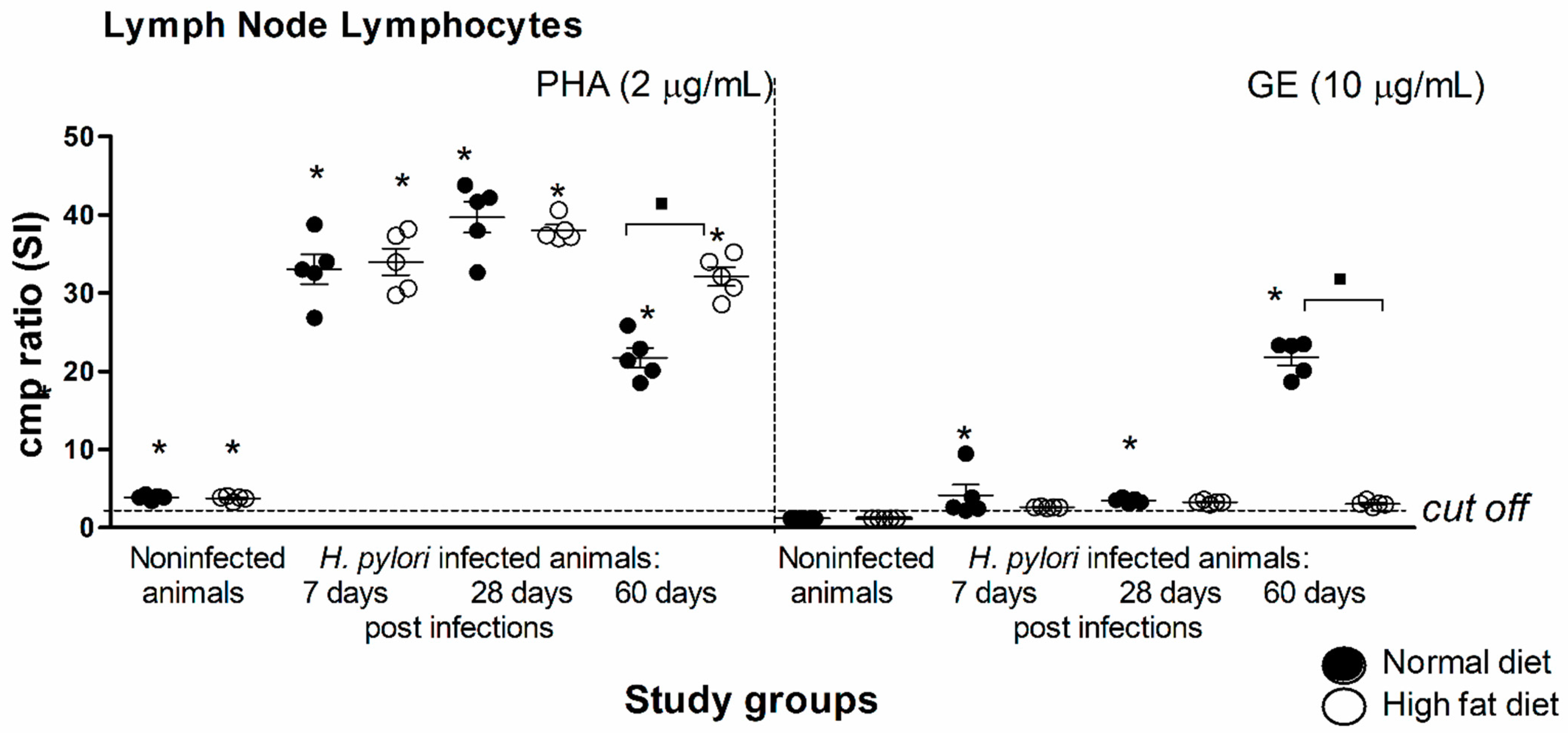
2.2. Inflammatory Process in Experimental Atherosclerosis Developed in Caviae porcellus Colonized with H. pylori
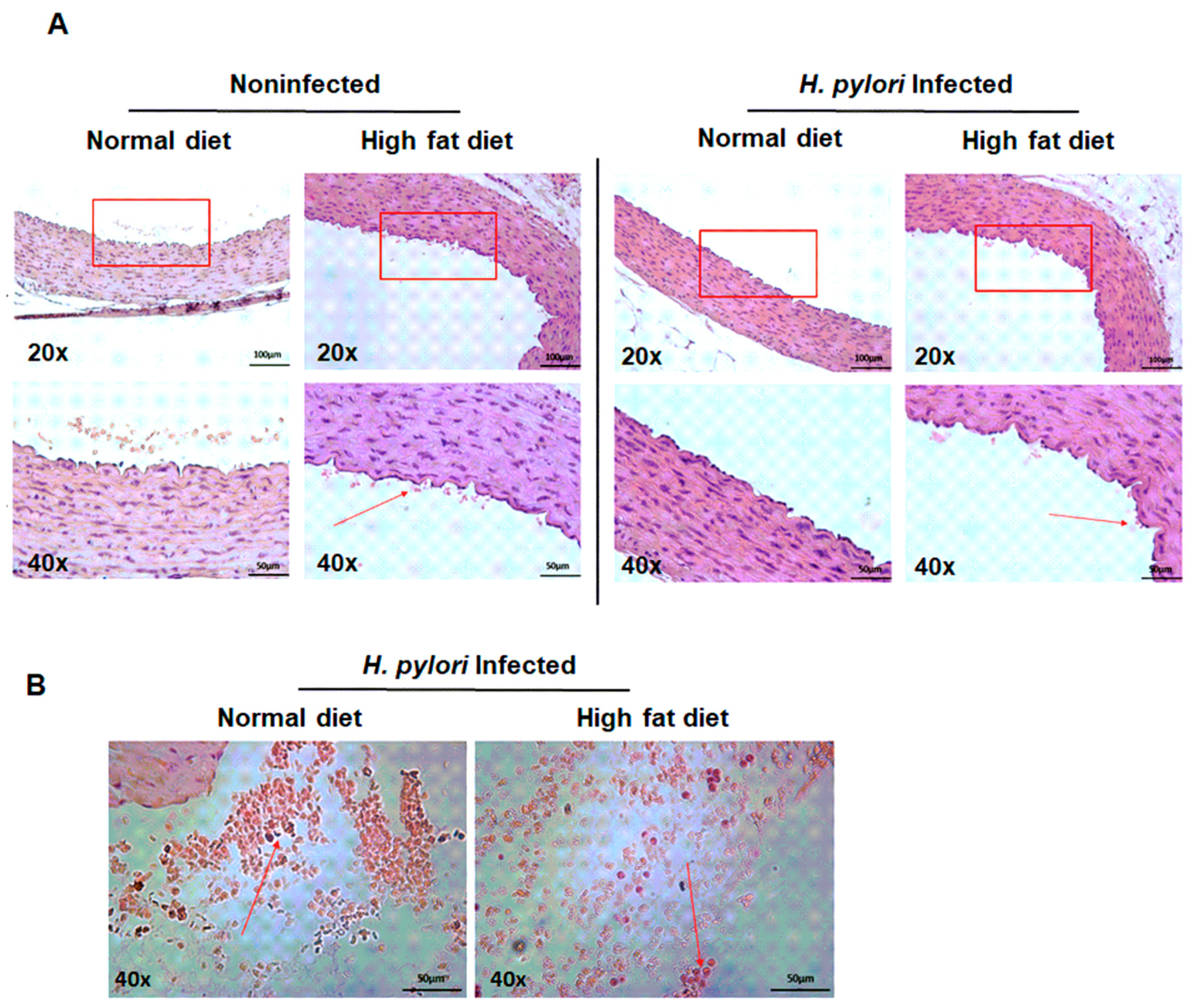
2.3. Diminished Vascular Elasticity in Caviae porcellus Colonized with H. pylori in Conjunction with a High-Fat Diet
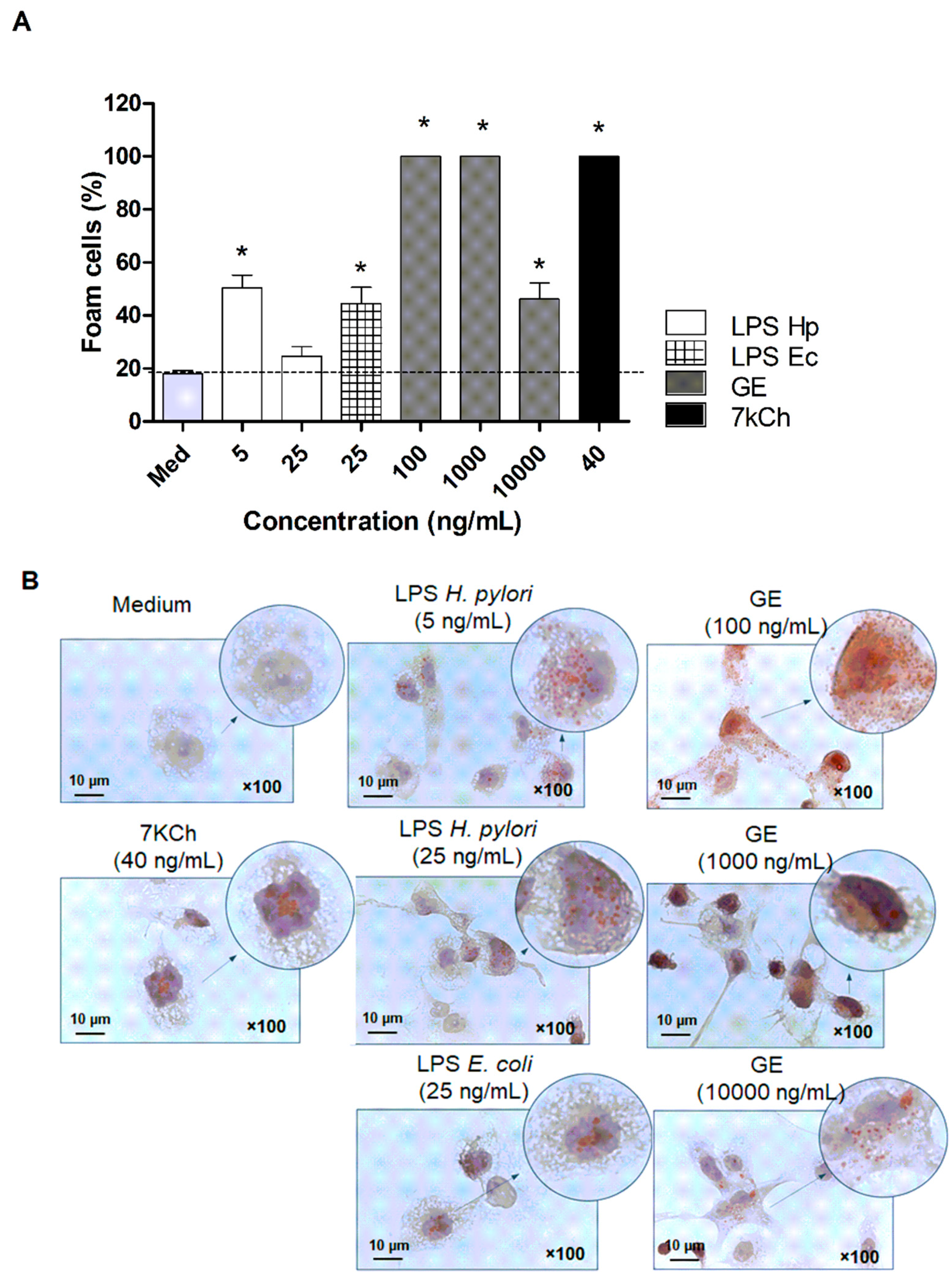
2.4. Established Role of H. pylori Components in Transformation of Macrophages into Foam Cells
2.5. In Vitro Model of Vascular Endothelial Response Driven by H. pylori Components
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. H. pylori Infection in Caviae porcellus (Guinea Pigs)
4.3. ELISA Assays: MMP-9, CRP
4.4. Determination of Oxidative Stress (MPO)
4.5. Cell Proliferation
4.6. Measurement of Arterial Stiffness
4.7. Cell Culture
4.8. Bacterial Stimuli
4.9. Foam Cell Formation
4.10. Cell Metabolic Activity/Viability Assay
4.11. Extracellular Matrix Production (Collagen I Concentration)
4.12. Cell Apoptosis
4.13. Erk Activation/Phosphorylation (pErk)
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4HNE | 4 hydroxynonenal |
| 7-kCh | 7-ketocholesterol |
| BSA | Bovine serum albumin |
| CagA | Cytotoxin associated gene A protein |
| CC3 | Cleaved Caspase 3 |
| CHD | Coronary Heart Disease |
| CRP | C-reactive protein |
| DAPI | 4’,6-diamidino-2-phenylindole |
| EGF | Epidermal growth factor |
| EGM-2 | Endothelial growth medium |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Enriched with fetal bovine serum |
| FGF | Hydrocortisone, fibroblast growth factor |
| FITC | Fluorescein isothiocyanate |
| GE | Glycine acid extract |
| HLO | Helicobacter-like organisms |
| Hsp | Heat shock protein |
| HUVEC | Human umbilical vein endothelial cells |
| ICAM-1 | Intracellular adhesion molecules 1 |
| ICAM-2 | Intracellular adhesion molecules 2 |
| IL-6 | Interleukin |
| LDL | Low-density lipoproteins |
| LPS | Lipopolysaccharide |
| MCP-1 | Macrophage chemotactic protein-1 |
| MMP-9 | Metalloproteinase |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear factor kappa B |
| oxLDL | Oxidized low-density lipoproteins |
| PBS | Phosphate buffered saline |
| PECAM | 1—platelet endothelial cell adhesion molecule 1 |
| pERK | Phosphorylated extracellular signal-regulated kinase |
| PHA | Phytohemagglutinin |
| SDS-PAGE | Sodium dodecyl sulphate—polyacrylamide gel electrophoresis |
| TCR | T-cell receptor |
| TGF-β | Transforming growth factor |
| THP-1 | Human monocyte leukemic cell line |
| TLR-4 | Toll-like receptor 4 |
| TMB | 3,3′5,5′tetrametylobenzydyne |
| TNF–α | Tumor necrosis factor |
| UreA | Urease subunit A |
| UreB | Urease subunit B |
| VacA | Vacuolating cytoxin A |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth actor |
References
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [Green Version]
- Kutuk, O.; Basaga, H. Inflammation meets oxidation: NF-κB as a mediator of initial lesion development in atherosclerosis. Trends Mol. Med. 2003, 9, 549–557. [Google Scholar] [CrossRef]
- Hajra, L.; Evans, A.I.; Chen, M.; Hyduk, S.J.; Collins, T.; Cybulsky, M.I. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. USA 2000, 97, 9052–9057. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.A.; Rosenfeld, M. Pathogens and atherosclerosis: Update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost. 2011, 106, 858–867. [Google Scholar] [CrossRef]
- Chmiela, M.; Gajewski, A.; Rudnicka, K. Helicobacter pylori vs coronary heart disease—searching for connections. World J. Cardiol. 2015, 7, 187–203. [Google Scholar] [CrossRef]
- Lawson, J.S. Multiple Infectious Agents and the Origins of Atherosclerotic Coronary Artery Disease. Front. Cardiovasc. Med. 2016, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Carnevale, R.; Nocella, C.; Petrozza, V.; Cammisotto, V.; Pacini, L.; Sorrentino, V.; Martinelli, O.; Irace, L.; Sciarretta, S.; Frati, G.; et al. Localization of lipopolysaccharide from Escherichia Coli into human atherosclerotic plaque. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Mendall, M.A.; Goggin, P.M.; Molineaux, N.; Levy, J.; Toosy, T.; Strachan, D.; Camm, A.J.; Northfield, T.C. Relation of Helicobacter pylori infection and coronary heart disease. Heart 1994, 71, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M. Prevalence of Helicobacter pylori infection in coronary artery disease and effect of its eradication an coronary lumen reduction after percutations coronaryangioplasty. Dig. Liver Dis. 2001, 33, 222–229. [Google Scholar] [CrossRef]
- Chmiela, M.; Kowalewicz-Kulbat, M.; Miszczak, A.; Wisniewska, M.; Rechcinski, T.; Kolodziej, K.; Kasprzak, J.; Wadstrom, T.; Rudnicka, W. A link betweenHelicobacter pyloriand/orChlamydiaspp. infections and atherosclerosis. FEMS Immunol. Med. Microbiol. 2003, 36, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Park, M.J.; Choi, S.H.; Kim, D.; Kang, S.J.; Chung, S.J.; Choi, S.Y.; Yoon, D.H.; Lim, S.H.; Kim, Y.S.; Yim, J.Y.; et al. Association between Helicobacter pylori Seropositivity and the Coronary Artery Calcium Score in a Screening Population. Gut Liver 2011, 5, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafaeimanesh, J.; Hejazi, S.F.; Damanpak, V.; Vahedian, M.; Sattari, M.; Seyyedmajidi, M. Association ofHelicobacter pylori Infection with Coronary Artery Disease: IsHelicobacter pyloria Risk Factor? Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Kelly, D.J. The Physiology and Metabolism of the Human Gastric Pathogen Helicobacter pylori. Adv. Microb. Physiol. 1998, 40, 137–189. [Google Scholar] [CrossRef]
- Semino-Mora, C.; Doi, S.Q.; Marty, A.; Simko, V.; Carlstedt, I.; Dubois, A. Intracellular and Interstitial Expression of Helicobacter pyloriVirulence Genes in Gastric Precancerous Intestinal Metaplasia and Adenocarcinoma. J. Infect. Dis. 2003, 187, 1165–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmiela, M.; Karwowska, Z.; Gonciarz, W.; Allushi, B.; Stączek, P. Host pathogen interactions in Helicobacter pylori related gastric cancer. World J. Gastroenterol. 2017, 23, 1521–1540. [Google Scholar] [CrossRef]
- Rudnicka, K.; Backert, S.; Chmiela, M. Genetic Polymorphisms in Inflammatory and Other Regulators in Gastric Cancer: Risks and Clinical Consequences. Curr. Top. Microbiol. Immunol. 2019, 421, 53–76. [Google Scholar] [CrossRef]
- Chmiela, M.; Czkwianianc, E.; Wadstrom, T.; Rudnicka, W. Role of Helicobacter pylori surface structures in bacterial interaction with macrophages. Gut 1997, 40, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Huangying, B.; Chen, Y.; Xie, Q.; Lin, G.; Wu, Y.; Feng, Y.; Li, J.; Zhuo, Y.; Zhang, P. CagA-Positive Helicobacter pylori Strains Enhanced Coronary Atherosclerosis by Increasing Serum OxLDL and HsCRP in Patients with Coronary Heart Disease. Dig. Dis. Sci. 2010, 56, 109–114. [Google Scholar] [CrossRef]
- Fiorentino, M.; Ding, H.; Blanchard, T.G.; Czinn, S.J.; Sztein, M.B.; Fasano, A. Helicobacter pylori-induced disruption of monolayer permeability and proinflammatory cytokine secretion in polarized human gastric epithelial cells. Infect. Immun. 2013, 81, 876–883. [Google Scholar] [CrossRef] [Green Version]
- Mnich, E.; Kowalewicz-Kulbat, M.; Sicińska, P.; Hinc, K.; Obuchowski, M.; Gajewski, A.; Moran, A.P.; Chmiela, M. Impact ofHelicobacter pylorion the healing process of the gastric barrier. World J. Gastroenterol. 2016, 22, 7536–7558. [Google Scholar] [CrossRef]
- Gonciarz, W.; Krupa, A.; Hinc, K.; Obuchowski, M.; Moran, A.P.; Gajewski, A.; Chmiela, M. The effect of Helicobacter pylori infection and different H. pylori components on the proliferation and apoptosis of gastric epithelial cells and fibroblasts. PLoS ONE 2019, 14, e0220636. [Google Scholar] [CrossRef] [Green Version]
- Gonciarz, W.; Krupa, A.; Chmiela, M. Proregenerative Activity of IL-33 in Gastric Tissue Cells Undergoing Helicobacter Pylori-Induced Apoptosis. Int. J. Mol. Sci. 2020, 21, 1801. [Google Scholar] [CrossRef] [Green Version]
- Bravo, D.; Hoare, A.; Soto, C.; A Valenzuela, M.; Quest, A.F. Helicobacter pyloriin human health and disease: Mechanisms for local gastric and systemic effects. World J. Gastroenterol. 2018, 24, 3071–3089. [Google Scholar] [CrossRef]
- Tani, M.; Kamata, Y.; Deushi, M.; Osaka, M.; Yoshida, M. 7-Ketocholesterol enhances leukocyte adhesion to endothelial cells via p38MAPK pathway. PLoS ONE 2018, 13, e0200499. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yamaguchi, T.; Ishihara, N.; Nakamura, S.; Tanaka, S.; Oka, R.; Imamura, H.; Sato, Y.; Ban, N.; Kawana, H.; et al. 7-Ketocholesterol induces ROS-mediated mRNA expression of 12-lipoxygenase, cyclooxygenase-2 and pro-inflammatory cytokines in human mesangial cells: Potential role in diabetic nephropathy. Prostaglandins Other Lipid Mediat. 2018, 134, 16–23. [Google Scholar] [CrossRef]
- Gajewski, A.; Gonciarz, W.; Hinc, K.; Obuchowski, M.; Chmiela, M. Dysfunction of gastric barrier in the milieu of 7-ketocholesterol, acetylsalicylic acid and Helicobacter pylori compounds. Potential implications in the development of coronary heart disease. Helicobacter 2018, 23, e12525. [Google Scholar]
- Krupa, A. Regulation of endothelial cells activation / apoptosis by Helicobacter pylori antigenic components. Helicobacter 2018, 23, e12525. [Google Scholar]
- Nedrud, J.G.; Czinn, S.J. Host, heredity and helicobacter. Gut 1999, 45, 323–324. [Google Scholar] [CrossRef] [Green Version]
- Miszczyk, E.; Walencka, M.; Rudnicka, K.; Matusiak, A.; Rudnicka, W.; Chmiela, M. Antigen-specific lymphocyte proliferation as a marker of immune response in guinea pigs with sustained Helicobacter pylori infection. Acta Biochim. Pol. 2014, 61, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Walencka, M.; Gonciarz, W.; Mnich, E.; Gajewski, A.; Stawerski, P.; Knapik-Dabrowicz, A.; Chmiela, M. The microbiological, histological, immunological and molecular determinants of Helicobacter pylori infection in guinea pigs as a convenient animal model to study pathogenicity of these bacteria and the infection dependent immune response of the host. Acta Biochim. Pol. 2015, 62, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjunnesson, H.; Sturegård, E.; Grubb, A.; Willén, R.; Wadström, T. Comparative study of Helicobacter pylori infection in guinea pigs and mice—elevation of acute-phase protein C3 in infected guinea pigs. FEMS Immunol. Med. Microbiol. 2001, 30, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Sjunnesson, H.; Sturegard, E.; Hynes, S.; Willen, R.; Feinstein, R.; Wadstrom, T. Five month persistence of Helicobacter pylori infection in guinea pigs. APMIS 2003, 111, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Ogburn, R.; Leite, J.O.; Ratliff, J.; Volek, J.S.; McGrane, M.M.; Fernandez, M.L. Effects of increased dietary cholesterol with carbo-hydrate restriction on hepatic lipid metabolism in Guinea pigs. Comp Med. 2012, 62, 109–115. [Google Scholar]
- Ye, P.; Cheah, I.K.; Halliwell, B. A high-fat and cholesterol diet causes fatty liver in guinea pigs. The role of iron and oxidative damage. Free. Radic. Res. 2013, 47, 602–613. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Lee, A. Spiral Organisms: What are They? A Microbiologic Introduction toHelicobacter pylori. Scand. J. Gastroenterol. 1991, 26, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Grebowska, A.; Rechcinski, T.; Bak-Romaniszyn, L.; Czkwianianc, E.; Moran, A.; Druszczynska, M.; Kowalewicz-Kulbat, M.; Owczarek, A.; Dziuba, M.; Krzemińska-Pakuła, M.; et al. Potential role of LPS in the outcome of Helicobacter pylori related diseases. Polish J. Microbiol. 2006, 55, 1. [Google Scholar]
- Mnich, E.; Gajewski, A.; Rudnicka, K.; Gonciarz, W.; Stawerski, P.; Hinc, K.; Obuchowski, M.; Chmiela, M. Immunoregulation of antigen presenting and secretory functions of monocytic cells by Helicobacter pylori antigens in relation to impairment of lymphocyte expansion. Acta Bioch. Pol. 2015, 62, 641–650. [Google Scholar] [CrossRef]
- Moran, A.P.; Helander, I.M.; Kosunen, T.U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J. Bacteriol. 1992, 174, 1370–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rechciński, T.; Chmiela, M.; Matecka-Panas, E.; Płaneta-Małsecka, I.; Rudnicka, W. Serological Indicators ofHelicobacter pyloriInfection in Adult Dyspeptic Patients and Healthy Blood Donors. Microbiol. Immunol. 1997, 41, 387–393. [Google Scholar] [CrossRef]
- Khan, S.; Rahman, H.N.A.; Okamoto, T.; Matsunaga, T.; Fujiwara, Y.; Sawa, T.; Yoshitake, J.; Ono, K.; Ahmed, K.A.; Rahaman, M.; et al. Promotion of atherosclerosis by Helicobacter cinaedi infection that involves macrophage-driven proinflammatory responses. Sci. Rep. 2014, 4, 4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrayoz, I.M.; Huang, J.-D.; Lee, J.W.; Pascual, I.; Rodríguez, I.R. 7-Ketocholesterol–Induced Inflammation: Involvement of Multiple Kinase Signaling Pathways via NFκB but Independently of Reactive Oxygen Species Formation. Investig. Opthalmol. Vis. Sci. 2010, 51, 4942–4955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovechkin, A.V.; Tyagi, N.; Rodriguez, W.E.; Hayden, M.R.; Moshal, K.S.; Tyagi, S.C. Role of matrix metalloproteinase-9 in endothelial apoptosis in chronic heart failure in mice. J. Appl. Physiol. 2005, 99, 2398–2405. [Google Scholar] [CrossRef] [Green Version]
- Adiguzel, E.; Ahmad, P.J.; Franco, C.; Bendeck, M.P. Collagens in the progression and complications of atherosclerosis. Vasc. Med. 2009, 14, 73–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Chen, B.; Wang, W.; Shen, T.; Qi, R. Thioredoxin1 Downregulates Oxidized Low-Density Lipoprotein-Induced Adhesion Molecule Expression via Smad3 Protein. PLoS ONE 2013, 8, e76226. [Google Scholar] [CrossRef] [Green Version]
- Gunn, M.; Stephens, J.C.; Thompson, J.R.; Rathbone, B.J.; Samani, N.J. Significant association of cagA positive Helicobacter pylori strains with risk of premature myocardial infarction. Heart 2000, 84, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo-Mbenza, B.; Nsenga, J.N.; Mokondjimobe, E.; Gombet, T.; Assori, I.N.; Ibara, J.R.; Ellenga-Mbolla, B.; Vangu, D.N.; Fuele, S.M. Helicobacter pylori infection is identified as a cardiovascular risk factor in Central Africans. Vasc. Heal. Risk Manag. 2012, 6, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkov, S.; Paro, M.M.K.; Vidjak, V.; Flegar-Mestric, Z. The Evaluation of New Biomarkers of Inflammation and Angiogenesis in Peripheral Arterial Disease. In Current Trends in Atherogenesis; Rezzani, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 97–120. [Google Scholar]
- Chalubinski, M.; Zemanek, K.; Skowron, W.; Wojdan, K.; Gorzelak, P.; Broncel, M. The effect of 7-ketocholesterol and 25-hydroxycholesterol on the integrity of the human aortic endothelial and intestinal epithelial barriers. Inflamm. Res. 2013, 62, 1015–1023. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-Reactive Protein and Low-Density Lipoprotein Cholesterol Levels in the Prediction of First Cardiovascular Events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, Y.; Lee, Y.; Cho, K.H.; Kim, K.H.; Chung, J.H. Cholesterol inhibits MMP-9 expression in human epidermal keratinocytes and HaCaT cells. FEBS Lett. 2007, 581, 3869–3874. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Hernandez-Anzaldo, S.; Brglez, V.; Hemmeryckx, B.; Leung, D.; Filep, J.G.; Vance, J.E.; Vance, D.E.; Kassiri, Z.; Lijnen, R.H.; Lambeau, G.; et al. Novel Role for Matrix Metalloproteinase 9 in Modulation of Cholesterol Metabolism. J. Am. Hear. Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- De Mello, W.C. Angiotensin (1–7) re-establishes impulse conduction in cardiac muscle during ischaemia-reperfusion. The role of the sodium pump. J. Renin-Angiotensin-Aldosterone Syst. 2004, 5, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Bernick, S.; Patek, P.R.; Ershoff, B.H.; Wells, A. Effect of Cholesterol Feeding on the Thyroid Gland and Vascular Structures of the Rabbit, Guinea Pig, Hamster and Rat. Am. J. Pathol. 1962, 41, 661–677. [Google Scholar] [PubMed]
- Morgan, E.; Casabianca, A.B.; Khouri, S.J.; Kalinoski, A.L.N. In vivo assessment of arterial stiffness in the isoflurane anesthetized spontaneously hypertensive rat. Cardiovasc. Ultrasound 2014, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.M.; Lim, S.H.; Han, Y.M.; Lee, H.; Seo, J.Y.; Park, H.E.; Kwak, M.-S.; Chung, G.E.; Choi, S.-Y.; Kim, J.S. Association between Helicobacter pylori infection and arterial stiffness: Results from a large cross-sectional study. PLoS ONE 2019, 14, e0221643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, Y.; Utsugi, M.; Yoshioka, E.; Horikawa, N.; Sato, T.; Gong, Y.; Kishi, R. Relationship of Helicobacter pylori Infection to Arterial Stiffness in Japanese Subjects. Hypertens. Res. 2005, 28, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Hansson, G.K.; Libby, P.; Schönbeck, U.; Yan, Z.-Q. Innate and Adaptive Immunity in the Pathogenesis of Atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Milioti, N.; Bermudez-Fajardo, A.; Penichet, M.L.; Oviedo-Orta, E. Antigen-Induced Immunomodulation in the Pathogenesis of Atherosclerosis. Clin. Dev. Immunol. 2008, 2008, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedgui, A.; Mallat, Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Kong, D.; Chen, H. miR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci. Rep. 2016, 6, 21789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Xia, Y.; Hu, B. Infection and atherosclerosis: TLR-dependent pathways. Cell. Mol. Life Sci. 2020, 77, 2751–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Tan, R.Z.; Chen, Y.; Wang, H.L.; Liu, Y.H.; Wen, D.; Fan, J.M. CagA promotes proliferation and secretion of extra-cellular matrix by inhibiting signaling pathway of apoptosis in rat glomerular mesangial cells. Renal Failure 2016, 38, 458–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusuma, S.; Zhao, S.; Gerecht, S. The extracellular matrix is a novel attribute of endothelial progenitors and of hypoxic mature endothelial cells. FASEB J. 2012, 26, 4925–4936. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Senger, D.R. Endothelial Extracellular Matrix. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.P.; Liu, P.; Li, Y.F.; Zhang, G.L.; Zeng, D.S.; Liu, D.L. LPS induces activation of the TLR-4 pathway in fibroblasts and promotes skin car formation through collagen I. Int. J. Clin. Exp. 2019, 12, 2121–2129, ISSN 1936-2625/IJCEP0091013. [Google Scholar]
- Shields, K.J.; Stolz, N.; Watkins, S.C.; Ahearn, J.M. Complement Proteins C3 and C4 Bind to Collagen and Elastin in the Vascular Wall: A Potential Role in Vascular Stiffness and Atherosclerosis. Clin. Transl. Sci. 2011, 4, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Trachootham, D.A.; Lu, W.; Ogasawara, A.; Valle, R.D.; Huang, P. Redox regulation of cell survival. Antioxidant Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-M.; Chen, A.F.; Zhang, K. Isolation and Primary Culture of Mouse Aortic Endothelial Cells. J. Vis. Exp. 2016, 118, 52965. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.; Lindgren, S.; Eriksson, S.; Wadström, T. Serum antibodies to Helicobacter hepaticus and Helicobacter pylori in patients with chronic liver disease. Gut 2000, 46, 410–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, I.; Ljungh, A.; Aleljung, P.; Wadström, T. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J. Clin. Microbiol. 1997, 35, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusek, P.; Gonciarz, W.; Krupa, A.; Mikołajczyk-Chmiela, M. Role of Bcl-2 in regulation of endothelial cell apoptosis induced by H. pylori antigenic components. Helicobacter 2019, 24, e12647. [Google Scholar]
- Krupa, A.; Dziuba, A.; Chmiela, M. Contribution of Helicobacter pylori to foam cell formation in experimental atherosclerosis. Helicobacter 2020, 25, e12745. [Google Scholar]
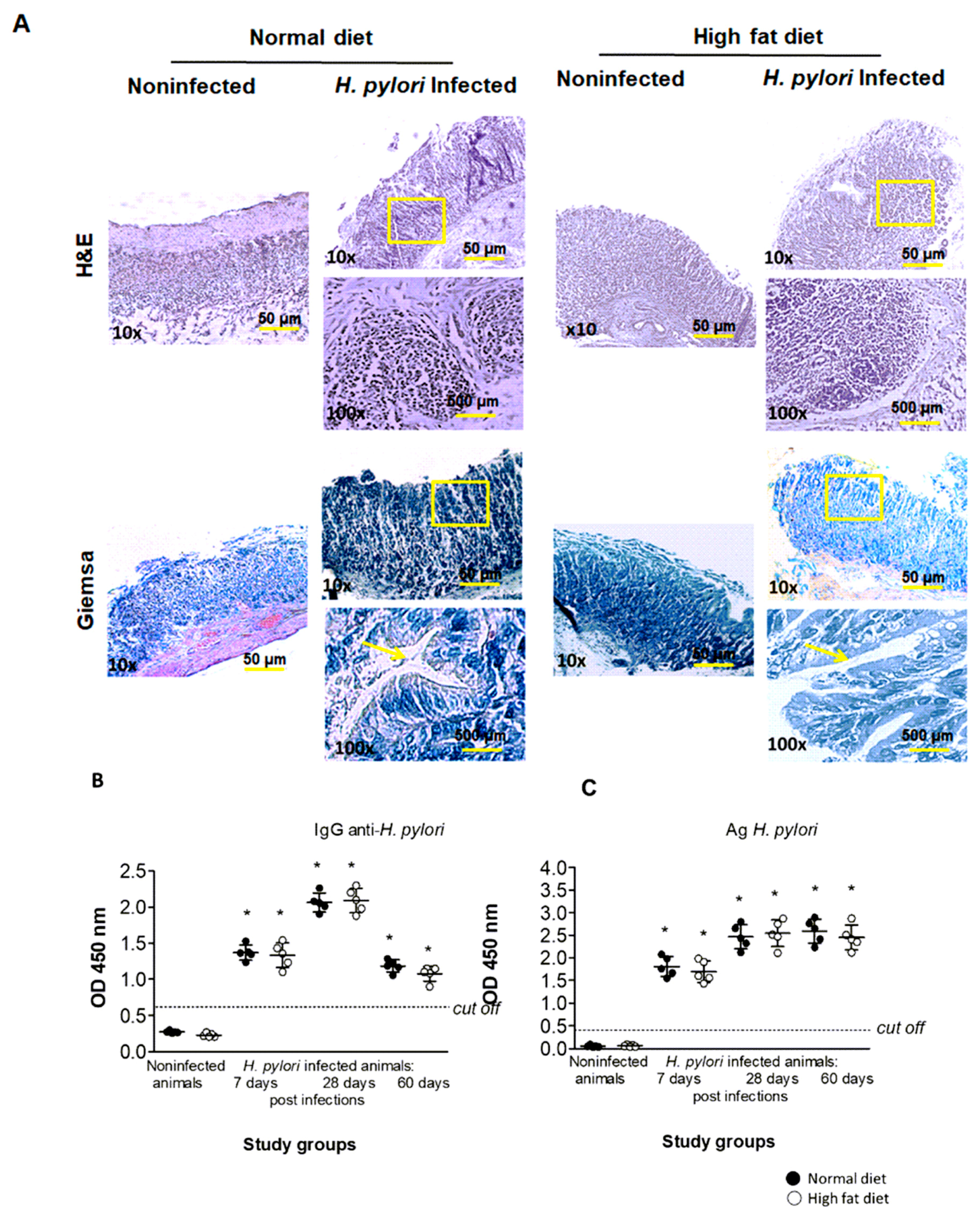




| Sample Diagnostic Assay | H. pylori CCUG 17874 | Brucella Broth | H. pylori CCUG 17874 | Brucella Broth | |||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Diet | High Fat Diet | ||||||||
| 7 Days Post Infection (n = 5) | 28 Days Post Infection (n = 5) | 60 Days Post Infection (n = 5) | Control (n = 5) | 7 Days Post Infection (n = 5) | 28 Days Post Infection (n = 5) | 60 Days Post Infection (n = 5) | Control (n = 5) | ||
| Histopathology | |||||||||
| Gastric tissue | HLO grading: | ||||||||
| -Giemsa | 1 1 1 2 1 | 1 2 1 2 2 | 1 2 2 1 0 | 0 0 0 0 1 | 1 1 1 1 2 | 1 2 2 1 1 | 2 1 2 2 0 | 0 0 0 0 0 | |
| Immune cell grading (H&E) | |||||||||
| -granulocytes | 1 1 1 1 1 | 1 2 1 2 1 | 2 1 2 2 2 | 0 0 0 0 0 | 1 1 1 1 1 | 1 2 1 2 2 | 2 2 2 2 2 | 0 0 0 0 0 | |
| -lymphocytes | 1 1 1 2 1 | 1 2 2 2 2 | 2 1 2 2 1 | 0 0 0 0 0 | 1 1 1 1 1 | 2 1 2 2 2 | 1 2 2 1 2 | 0 0 0 0 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, A.; Gonciarz, W.; Rusek-Wala, P.; Rechciński, T.; Gajewski, A.; Samsel, Z.; Dziuba, A.; Śmiech, A.; Chmiela, M. Helicobacter pylori Infection Acts Synergistically with a High-Fat Diet in the Development of a Proinflammatory and Potentially Proatherogenic Endothelial Cell Environment in an Experimental Model. Int. J. Mol. Sci. 2021, 22, 3394. https://doi.org/10.3390/ijms22073394
Krupa A, Gonciarz W, Rusek-Wala P, Rechciński T, Gajewski A, Samsel Z, Dziuba A, Śmiech A, Chmiela M. Helicobacter pylori Infection Acts Synergistically with a High-Fat Diet in the Development of a Proinflammatory and Potentially Proatherogenic Endothelial Cell Environment in an Experimental Model. International Journal of Molecular Sciences. 2021; 22(7):3394. https://doi.org/10.3390/ijms22073394
Chicago/Turabian StyleKrupa, Agnieszka, Weronika Gonciarz, Paulina Rusek-Wala, Tomasz Rechciński, Adrian Gajewski, Zuzanna Samsel, Anna Dziuba, Agnieszka Śmiech, and Magdalena Chmiela. 2021. "Helicobacter pylori Infection Acts Synergistically with a High-Fat Diet in the Development of a Proinflammatory and Potentially Proatherogenic Endothelial Cell Environment in an Experimental Model" International Journal of Molecular Sciences 22, no. 7: 3394. https://doi.org/10.3390/ijms22073394
APA StyleKrupa, A., Gonciarz, W., Rusek-Wala, P., Rechciński, T., Gajewski, A., Samsel, Z., Dziuba, A., Śmiech, A., & Chmiela, M. (2021). Helicobacter pylori Infection Acts Synergistically with a High-Fat Diet in the Development of a Proinflammatory and Potentially Proatherogenic Endothelial Cell Environment in an Experimental Model. International Journal of Molecular Sciences, 22(7), 3394. https://doi.org/10.3390/ijms22073394







