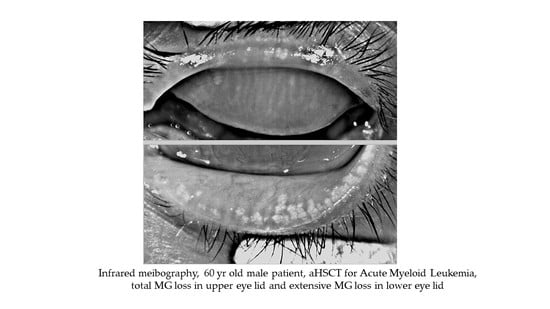
Meibomian Gland Dysfunction in Ocular Graft vs. Host Disease: A Need for Pre-Clinical Models and Deeper Insights
Abstract
:1. Introduction
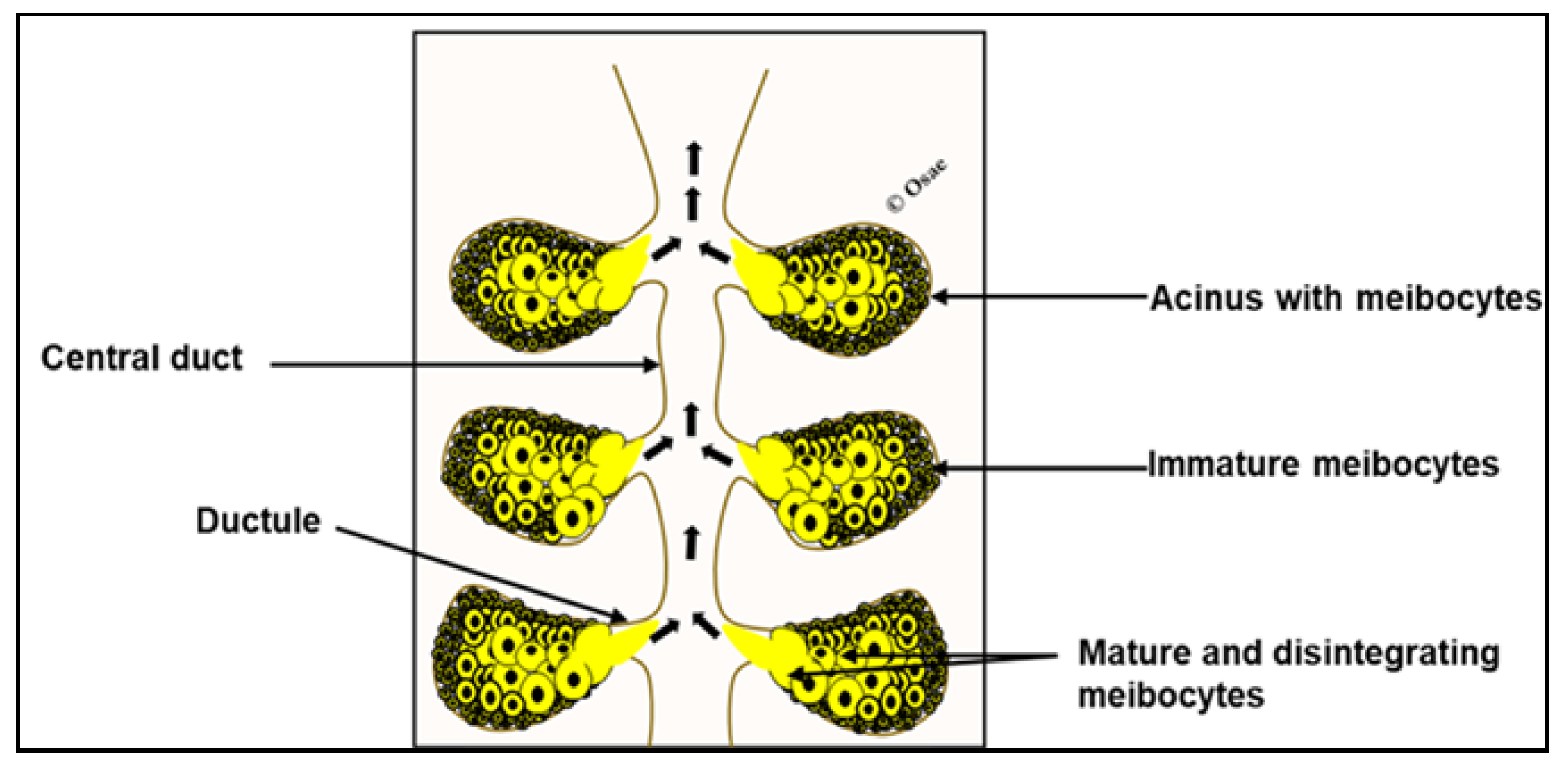
2. Overview of Meibomian Gland Biology
3. Secretory and Postulated Regeneration Properties of the Meibomian Gland
4. Pre-aHSCT Conditioning Regimen Can Affect Normal Meibomian Gland Health
5. Evidence of Inflammatory Immune Response in oGvHD and the Meibomian Gland
6. Pre-Clinical Models of oGvHD and the Meibomian Gland
7. Discussion
8. Conclusions
9. Literature Search Strategy
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| aHSTC | Allogeneic hematopoietic stem cell transplantation |
| CD | Cluster of Differentiation |
| GvHD | Graft-vs-host disease |
| HLA | Human leukocyte antigen |
| MHC | Major histocompatibility complex |
| MiHA | Minor histocompatibility antigen |
| MDA | Malondialdehyde |
| MGD | Meibomian glad dysfunction |
| oGvHD | Ocular graft-vs-host-disease |
References
- Dean, R.M.; Bishop, M.R. Graft-versus-host disease: Emerging concepts in prevention and therapy. Curr. Hematol. Rep. 2003, 2, 287–294. [Google Scholar]
- Choi, S.W.; Levine, J.E.; Ferrara, J.L. Pathogenesis and management of graft-versus-host disease. Immunol. Allergy Clin. 2010, 30, 75–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikari, H.; Antin, J.H.; Dana, R. Ocular graft-versus-host disease: A review. Surv. Ophthalmol. 2013, 58, 233–251. [Google Scholar] [CrossRef]
- Milosevic, S.; Bachnick, B.; Karim, K.; Bornkamm, G.W.; Witter, K.; Gerbitz, A.; Mautner, J.; Behrends, U. Identification of MHC II-restricted minor histocompatibility antigens after HLA-identical stem-cell transplantation. Transplantation 2010, 90, 1030–1035. [Google Scholar] [CrossRef]
- Magenau, J.; Runaas, L.; Reddy, P. Advances in understanding the pathogenesis of graft-versus-host disease. Br. J. Haematol. 2016, 173, 190–205. [Google Scholar] [CrossRef]
- Munir, S.Z.; Aylward, J. A review of ocular graft-versus-host disease. Optom. Vis. Sci. 2017, 94, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Engel, L.; Wittig, S.; Bock, F.; Sauerbier, L.; Scheid, C.; Holtick, U.; Chemnitz, J.-M.; Hallek, M.; Cursiefen, C.; Steven, P. Meibography and meibomian gland measurements in ocular graft-versus-host disease. BMT 2015, 50, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Dietrich-Ntoukas, T.; Cursiefen, C.; Westekemper, H.; Eberwein, P.; Reinhard, T.; Bertz, H.; Nepp, J.; Lawitschka, A.; Heiligenhaus, A.; Seitz, B.; et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: Report from the German–Austrian–Swiss Consensus Conference on Clinical Practice in Chronic GVHD. Cornea 2012, 31, 299–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; De Paiva, C.S.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating stress induces T cell-mediated Sjögren’s syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, M.E.; Beuerman, R.W.; Fox, R.I.; Gao, J.; Mircheff, A.K.; Pflugfelder, S.C. A unified theory of the role of the ocular surface in dry eye. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2; Springer: Boston, MA, USA, 1998; pp. 643–651. [Google Scholar]
- Inamoto, Y.; Valdés-Sanz, N.; Ogawa, Y.; Alves, M.; Berchicci, L.; Galvin, J.; Greinix, H.; Hale, G.A.; Horn, B.; Kelly, D.; et al. Ocular graft-versus-host disease after hematopoietic cell transplantation: Expert review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Transplant Complications Working Party of the EBMT. BMT 2019, 54, 662–673. [Google Scholar] [CrossRef]
- Laterza, L.; Rizzatti, G.; Gaetani, E.; Chiusolo, P.; Gasbarrini, A. The gut microbiota and immune system relationship in human graft-versus-host disease. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016025. [Google Scholar] [CrossRef] [Green Version]
- Giannaccare, G.; Pellegrini, M.; Taroni, L.; Bernabei, F.; Senni, C.; Grendele, A.; Scorcia, V.; Campos, E.C. Corneal biomechanical alterations in patients with chronic ocular graft versus-host disease. PLoS ONE 2019, 14, e0213117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaraman, K.R.; Jivrajka, R.V.; Soin, K.; Bouchard, C.S.; Movahedan, A.; Shorter, E.; Jain, S.; Jacobs, D.S.; Djalilian, A.R. Superior limbic keratoconjunctivitis-like inflammation in patients with chronic graft-versus-host disease. Ocul. Surf. 2016, 14, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Scorcia, V.; Campos, E. Ocular surface system alterations in ocular graft-versus-host disease: All the pieces of the complex puzzle. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Copsel, S.N.; Lightbourn, C.O.; Barreras, H.; Lohse, I.; Wolf, D.; Bader, C.S.; Manov, J.; Kale, B.J.; Shah, D.; Brothers, S.P.; et al. BET Bromodomain inhibitors which permit Treg function enable a combinatorial strategy to suppress GVHD in pre-clinical allogeneic HSCT. Front. Immunol. 2019, 9, 3104. [Google Scholar] [CrossRef] [PubMed]
- Boieri, M.; Shah, P.; Dressel, R.; Inngjerdingen, M. The role of animal models in the study of hematopoietic stem cell transplantation and GvHD: A historical overview. Front. Immunol. 2016, 7, 333. [Google Scholar] [CrossRef] [Green Version]
- Shamloo, K.; Barbarino, A.; Alfuraih, S.; Sharma, A. Graft versus host disease-associated dry eye: Role of ocular surface mucins and the effect of rebamipide, a mucin secretagogue. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4511–4519. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.S.; Clouthier, S.G.; Ferrara, J.L.; Stepan, A.; Mian, S.I.; Ahmad, A.Z.; Elner, V.M. Lacrimal gland involvement in graft-versus-host disease: A murine model. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2692–2697. [Google Scholar] [CrossRef] [Green Version]
- Pérez, R.L.; Pérez-Simón, J.A.; Caballero-Velazquez, T.; Flores, T.; Carrancio, S.; Herrero, C.; Blanco, B.; Gutierrez-Cosio, S.; Cañete-Campos, C.; González, F.C.; et al. Limbus damage in ocular graft-versus-host disease. Biol. Blood Marrow Transplant. 2011, 17, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Perez, V.L.; Barsam, A.; Duffort, S.; Urbieta, M.; Barreras, H.; Lightbourn, C.; Komanduri, K.V.; Levy, R.B. Novel scoring criteria for the evaluation of ocular graft-versus-host disease in a preclinical allogeneic hematopoietic stem cell transplantation animal model. Biol. Blood Marrow Transplant. 2016, 22, 1765–1772. [Google Scholar] [CrossRef] [Green Version]
- Ohigashi, H.; Hashimoto, D.; Hayase, E.; Takahashi, S.; Ara, T.; Yamakawa, T.; Sugita, J.; Onozawa, M.; Nakagawa, M.; Teshima, T. Ocular instillation of vitamin A–coupled liposomes containing HSP47 siRNA ameliorates dry eye syndrome in chronic GVHD. Blood Adv. 2019, 3, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Millar, T.J.; Schuett, B.S. The real reason for having a meibomian lipid layer covering the outer surface of the tear film—A review. Exp. Eye Res. 2015, 137, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P. Antimicrobial role of human meibomian lipids at the ocular surface. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7272–7277. [Google Scholar] [CrossRef] [Green Version]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [Green Version]
- Osae, E.A.; Ablordeppey, R.K.; Horstmann, J.; Kumah, D.B.; Steven, P. Clinical Dry Eye and Meibomian Gland Features Among Dry Eye Patients in Rural and Urban Ghana. Clin. Ophthalmol. (Auckl. N. Z.) 2020, 14, 4055. [Google Scholar] [CrossRef]
- Andersen, H.; Ehlers, N.; Matthiessen, M.E. Histochemistry and development of the human eyelids. Acta Ophthalmol. 1965, 43, 642–668. [Google Scholar] [CrossRef] [PubMed]
- Byun, T.H.; Kim, J.T.; Park, H.W.; Kim, W.K. Timetable for upper eyelid development in staged human embryos and fetuses. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2011, 294, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Nien, C.J.; Massei, S.; Lin, G.; Liu, H.; Paugh, J.R.; Liu, C.-Y.; Kao, W.W.Y.; Brown, D.J.; Jester, J.V. The development of meibomian glands in mice. Mol. Vis. 2010, 16, 1132. [Google Scholar]
- Parfitt, G.J.; Lewis, P.N.; Young, R.D.; Richardson, A.; Lyons, J.G.; Di Girolamo, N.; Jester, J.V. Renewal of the holocrine meibomian glands by label-retaining, unipotent epithelial progenitors. Stem Cell Rep. 2016, 7, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Argüeso, P. Proteolytic activity in the meibomian gland: Implications to health and disease. Exp. Eye Res. 2017, 163, 53–57. [Google Scholar] [CrossRef]
- Olami, Y.; Zajicek, G.; Cogan, M.; Gnessin, H.; Pe’er, J. Turnover and migration of meibomian gland cells in rats’ eyelids. Ophthalmic Res. 2001, 33, 170–175. [Google Scholar] [CrossRef]
- Sabuncuoğlu, S.; Kuşkonmaz, B.; Çetinkaya, D.U.; Özgüneş, H. Evaluation of oxidative and antioxidative parameters in pediatric hematopoietic SCT patients. BMT 2012, 47, 651–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, T.L.; Benvegnú, D.M.; Bonfanti, G.; Frediani, A.V.; Pereira, D.V.; Rocha, J.B. Oxidative stress and δ-ALA-D activity in different conditioning regimens in allogeneic bone marrow transplantation patients. Clin. Biochem. 2009, 42, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef] [Green Version]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, O.M.; Dogru, M.; Matsumoto, Y.; Igarashi, A.; Kojima, T.; Wakamatsu, T.H.; Inaba, T.; Shimizu, T.; Shimazaki, J.; Tsubota, K. Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PLoS ONE 2014, 9, e99328. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, M.; Babic, A. The European Blood and Marrow Transplantation Textbook for Nurses: Under the Auspices of EBMT; Springer: New York, NY, USA, 2018. [Google Scholar]
- Kumar, S.; Juresic, E.; Barton, M.; Shafiq, J. Management of skin toxicity during radiation therapy: A review of the evidence. J. Med. Imaging Radiat. Oncol. 2010, 54, 264–279. [Google Scholar] [CrossRef]
- Hirst, L.W.; Jabs, D.A.; Tutschka, P.J.; Green, W.R.; Santos, G.W. The eye in bone marrow transplantation: I. Clinical study. Arch. Ophthalmol. 1983, 101, 580–584. [Google Scholar] [CrossRef]
- Holler, E. Risk assessment in haematopoietic stem cell transplantation: GvHD prevention and treatment. Best Pract. Res. Clin. Haematol. 2007, 20, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Herretes, S.; Ross, D.B.; Duffort, S.; Barreras, H.; Yaohong, T.; Saeed, A.M.; Murillo, J.C.; Komanduri, K.V.; Levy, R.B.; Perez, V.L. Recruitment of donor T cells to the eyes during ocular GVHD in recipients of MHC-matched allogeneic hematopoietic stem cell transplants. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2348–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, Y.; Kim, S.K.; Dana, R.; Clayton, J.; Jain, S.; Rosenblatt, M.I.; Perez, V.L.; Shikari, H.; Riemens, A.; Tsubota, K. International chronic ocular graft-vs-host-disease (GVHD) consensus group: Proposed diagnostic criteria for chronic GVHD (Part I). Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, S.C.; Raja, H.; Nau, C.B.; Shen, J.F.; Schornack, M.M. Ocular graft-versus-host disease: A review. Eye Contact Lens. 2015, 41, 256–261. [Google Scholar] [CrossRef]
- Poe, J.C.; Jia, W.; Di Paolo, J.A.; Reyes, N.J.; Kim, J.Y.; Su, H.; Sundy, J.S.; Cardones, A.R.; Perez, V.L.; Chen, B.J.; et al. SYK inhibitor entospletinib prevents ocular and skin GVHD in mice. JCI Insight 2018, 3, e122430. [Google Scholar] [CrossRef]
- Hwang, H.S.; Ha, M.; Kim, H.-S.; Na, K.-S. Longitudinal analysis of meibomian gland dropout in patients with ocular graft-versus-host disease. Ocul. Surf. 2019, 17, 464–469. [Google Scholar] [CrossRef]
- Kusne, Y.; Temkit, M.H.; Khera, N.; Patel, D.R.; Shen, J.F. Conjunctival subepithelial fibrosis and meibomian gland atrophy in ocular graft-versus-host disease. Ocul. Surf. 2017, 15, 784–788. [Google Scholar] [CrossRef]
- Giannaccare, G.; Bonifazi, F.; Sessa, M.; Fresina, M.; Arpinati, M.; Bandini, G.; Versura, P. Dry eye disease is already present in hematological patients before hematopoietic stem cell transplantation. Cornea 2016, 35, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Bonifazi, F.; Sebastiani, S.; Sessa, M.; Pellegrini, M.; Arpinati, M.; Moscardelli, F.; Versura, P.; Campos, E. Meibomian gland dropout in hematological patients before hematopoietic stem cell transplantation. Cornea 2018, 37, 1264–1269. [Google Scholar] [CrossRef]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid–protein interactions in health and disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, N.; Saban, D.R. Pathogenesis of meibomian gland dysfunction (MGD) requires the T cell-neutrophil axis, in the allergy setting. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1431. [Google Scholar]
- Amber, K.T.; Shiman, M.I.; Badiavas, E.V. The use of antioxidants in radiotherapy-induced skin toxicity. Integr. Cancer Ther. 2014, 13, 38–45. [Google Scholar] [CrossRef]
- Osae, E.A.; Bullock, T.; Chintapalati, M.; Brodesser, S.; Hanlon, S.; Redfern, R.; Steven, P.; Smith, C.W.; Rumbaut, R.E.; Burns, A.R. Obese Mice with Dyslipidemia Exhibit Meibomian Gland Hypertrophy and Alterations in Meibum Composition and Aqueous Tear Production. Int. J. Mol. Sci. 2020, 21, 8772. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Lu, H.; McMahon, A.; Eule, J.C. Toward an animal model of the human tear film: Biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6881–6896. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C.; Foulks, G.N. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 2010, 91, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Purpose | Model Description | Pre-Conditioning Regimen | Main Ocular Findings | Therapy/OutCome | |||
|---|---|---|---|---|---|---|---|---|
| Cornea | Conjunctiva | Lacrimal Gland | Meibomian Gland | |||||
| [22] | To develop a novel topical antifibrotic treatment against ocular chronic GVHD using vitamin A-coupled liposomes containing short interfering RNA against heat shock protein 47 (VA-lip HSP47) eye drops. | Minor histocompatibility-antigen mismatched mouse model Donor: B10.D2 (H-2d) recipient: BALB/c (H-2d) | Total body irradiation | No information | No information | Infiltration of HSP47+ fibroblasts Increased fibrosis Increased collagen deposition | No information | Instillation of VA-lip HSP47 agent reduced fibrosis, collagen deposition and restored normal levels of tear production |
| [18] | To investigate the role of ocular surface glycocalyx and mucins in graft versus host disease (GVHD)-associated dry eye and ameliorative effect of topical rebamipide, a mucin secretagogue, on GVHD-associated dry eye | Major histocompatibility class I mismatch Donor: C57BL/6 Ly 5.2+ Recipients: B6D2F1 (F1) | Total body irradiation | Presence of dry eye phenotype with keratopathy indicated by significant punctate and plaque corneal staining Reduced glycocalyx density and thickness Reduced Muc 1 and M4 and increased Muc 16 | Decrease in palpebral conjunctiva goblet cells | Reduced tear film volume with concomitant reduction in Muc5ac | No information | Instillation of 2% rebamipide in balance salt solution vehicle twice daily (left eye only) attenuated reduction in tear production and corneal damage |
| [45] | To evaluate the efficacy of entospletinib (ENTO), tyrosine kinase SYK inhibitor on the clinical (clinical and skin) aspects of GVHD | Donor: C57BL/6 (H2b) Recipient: BALB/c (H2kd) Clarification on matching status need | Total body irradiation | No specific information | Chemosis, redness | No specific information | Eyelid edema and blepharitis but no direct mention of the meibomian glands | ENTO administration resulted in profound improvements in clinical eye as well as other systemic GvHD scores. |
| [21] | To develop a novel clinical scoring criterion for identifying degrees of ocular pathology at both the ocular surface and adnexa in oGvHD | MHC-matched, minor transplantation antigen–mismatched allogeneic model of matched unrelated donor Donors: B6 mice (H-2b, Thy1.1)//(eGFP) B6 transgenic (H2b) Recipients: C3H.SW (H2b) | Total body irradiation | Corneal ulceration, epithelial haze, corneo-limbal eGFP+ immune cell infiltrates | No information | No specific information | Eye lid oedema and closure with eGFP+ immune cell infiltrates but no specific mention of the meibomian gland | No information |
| [42] | To identify the kinetics and origin of ocular infiltrating T cells in a preclinical model of graft-versus-host disease (GVHD) that induces eye tissue damage. | Major histocompatibility complex-matched, minor histocompatibility-mismatched hematopoietic stem cell transplant mouse model. Donor: C56BL/6 mice (H2b, Ly9.1−) Recipient: C3H.SW, H2b, Ly9.1+ | Total body irradiation | Epitheliopathy with increased staining 3–4wks post-transplant Infiltration of CD4+ and CD8+ T cells, macrophages, monocytes and neutrophils Upregulation of IFNy, TNFα and IL-6 genes | Reduced goblet cells density Apparent atrophy of fornix | Infiltration of T cells and macrophages | No information | No information |
| [20] | To establishes a model of GVHD with cornea and limbus involvement | Major/sex mismatch and Donor: Male C57BL/6 (H2b) Recipient: Female BALB/c (H2k) | Total body irradiation | Atrophic epitheliopathy with vacuolization Stromal oedema, neovascularization, and inflammatory/lymphocytic infiltrates Limbus show epithelial satellitosis | Focal epithelial loss, lymphocytic exocytosis, necrosis Mononuclear cells and microvesicular infiltrates | Apparent ductal fibrosis Ductal/Interlobu-lar infiltration with eosinophils | Crusted/erythe-matosus eyelids but no direct mention of the meibomian gland | No information |
| [19] | To describe lacrimal gland involvement in graft versus-host disease | Major histocompatibility class I mismatch Donors: C57BL/6 Ly 5.2+ (Allogeneic), B6D2F1 (F1) (Syngeneic) Recipients: B6D2F1 (F1) | Total body irradiation | No information | No information | Periductal inflammation fibrosis, apoptosis, accumulation of ductal debris and stasis of ducts Infiltration of of CD3+, CD8+ and CD4+ positive T cells Overall higher disease scores in the allogeneic group | Lid inflammation but no direct mention of the meibomian gland involvement | No information |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appenteng Osae, E.; Steven, P. Meibomian Gland Dysfunction in Ocular Graft vs. Host Disease: A Need for Pre-Clinical Models and Deeper Insights. Int. J. Mol. Sci. 2021, 22, 3516. https://doi.org/10.3390/ijms22073516
Appenteng Osae E, Steven P. Meibomian Gland Dysfunction in Ocular Graft vs. Host Disease: A Need for Pre-Clinical Models and Deeper Insights. International Journal of Molecular Sciences. 2021; 22(7):3516. https://doi.org/10.3390/ijms22073516
Chicago/Turabian StyleAppenteng Osae, Eugene, and Philipp Steven. 2021. "Meibomian Gland Dysfunction in Ocular Graft vs. Host Disease: A Need for Pre-Clinical Models and Deeper Insights" International Journal of Molecular Sciences 22, no. 7: 3516. https://doi.org/10.3390/ijms22073516
APA StyleAppenteng Osae, E., & Steven, P. (2021). Meibomian Gland Dysfunction in Ocular Graft vs. Host Disease: A Need for Pre-Clinical Models and Deeper Insights. International Journal of Molecular Sciences, 22(7), 3516. https://doi.org/10.3390/ijms22073516