Sacubitril/Valsartan Improves Diastolic Function But Not Skeletal Muscle Function in a Rat Model of HFpEF
Abstract
1. Introduction
2. Results
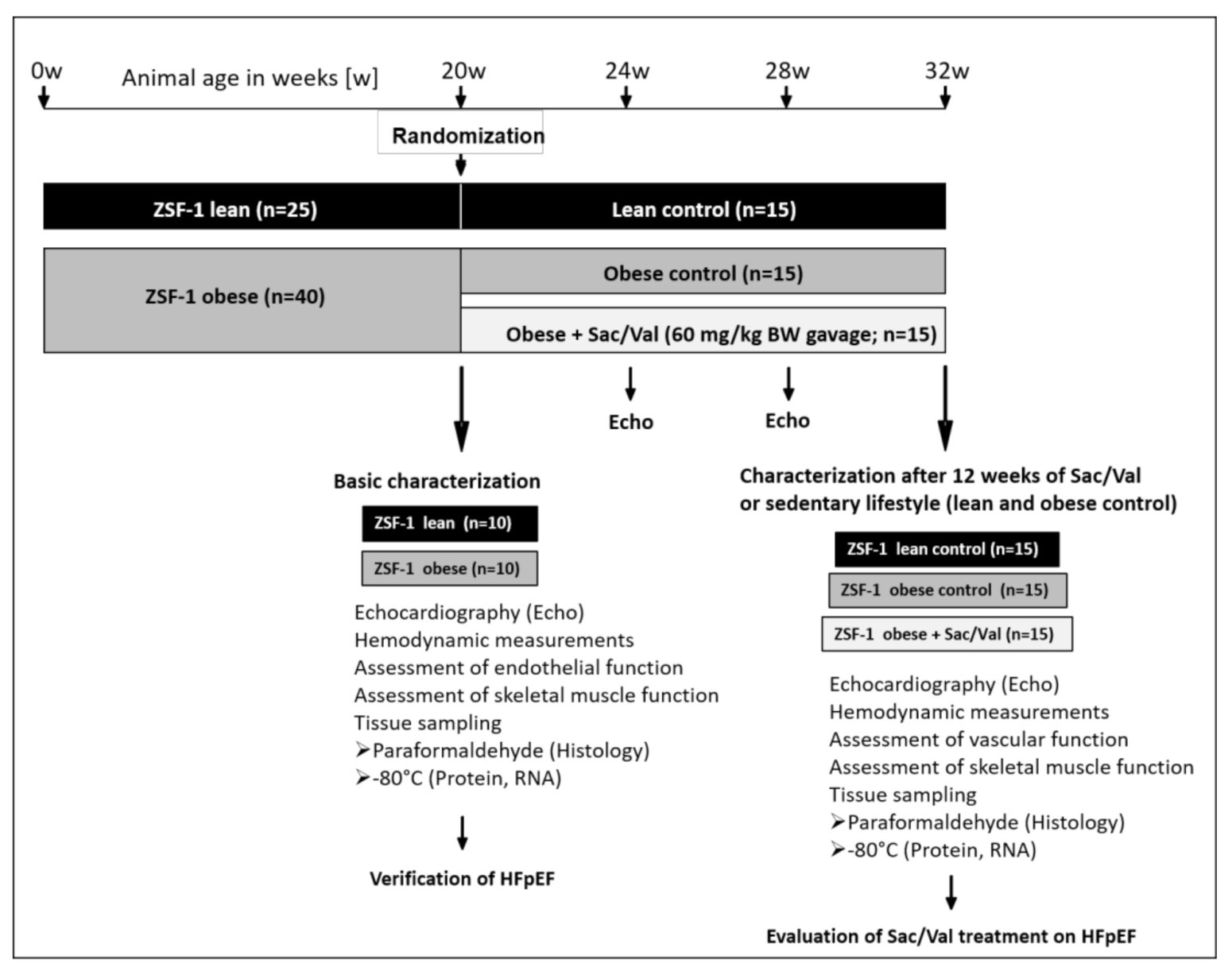
2.1. Baseline Animal Characteristics at 20 Weeks of Age
2.2. Animal Characteristics at 32 Weeks of Age (After 12-Week Sac/Val or Control)
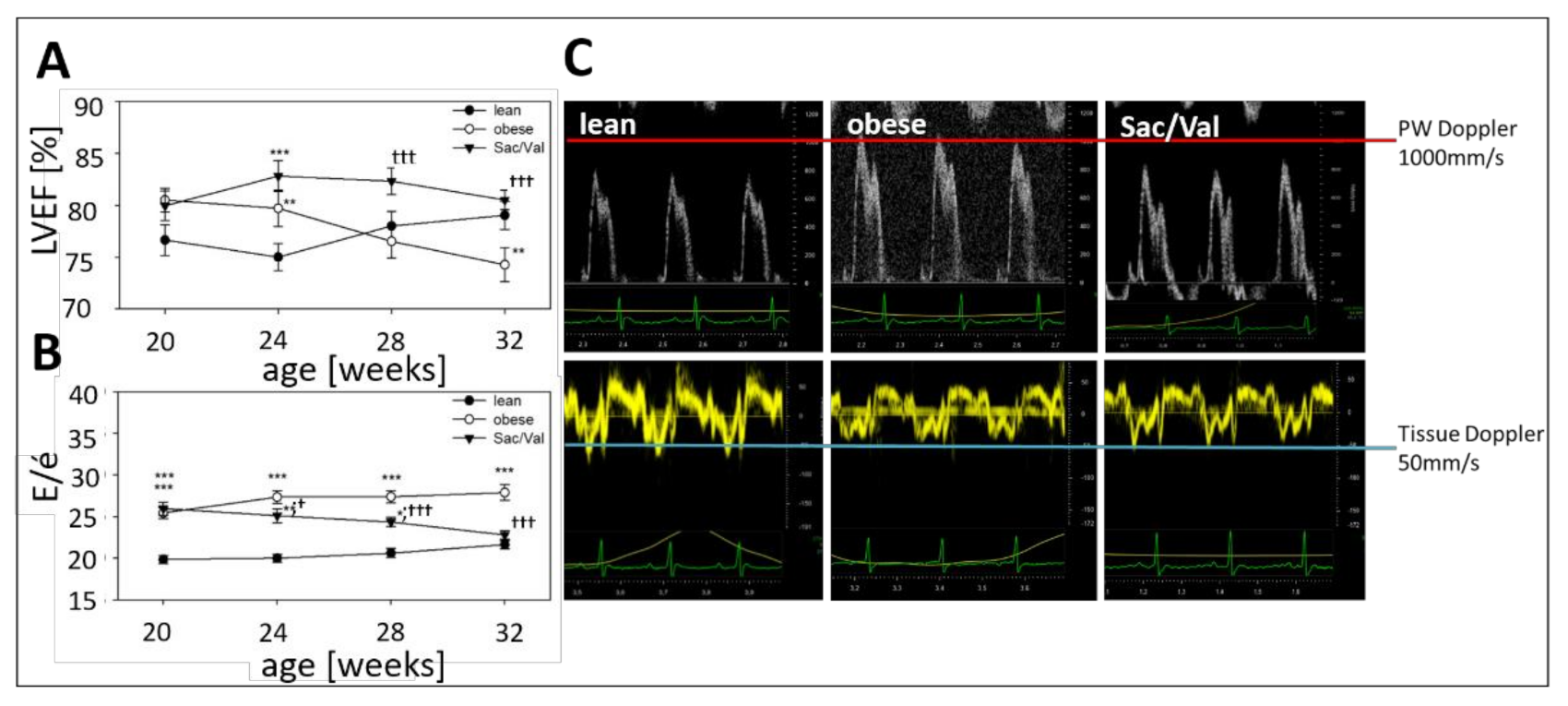
2.3. Impact of Sac/Val on Systolic and Diastolic Function
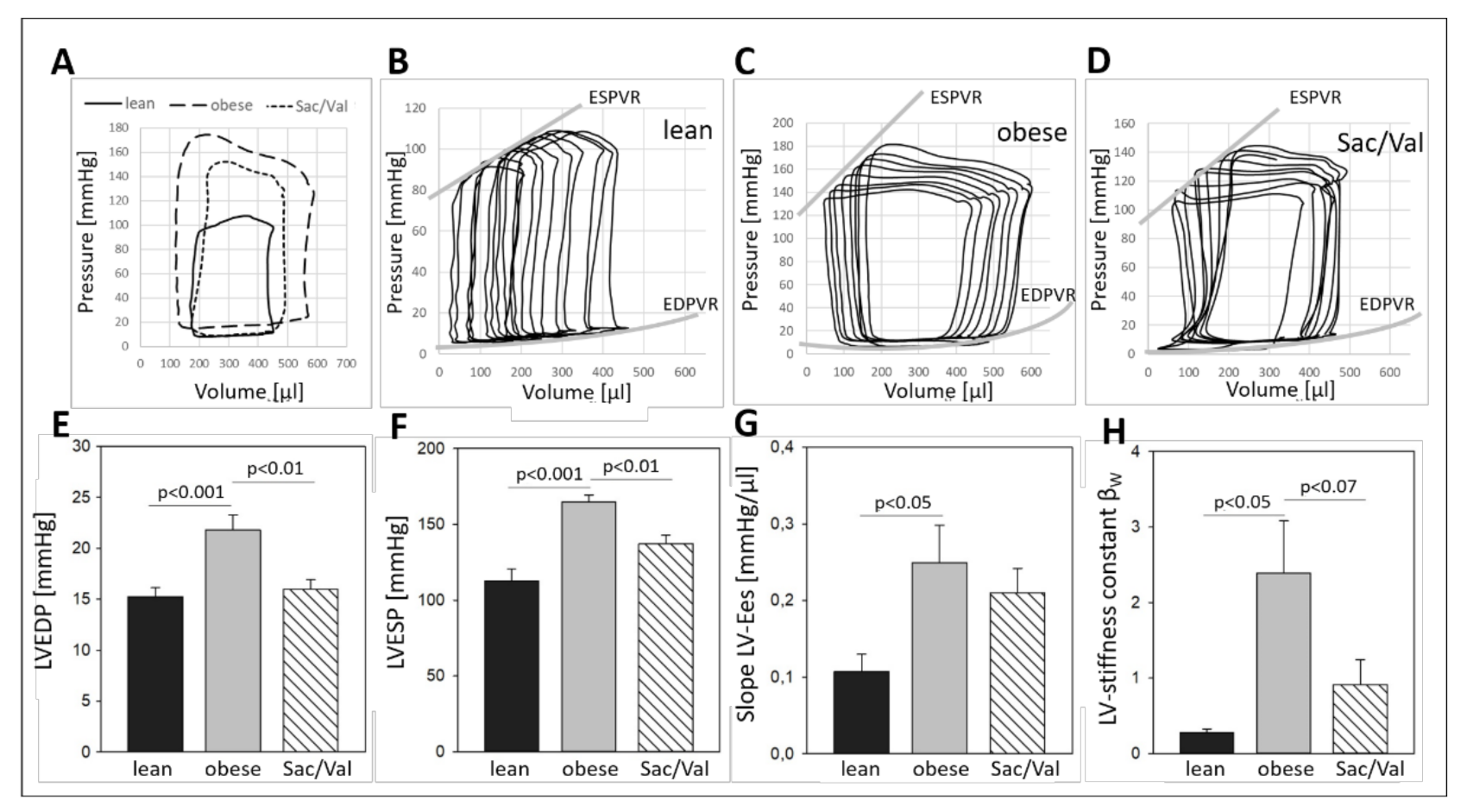
2.4. Impact of Sac/Val on Invasively Determined Hemodynamics
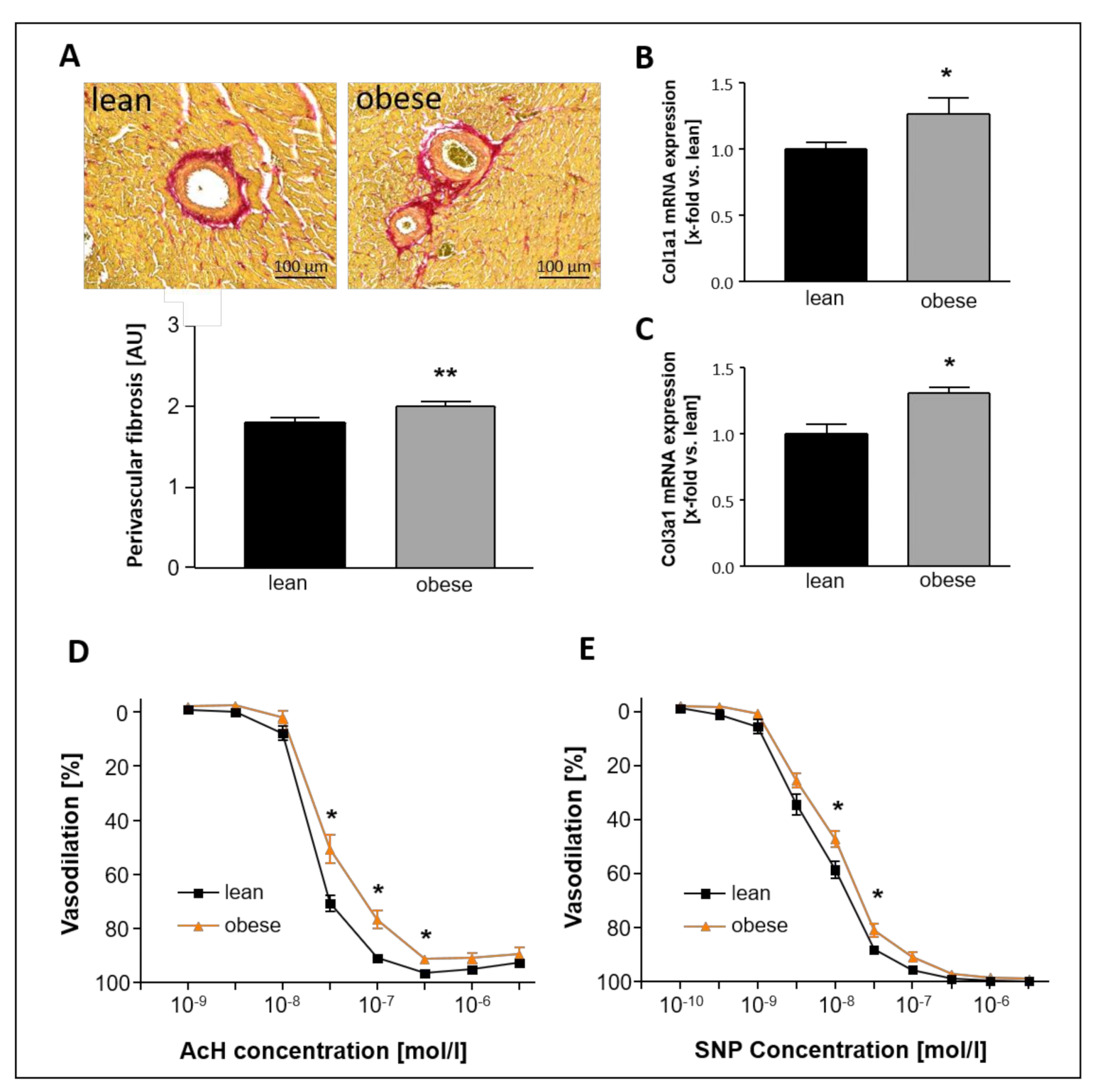
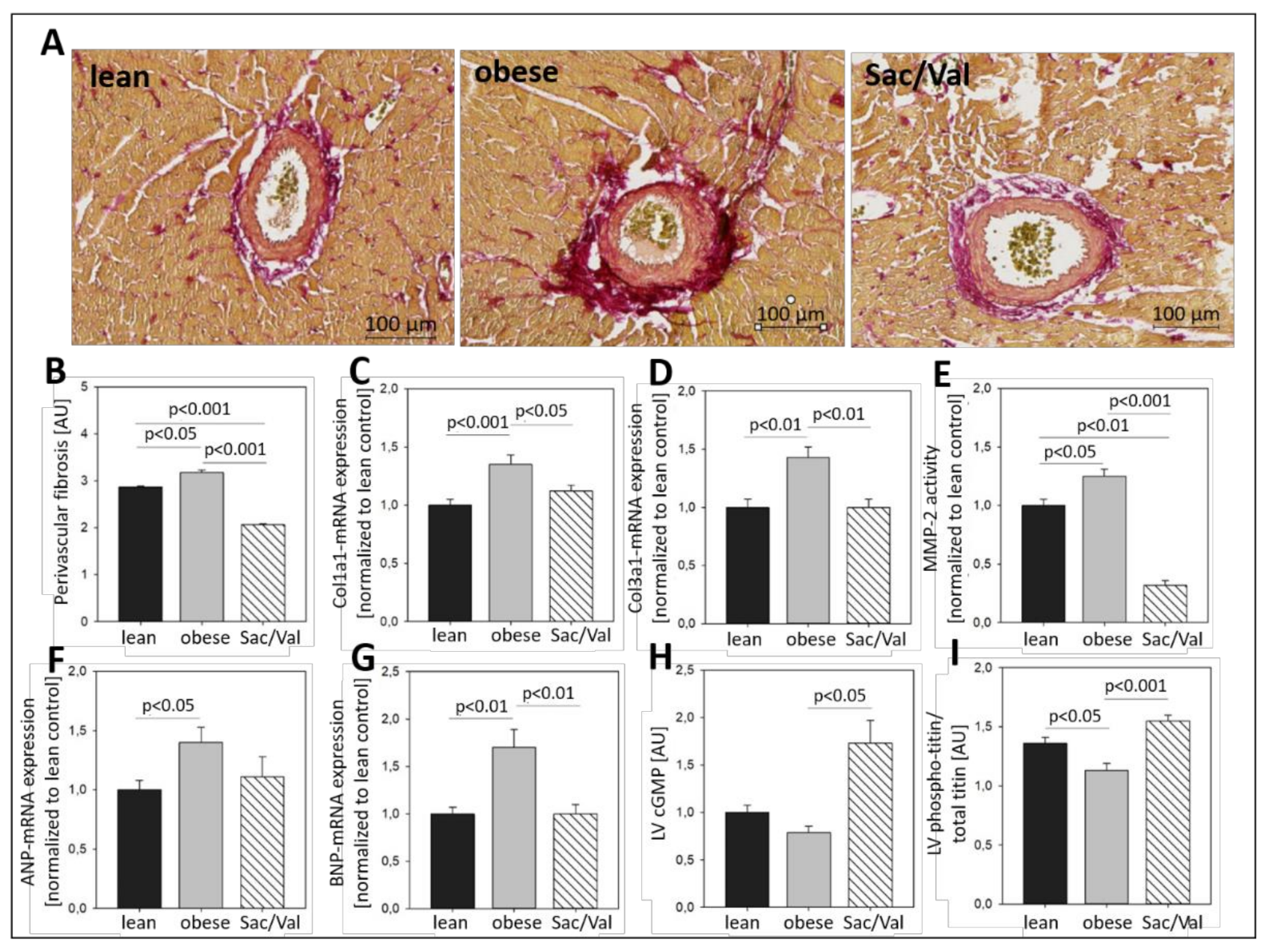
2.5. Impact of Sac/Val on Myocardial Fibrosis and Titin Phosphorylation
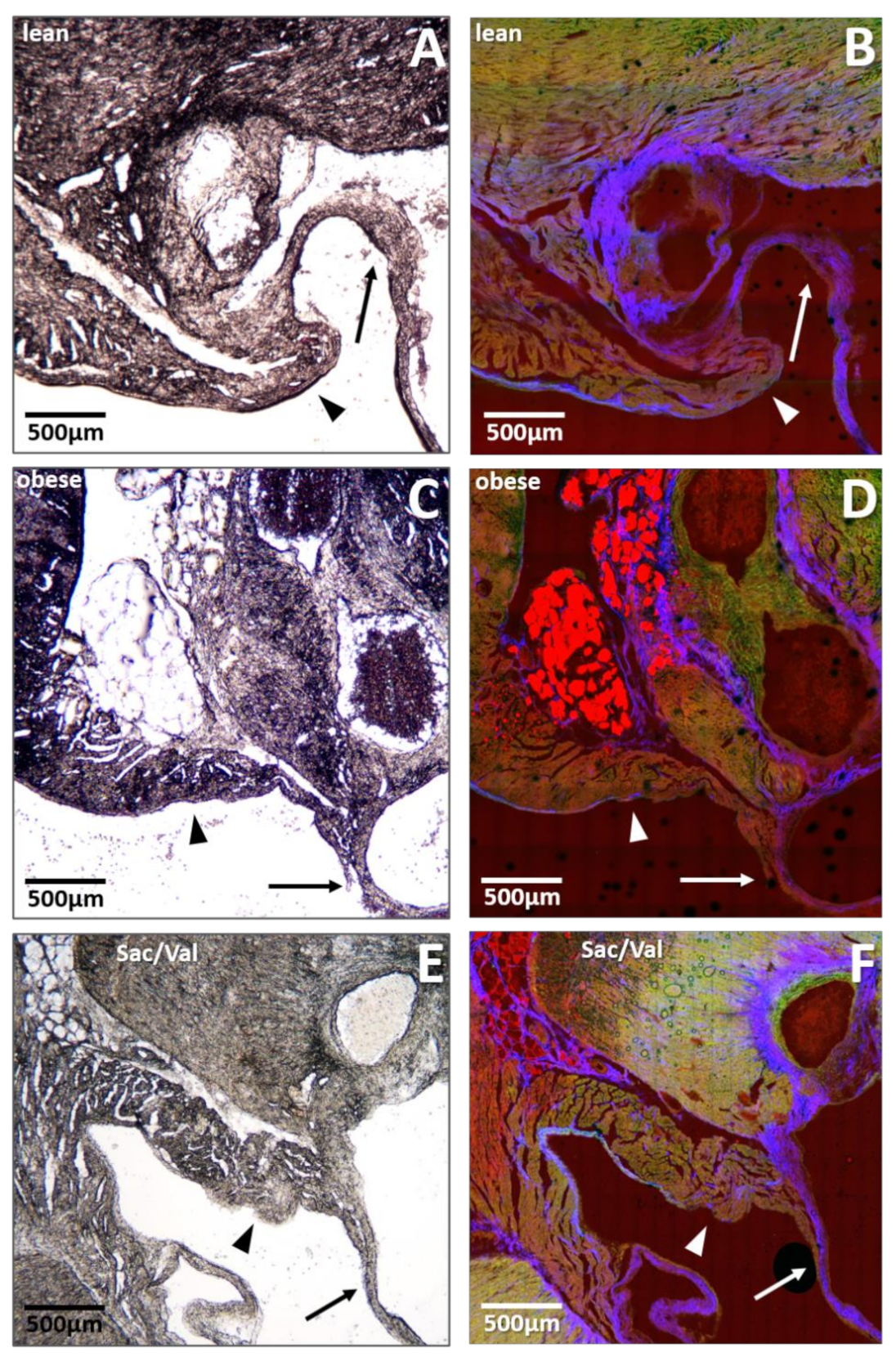
2.6. Impact of Sac/Val on Fat Deposition within the Mitral Valve Annulus
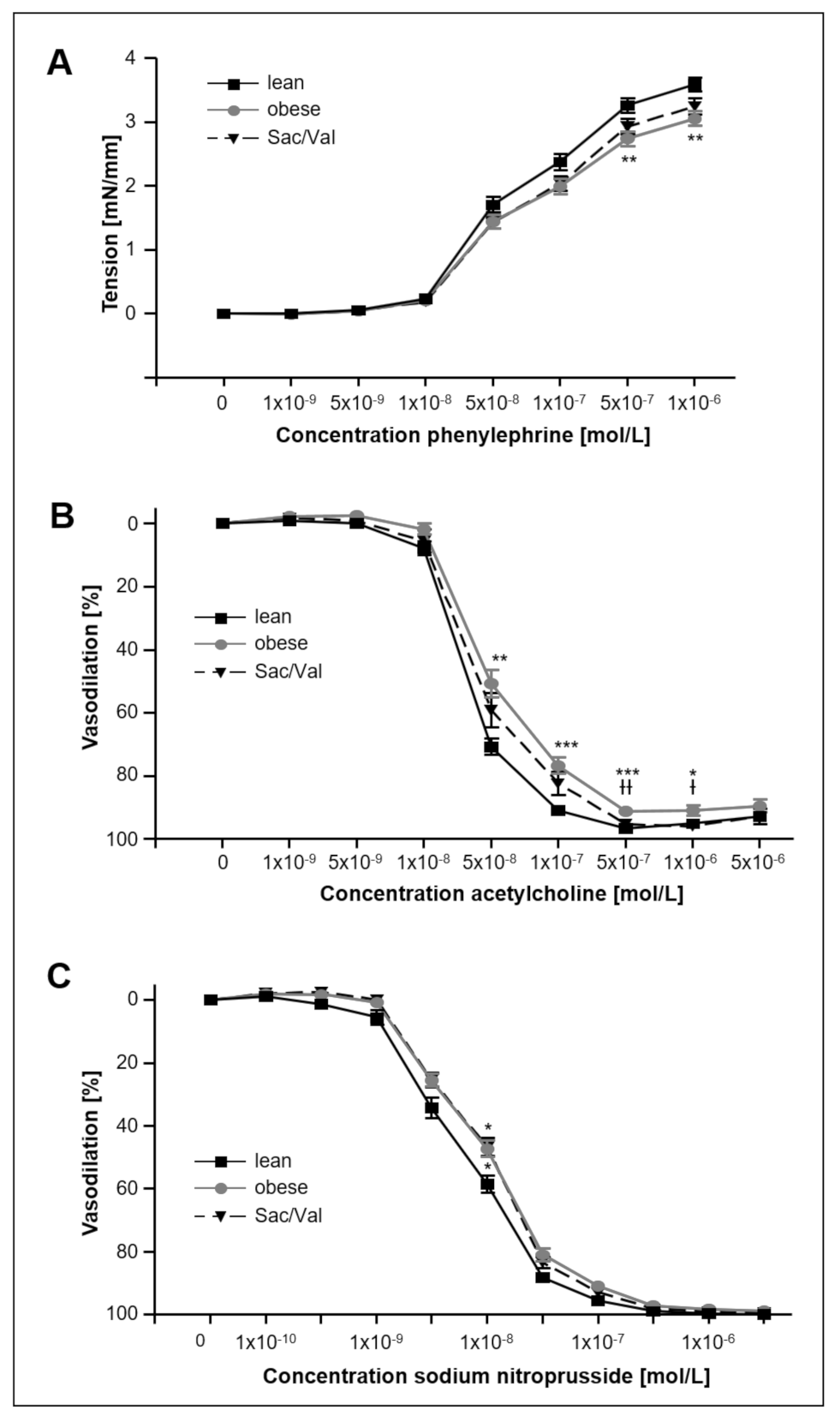
2.7. Impact of Sac/Val on Vascular Function
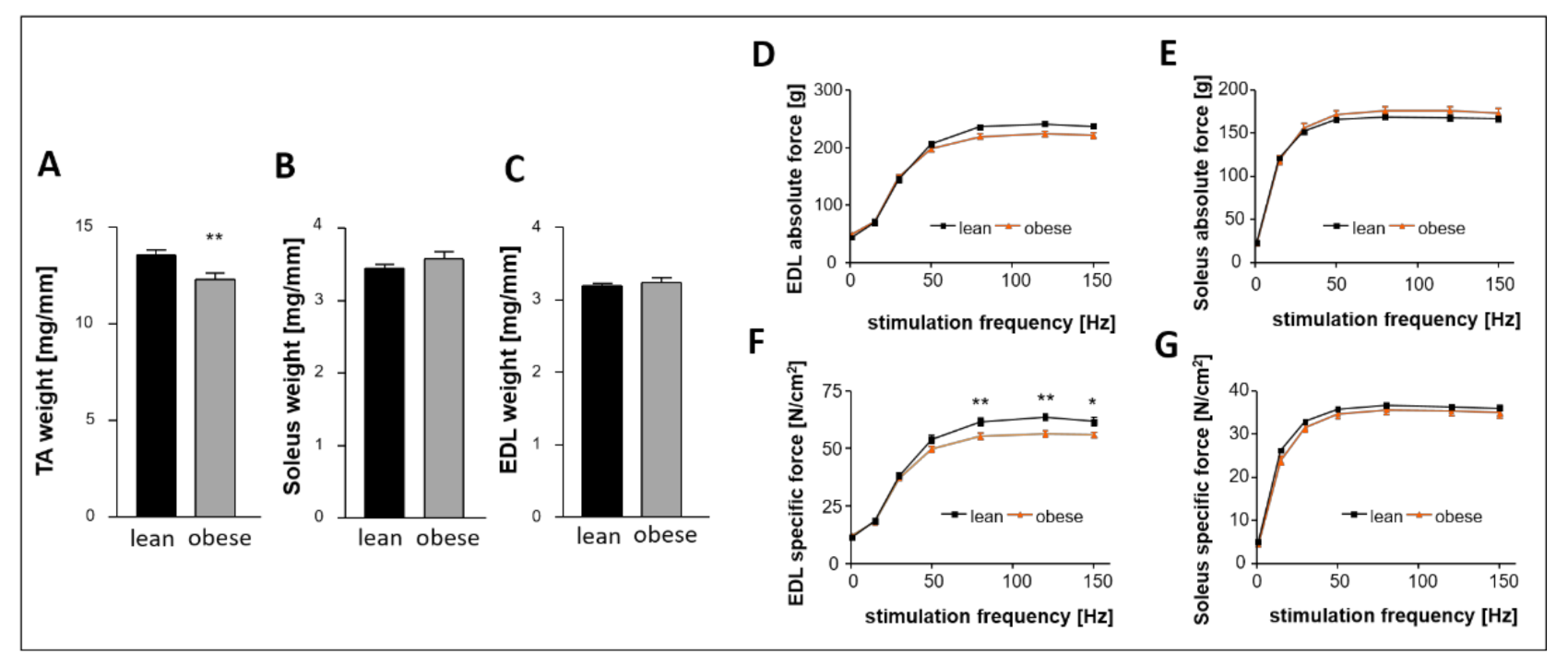
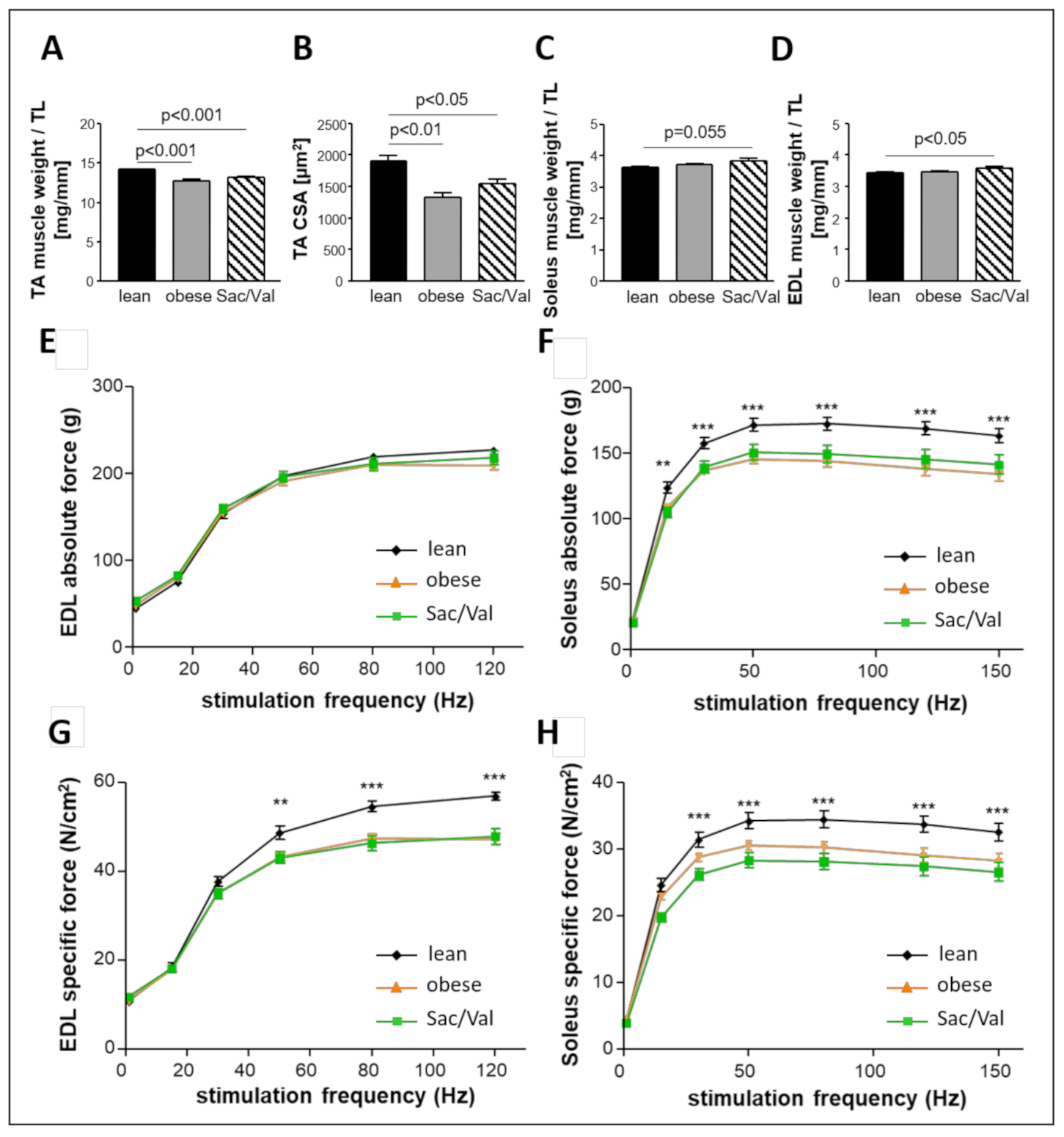
2.8. Impact of Sac/Val on Skeletal Muscle Atrophy and Skeletal Muscle Function
3. Discussion
- (a)
- A 12-week treatment of obese ZSF1 rats with Sac/Val improved diastolic dysfunction, which became significant after four weeks of treatment and continued to improve until final assessment.
- (b)
- After 12 weeks of Sac/Val treatment, we observed significantly lower LV pressure in systole (LVESP) and diastole (LVEDP) as well as reduced left ventricular stiffness compared to the obese control group.
- (c)
- Sac/Val treatment significantly reduced left ventricular collagen expression levels and, in particular, perivascular fibrosis. Moreover, Sac/Val restored LV titin phosphorylation, suggesting both reduced fibrosis and normalized phosphorylation levels of titin to be key modules of the preserved left ventricular elasticity.
- (d)
- Although Sac/Val treatment did not affect body weight, we monitored reduced heart weight and decreased myocardial fat deposition. Reduced fat deposition at the site of the mitral valve annulus in the Sac/Val-treated group might indicate an enhanced mobility of this structure, possibly being involved in the improvement of diastolic function.
- (e)
- Sac/Val treatment slightly improved endothelial-dependent vasodilation in carotid arteries compared to the obese control group.
- (f)
- Sac/Val treated obese rats neither differed in skeletal muscle weight nor in skeletal muscle function compared to the obese control.
3.1. The Female, Obese ZSF1 Rat as Research Model for HFpEF
3.2. Impact of Sac/Val on the Myocardium
3.3. Sac/Val Slightly Improves the Endothelial Function
3.4. Sac/Val Neither Influences Skeletal Muscle Atrophy nor Skeletal Muscle Function
4. Materials and Methods
4.1. Animals
4.2. Echocardiography
4.3. Hemodynamic Measurements and Pressure-Volume Analysis
4.4. Skeletal Muscle Function
4.5. Assessment of Carotid Artery Function
4.6. Quantification of Hba1c, NT-proBNP and cGMP
4.7. Immunohistochemistry
4.8. Assessment of Titin Phosphorylation
4.9. Zymography
4.10. RNA Isolation and Quantitative Real-Time PCR
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Taylor, A.L. Heart Failure in Women. Curr. Heart Fail. Rep. 2015, 12, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Donal, E.; Kraigher-Krainer, E.; Vasan, R.S. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2011, 13, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, G.; Heinonen, I.; Lourenco, A.P.; Duncker, D.J.; Falcao-Pires, I. Animal models of heart failure with preserved ejection fraction. Neth. Heart J. 2016, 24, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Schauer, A.; Draskowski, R.; Jannasch, A.; Kirchhoff, V.; Goto, K.; Mannel, A.; Barthel, P.; Augstein, A.; Winzer, E.; Tugtekin, M.; et al. ZSF1 rat as animal model for HFpEF: Development of reduced diastolic function and skeletal muscle dysfunction. ESC Heart Fail. 2020, 7, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.R.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.C.; et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: A post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017, 5, 333–340. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Liu, Q.; Shan, Q. Effects of renin-angiotensin-aldosterone system inhibitors on mortality, hospitalization, and diastolic function in patients with HFpEF. A meta-analysis of 13 randomized controlled trials. Herz 2016, 41, 76–86. [Google Scholar] [CrossRef]
- Solomon, S.D.; Janardhanan, R.; Verma, A.; Bourgoun, M.; Daley, W.L.; Purkayastha, D.; Lacourciere, Y.; Hippler, S.E.; Fields, H.; Naqvi, T.Z.; et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: A randomised trial. Lancet 2007, 369, 2079–2087. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Ohte, N. The effect of beta-blockers on mortality in heart failure with preserved ejection fraction: A meta-analysis of observational cohort and randomized controlled studies. Int. J. Cardiol. 2017, 228, 4–10. [Google Scholar] [CrossRef]
- Kovács, Á.; Alogna, A.; Post, H.; Hamdani, N. Is enhancing cGMP-PKG signalling a promising therapeutic target for heart failure with preserved ejection fraction? Neth. Heart J. 2016, 24, 268–274. [Google Scholar] [CrossRef]
- Solomon, S.D.; Rizkala, A.R.; Gong, J.; Wang, W.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; et al. Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial. JACC. Heart Fail. 2017, 5, 471–482. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Jackson, A.M.; Lam, C.S.P.; Redfield, M.M.; Anand, I.S.; Ge, J.; Lefkowitz, M.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation 2020, 141, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Wachter, R.; Shah, S.J.; Cowie, M.R.; Szecsödy, P.; Shi, V.; Ibram, G.; Zhao, Z.; Gong, J.; Klebs, S.; Pieske, B. Angiotensin receptor neprilysin inhibition versus individualized RAAS blockade: Design and rationale of the PARALLAX trial. ESC Heart Fail. 2020, 7, 856–864. [Google Scholar] [CrossRef]
- Nguyen, I.T.N.; Brandt, M.M.; van de Wouw, J.; van Drie, R.W.A.; Wesseling, M.; Cramer, M.J.; de Jager, S.C.A.; Merkus, D.; Duncker, D.J.; Cheng, C.; et al. Both male and female obese ZSF1 rats develop cardiac dysfunction in obesity-induced heart failure with preserved ejection fraction. PLoS ONE 2020, 15, e0232399. [Google Scholar] [CrossRef]
- Burkhoff, D.; Mirsky, I.; Suga, H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: A guide for clinical, translational, and basic researchers. Am. J. Physiol. Heart Circ. Physiol 2005, 289, H501–H512. [Google Scholar] [CrossRef]
- Pacher, P.; Nagayama, T.; Mukhopadhyay, P.; Bátkai, S.; Kass, D.A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 2008, 3, 1422–1434. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Claggett, B.L.; O’Meara, E.; Prescott, M.F.; Pfeffer, M.A.; Shah, S.J.; Redfield, M.M.; Zannad, F.; Chiang, L.M.; Rizkala, A.R.; et al. Effect of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients With HFpEF. J. Am. Coll. Cardiol. 2020, 76, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Croteau, D.; Qin, F.; Chambers, J.M.; Kallick, E.; Luptak, I.; Panagia, M.; Pimentel, D.R.; Siwik, D.A.; Colucci, W.S. Differential Effects of Sacubitril/Valsartan on Diastolic Function in Mice With Obesity-Related Metabolic Heart Disease. JACC Basic Transl. Sci. 2020, 5, 916–927. [Google Scholar] [CrossRef]
- Suematsu, Y.; Miura, S.i.; Goto, M.; Matsuo, Y.; Arimura, T.; Kuwano, T.; Imaizumi, S.; Iwata, A.; Yahiro, E.; Saku, K. LCZ696, an angiotensin receptorÔÇôneprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur. J. Heart Fail. 2016, 18, 386–393. [Google Scholar] [CrossRef]
- Schilling, J.D. Trimming the Fat in HFpEF: Learning How to Target the Metabolic Disease Phenotype. JACC Basic Transl. Sci. 2020, 5, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Morant-Talamante, N.; Lupón, J. Neprilysin and Natriuretic Peptide Regulation in Heart Failure. Curr. Heart Fail. Rep. 2016, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Aimo, A.; Castiglione, V.; Vergaro, G.; Georgiopoulos, G.; Saccaro, L.F.; Lombardi, C.M.; Passino, C.; Cerbai, E.; Metra, M.; et al. Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 1795–1807. [Google Scholar] [CrossRef]
- Krüger, M.; Kötter, S.; Grützner, A.; Lang, P.; Andresen, C.; Redfield, M.M.; Butt, E.; dos Remedios, C.G.; Linke, W.A. Protein Kinase G Modulates Human Myocardial Passive Stiffness by Phosphorylation of the Titin Springs. Cir. Res. 2009, 104, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Franssen, C.; Lourenco, A.; Falcao-Pires, I.; Fontoura, D.; Leite, S.; Plettig, L.; Lopez, B.; Ottenheijm, C.A.; Becher, P.M.; et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ. Heart Fail. 2013, 6, 1239–1249. [Google Scholar] [CrossRef]
- Michel, K.; Herwig, M.; Werner, F.; Spiranec Spes, K.; Abeßer, M.; Schuh, K.; Dabral, S.; Mügge, A.; Baba, H.A.; Skryabin, B.V.; et al. C-type natriuretic peptide moderates titin-based cardiomyocyte stiffness. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef]
- Wu, C.K.; Lee, J.K.; Hsu, J.C.; Su, M.Y.; Wu, Y.F.; Lin, T.T.; Lan, C.W.; Hwang, J.J.; Lin, L.Y. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 445–454. [Google Scholar] [CrossRef]
- Kang, D.; Park, S.; Shin, S.; Hong, G.; Lee, S.; Kim, M.; Yun, S.; Song, J.; Park, S.; Kim, J. Angiotensin Receptor Neprilysin Inhibitor for Functional Mitral Regurgitation. Circulation 2019, 139, 1354–1365. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Schmederer, Z.; Rolim, N.; Bowen, T.S.; Linke, A.; Wisloff, U.; Adams, V. Endothelial function is disturbed in a hypertensive diabetic animal model of HFpEF: Moderate continuous vs. high intensity interval training. Int. J. Cardiol. 2018, 273, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.; Cerqueira, R.J.; Ibarrola, J.; Fontoura, D.; Fern+índez-Celis, A.; Zannad, F.; Falc+úo-Pires, I.; Paulus, W.J.; Leite-Moreira, A.F.; Rossignol, P.; et al. Arterial Remodeling and Dysfunction in the ZSF1 Rat Model of Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e005596. [Google Scholar] [CrossRef] [PubMed]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; de Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.M.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Adams, V.; Linke, A.; Winzer, E. Skeletal muscle alterations in HFrEF vs HFpEF. Curr. Heart Fail. Rep. 2017, 14, 489–497. [Google Scholar] [CrossRef]
- Goto, K.; Schauer, A.; Augstein, A.; Methawasin, M.; Granzier, H.; Halle, M.; van Craenenbroeck, E.M.; Rolim, N.; Gielen, S.; Pieske, B.; et al. Muscular changes in animal models of heart failure with preserved ejection fraction: What comes closest to the patient? ESC Heart Fail. 2020. [Google Scholar] [CrossRef]
- Mirsky, I.; Pasipoularides, A. Clinical assessment of diastolic function. Prog. Cardiovasc. Dis. 1990, 32, 291–318. [Google Scholar] [CrossRef]
- Dai, Z.; Aoki, T.; Fukumoto, Y.; Shimokawa, H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J. Cardiol. 2012, 60, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Buttner, P.; Galli, R.; Husser, D.; Bollmann, A. Label-free Imaging of Myocardial Remodeling in Atrial Fibrillation Using Nonlinear Optical Microscopy: A Feasibility Study. J. Atr. Fibrillation 2018, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Pezacki, J.P.; Blake, J.A.; Danielson, D.C.; Kennedy, D.C.; Lyn, R.K.; Singaravelu, R. Chemical contrast for imaging living systems: Molecular vibrations drive CARS microscopy. Nat. Chem. Biol. 2011, 7, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Vogler, N.; Heuke, S.; Bocklitz, T.W.; Schmitt, M.; Popp, J. Multimodal Imaging Spectroscopy of Tissue. Annu. Rev. Anal. Chem. 2015, 8, 359–387. [Google Scholar] [CrossRef]
- Vanzi, F.; Sacconi, L.; Cicchi, R.; Pavone, F.S. Protein conformation and molecular order probed by second-harmonic-generation microscopy. J. Biomed. Opt. 2012, 17, 60901. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
| Physiology | Lean (n = 10) | Obese (n = 10) | p-Value |
|---|---|---|---|
| Body weight (g) | 239 ± 4 | 474 ± 8 | <0.001 |
| Tibia length (TL, mm) | 36.25 ± 0.19 | 36.62 ± 0.12 | 0.8 |
| Heart weight/TL (mg/mm) | 23.05 ± 0.31 | 32.08 ± 0.34 | <0.001 |
| Kidney weight/TL (mg/mm) | 24.85 ± 0.65 | 41.34 ± 1.08 | <0.001 |
| Serum glucose (mg/dL) | 275 ± 14 | 444 ± 35 | <0.001 |
| Serum NT-proBNP (pg/mL) | 42.67 ± 10.13 | 99.94 ± 22.62 | 0.03 |
| Echocardiography | |||
| Left ventricle (LV) weight (mg) | 721 ± 21 | 1009 ± 28 | <0.001 |
| LVEF (%) | 77 ± 2 | 80 ± 1 | 0.06 |
| LVFS (%) | 57 ± 2 | 60 ± 2 | 0.32 |
| LVESV (µL) | 339 ± 12 | 422 ± 18 | <0.001 |
| LVEDV (µL) | 444 ± 18 | 525 ± 21 | <0.01 |
| E/é | 19.8 ± 0.5 | 25.0 ± 0.7 | <0.001 |
| E/A | 1.3 ± 0.04 | 1.5 ± 0.1 | 0.07 |
| LAA (mm2) | 11.4 ± 0.6 | 11.3 ± 0.6 | 0.6 |
| LVAW (mm) | 1.8 ± 0.1 | 2.2 ± 0.1 | <0.001 |
| LVPW (mm) | 1.6 ± 0.1 | 2.0 ± 0.1 | <0.001 |
| LVEDD (mm) | 6.4 ± 0.2 | 7.1 ± 0.1 | 0.06 |
| Invasive hemodynamics | |||
| Ascending aorta | |||
| SAP (mmHg) | 116 ± 5 | 152 ± 8 | <0.01 |
| DAP (mmHg) | 86 ± 4 | 98 ± 6 | 0.1 |
| MAP (mmHg) | 99 ± 6 | 116 ± 5 | 0.07 |
| Left ventricle | |||
| Heart frequency [bpm) | 238 ± 7 | 217 ± 8 | 0.1 |
| LVEDP (mmHg) | 15 ± 2 | 20 ± 1 | <0.05 |
| LVESP (mmHg) | 108 ± 7 | 146 ± 6 | <0.01 |
| LVEDV (µL) | 367 ± 25 | 441 ± 13 | <0.01 |
| LVESV (µL) | 106 ± 16 | 136 ± 16 | 0.2 |
| dP/dt max (mmHg/s) | 5986 ± 378 | 9421 ± 914 | <0.001 |
| dP/dt min (mmHg/s) | −5588 ± 181 | −7361 ± 183 | <0.001 |
| dV/dt max (µL/s) | 11,404 ± 1044 | 10,505 ± 1478 | 0.7 |
| dV/dt min (µL/s) | −8424 ± 569 | −10,168 ± 1154 | 0.3 |
| Tau (ms) | 18 ± 1 | 17 ± 1 | 0.7 |
| Right ventricle | |||
| RVEDP (mmHg) | 16 ± 1 | 17 ± 5 | 0.7 |
| RVESP (mmHg) | 42 ± 3 | 46 ± 5 | 0.5 |
| RVEDV (µL) | 108 ± 24 | 173 ± 28 | <0.05 |
| RVESV (µL) | 79 ± 19 | 120 ± 17 | <0.05 |
| dP/dt max (mmHg/s) | 1680 ± 231 | 2285 ± 118 | 0.08 |
| dP/dt min (mmHg/s) | −1250 ± 159 | −2355 ± 471 | <0.05 |
| dV/dt max (µL/s) | 909 ± 76 | 1297 ± 346 | 0.2 |
| dV/dt min (µL/s) | −1009 ± 152 | −1192 ± 233 | 0.5 |
| Tau (ms) | 38 ± 5 | 34 ± 9 | 0.5 |
| Physiology | Lean (n = 15) | Obese (n = 15) | Sac/Val (n = 13) |
|---|---|---|---|
| Body weight [g) | 265 ± 4 | 559 ± 9 *** | 563 ± 8 *** |
| Tibia length (TL, mm) | 38.2 ± 0.1 | 38.0 ± 0.1 | 37.6 ± 0.2 |
| Heart weight/TL (mg/mm) | 23.54 ± 0.31 | 35.44 ± 0.50 *** | 33.22 ± 0.42 ***,ƗƗ |
| Kidney weight/TL (mg/mm) | 23.96 ± 0.45 | 43.21 ± 1.01 *** | 47.51 ± 1.03 ***, ƗƗ |
| Serum glucose (mg/dl) | 324 ± 20 | 571 ± 21 *** | 577 ± 31 *** |
| Serum HbA1c (ng/ml) | 4.69 ± 0.43 | 7.35 ± 0.95 * | 5.95 ± 0.70 |
| Serum NT-proBNP (pg/ml) | 96.96 ± 9.42 | 209.04 ± 38.26 * | 154.21 ± 28.12 |
| Echocardiography | |||
| LV weight (mg) | 890 ± 11 | 1274 ± 20 *** | 1070 ± 13 ***,ƗƗƗ |
| LVEF (%) | 79.0 ± 1.3 | 74.3 ± 1.2 ** | 80.5 ± 0.9 ƗƗƗ |
| LVFS (%) | 52 ± 1.7 | 54 ± 1.3 | 53 ± 1.4 |
| LVESV (µL) | 441 ± 28 | 511 ± 17 * | 469 ± 16 |
| LVEDV (µL) | 555 ± 34 | 691 ± 25 *** | 585 ± 17 ƗƗƗ |
| E/é | 21.6 ± 0.5 | 27.9 ± 0.7 *** | 22.8 ± 0.5 ƗƗƗ |
| E/A | 1.2 ± 0.1 | 1.4 ± 0.1 ** | 1.4 ± 0.1 |
| LAA (mm2) | 17 ± 1.1 | 21 ± 0.8 ** | 19 ± 1.2 |
| LVAW (mm) | 1.76 ± 0.06 | 2.03 ± 0.07 ** | 1.86 ± 0.05 |
| LVPW (mm) | 1.66 ± 0.08 | 2.02 ± 0.09 ** | 1.95 ± 0.07 * |
| LVEDD (mm) | 6.67 ± 0.11 | 7.97 ± 0.19 *** | 7.96 ± 0.21 *** |
| Invasive Hemodynamics | n = 12 | n = 11 | n = 9 |
| Ascending aorta | |||
| SAP (mmHg) | 125 ± 6 | 180 ± 4 *** | 154 ± 4 **, ƗƗƗ |
| DAP (mmHg) | 95 ± 5 | 113 ± 3 ** | 102 ± 3 ƗƗ |
| MAP (mmHg) | 105 ± 6 | 136 ± 3 *** | 120 ± 3 *, ƗƗ |
| Left ventricle | |||
| Heart frequency (bpm) | 226 ± 6 | 210 ± 14 | 222 ± 9 |
| LVEDP (mmHg) | 15 ± 1 | 22 ± 1*** | 16 ± 1 ƗƗ |
| LVESP (mmHg) | 113 ± 8 | 165 ± 5 *** | 137 ± 10 ƗƗ |
| LVEDV (µL) | 434 ± 29 | 622 ± 47** | 475 ± 27 Ɨ |
| LVESV (µL) | 134 ± 17 | 291 ± 31 *** | 172 ± 18 ƗƗ |
| dP/dt max (mmHg/s) | 5791 ± 310 | 9864 ± 411 *** | 10,042 ± 412 *** |
| dP/dt min (mmHg/s) | −6055 ± 411 | −8148 ± 323 *** | −7549 ± 483 * |
| dV/dt max (µL/s) | 14,972 ± 1752 | 15,263 ± 926 | 12,636 ± 1622 |
| dV/dt min (µL/s) | −12,495 ± 1344 | −13,666 ± 1874 | −11,082 ± 887 |
| Tau (ms) | 17 ± 1 | 17 ± 1 | 17 ± 1 |
| Right ventricle | |||
| RVEDP (mmHg) | 15 ± 0.7 | 16 ± 0.8 | 18 ± 1.8 |
| RVESP (mmHg) | 35 ± 0.9 | 44 ± 1.3 *** | 45 ± 3.4 ** |
| RVEDV (µL) | 153 ± 15 | 314 ± 26 *** | 204 ± 17 ƗƗ |
| RVESV (µL) | 113 ± 14 | 200 ± 24 ** | 161 ± 17 * |
| dP/dt max (mmHg/s) | 1193 ± 23 | 2123 ± 81 *** | 2293 ± 79 *** |
| dP/dt min (mmHg/s) | −1008 ± 45 | −1248 ± 94 * | −1159 ± 84 |
| dV/dt max (µL/s) | 1441 ± 157 | 2285 ± 198 ** | 1103 ± 137 ƗƗƗ |
| dV/dt min (µL/s) | −1283 ± 153 | −2331 ± 197 *** | −1182 ± 127 ƗƗƗ |
| Tau (ms) | 40 ± 2 | 39 ± 3 | 40 ± 4 |
| Gene | Primer 1 | Primer 2 | Gene bank ID |
|---|---|---|---|
| ANP | TCCCGTATACAGTGCGGTGTC | GGAGGCATGACCTCATCTTC | NM_012612 |
| BNP | ACAATCCACGATGCAGAAGC | GAAGGCGCTGTCTTGAGACC | NM_031545 |
| Col1A1 | CTGCACGAGTCACACCGGAA | CCAATGTCCAAGGGAGCCAC | NM_053304 |
| Col3A1 | TGGCTGCACTAAACACACTG | CCAATGTCATAGGGTGCGAT | NM_032085 |
| Rpl-32 | GGTGAAGCCCAAGATCGTCAA | TCTGGGTTTCCGCCAGTTTC | NM_013226.2 |
| Polr2a | GGTATTGAGCAGATCAGCAAGG | CAATGCCCAGTACCGTGAAG | XM_343922 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schauer, A.; Adams, V.; Augstein, A.; Jannasch, A.; Draskowski, R.; Kirchhoff, V.; Goto, K.; Mittag, J.; Galli, R.; Männel, A.; et al. Sacubitril/Valsartan Improves Diastolic Function But Not Skeletal Muscle Function in a Rat Model of HFpEF. Int. J. Mol. Sci. 2021, 22, 3570. https://doi.org/10.3390/ijms22073570
Schauer A, Adams V, Augstein A, Jannasch A, Draskowski R, Kirchhoff V, Goto K, Mittag J, Galli R, Männel A, et al. Sacubitril/Valsartan Improves Diastolic Function But Not Skeletal Muscle Function in a Rat Model of HFpEF. International Journal of Molecular Sciences. 2021; 22(7):3570. https://doi.org/10.3390/ijms22073570
Chicago/Turabian StyleSchauer, Antje, Volker Adams, Antje Augstein, Anett Jannasch, Runa Draskowski, Virginia Kirchhoff, Keita Goto, Jeniffer Mittag, Roberta Galli, Anita Männel, and et al. 2021. "Sacubitril/Valsartan Improves Diastolic Function But Not Skeletal Muscle Function in a Rat Model of HFpEF" International Journal of Molecular Sciences 22, no. 7: 3570. https://doi.org/10.3390/ijms22073570
APA StyleSchauer, A., Adams, V., Augstein, A., Jannasch, A., Draskowski, R., Kirchhoff, V., Goto, K., Mittag, J., Galli, R., Männel, A., Barthel, P., Linke, A., & Winzer, E. B. (2021). Sacubitril/Valsartan Improves Diastolic Function But Not Skeletal Muscle Function in a Rat Model of HFpEF. International Journal of Molecular Sciences, 22(7), 3570. https://doi.org/10.3390/ijms22073570







