Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines
Abstract
1. Introduction
2. Results
2.1. Methyl-Donors Affect Tumor Cell Proliferation
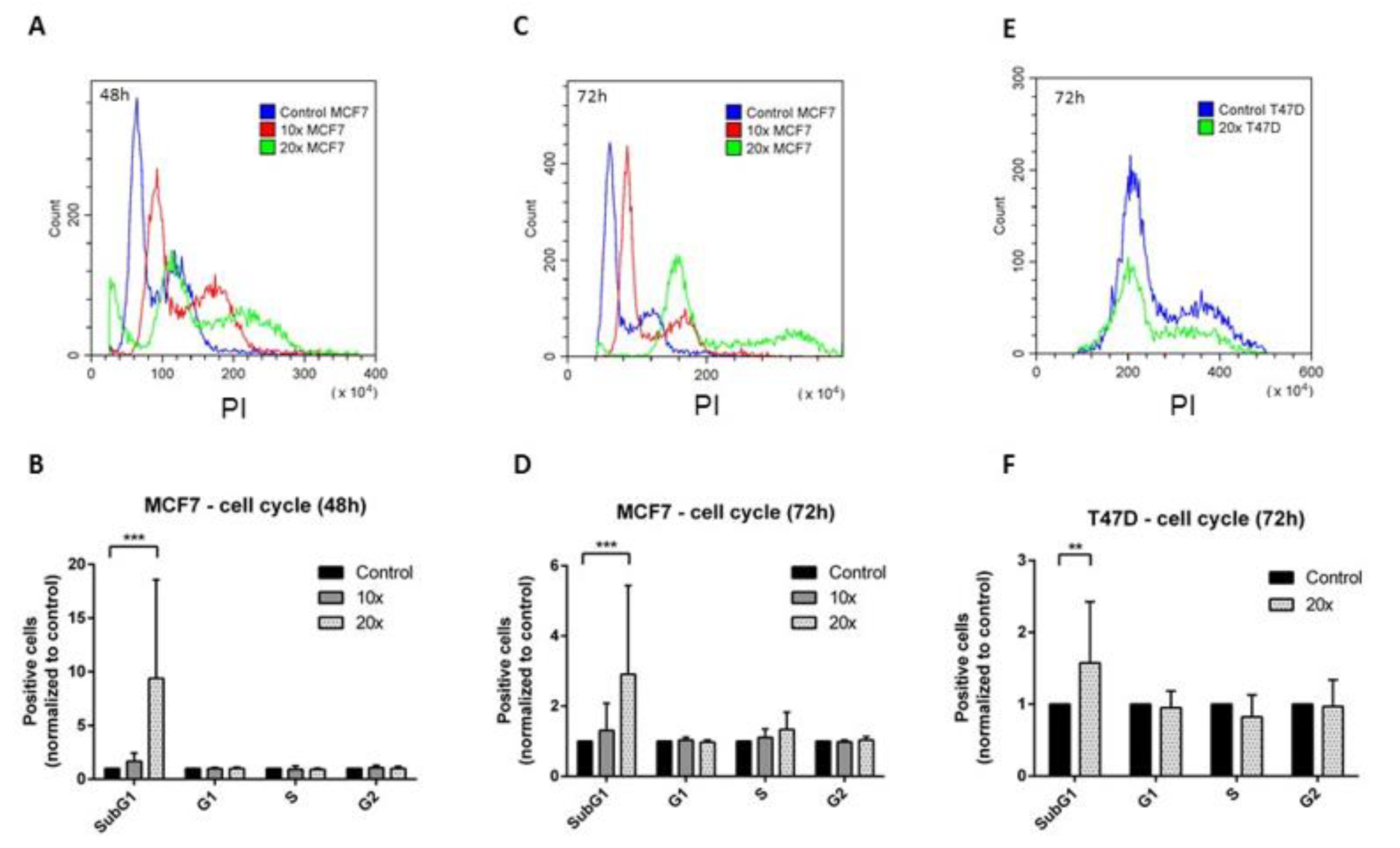
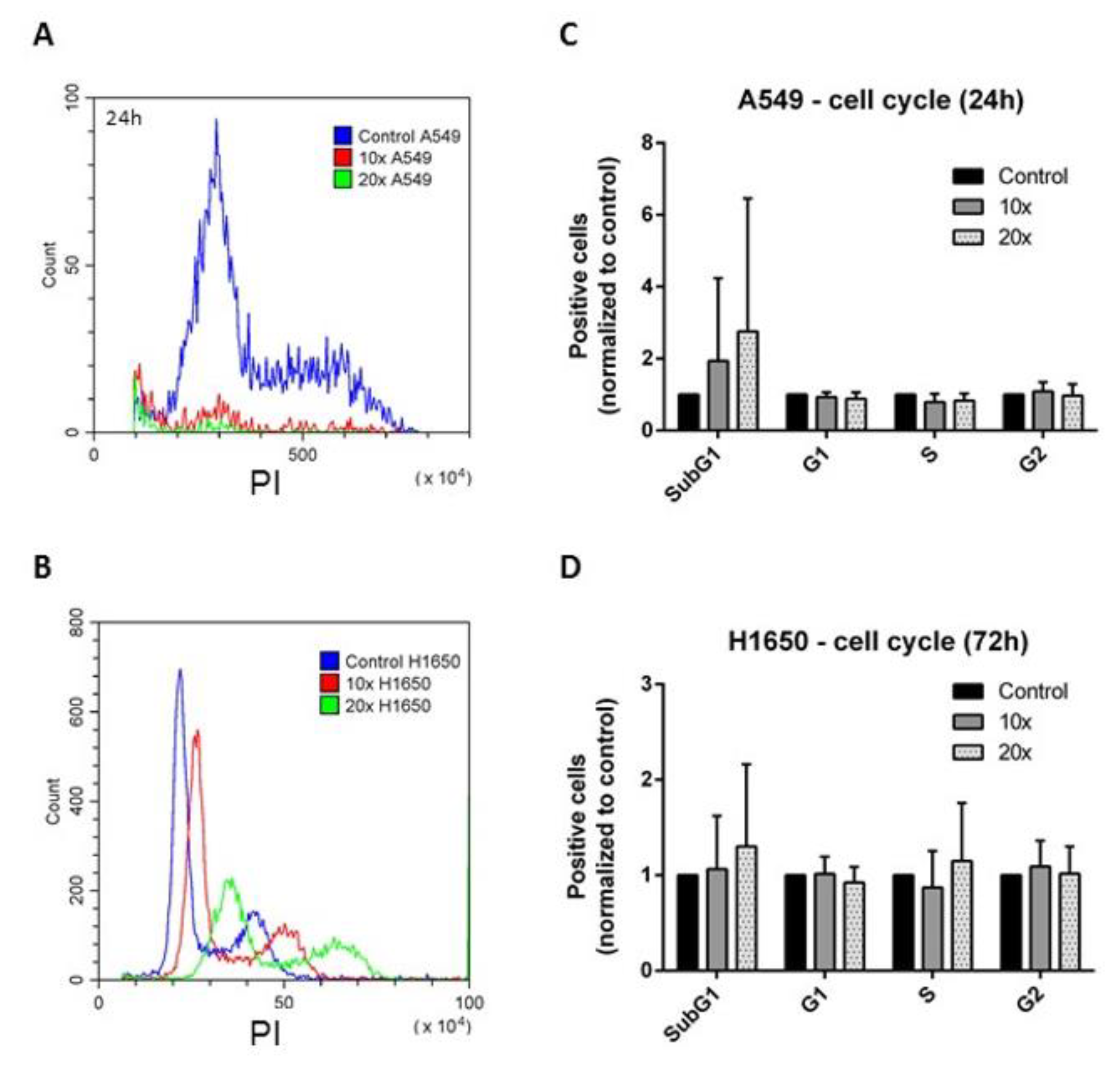
2.2. The Effects of the Methyl-Donors on the Cell Cycle
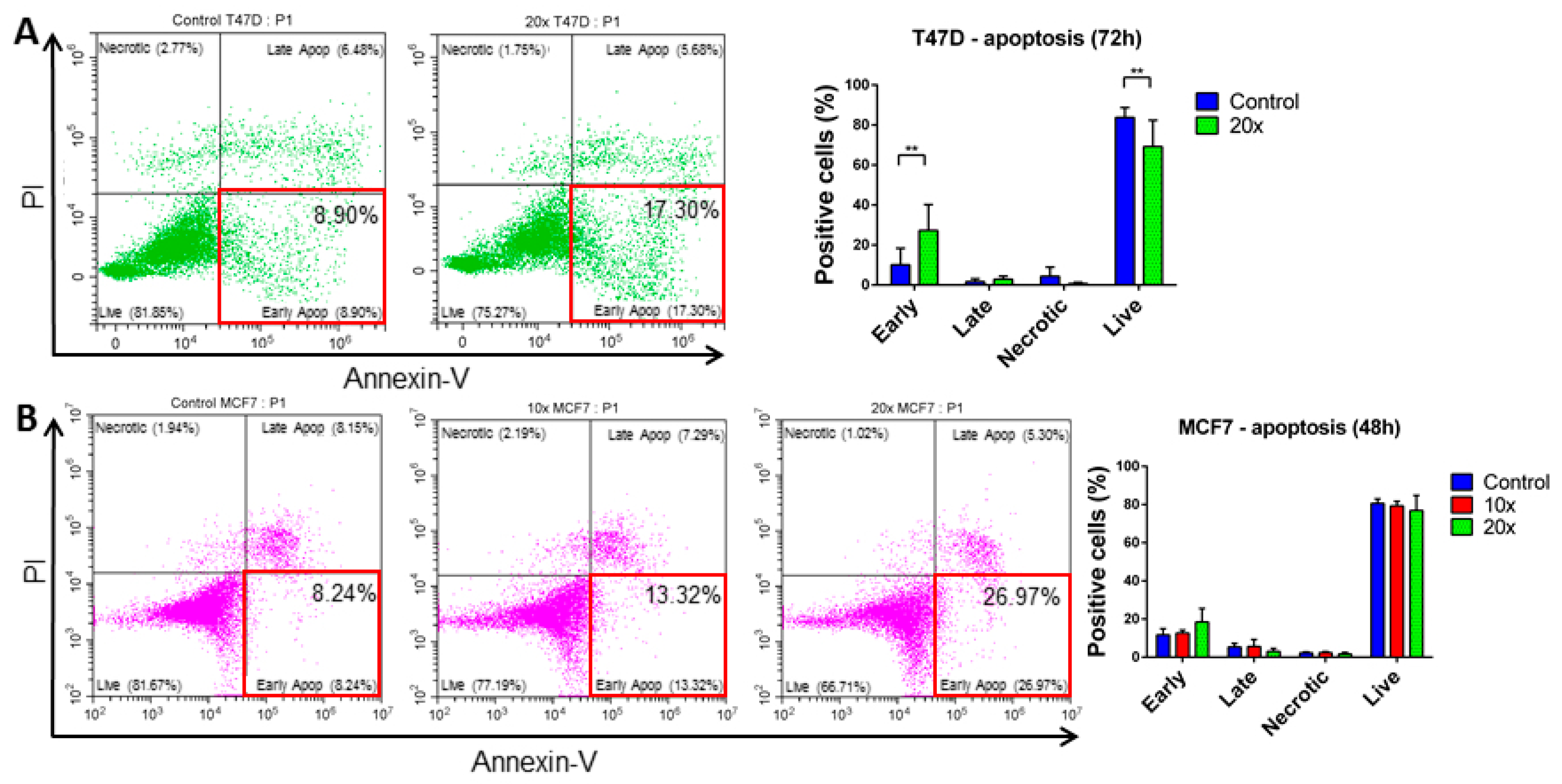
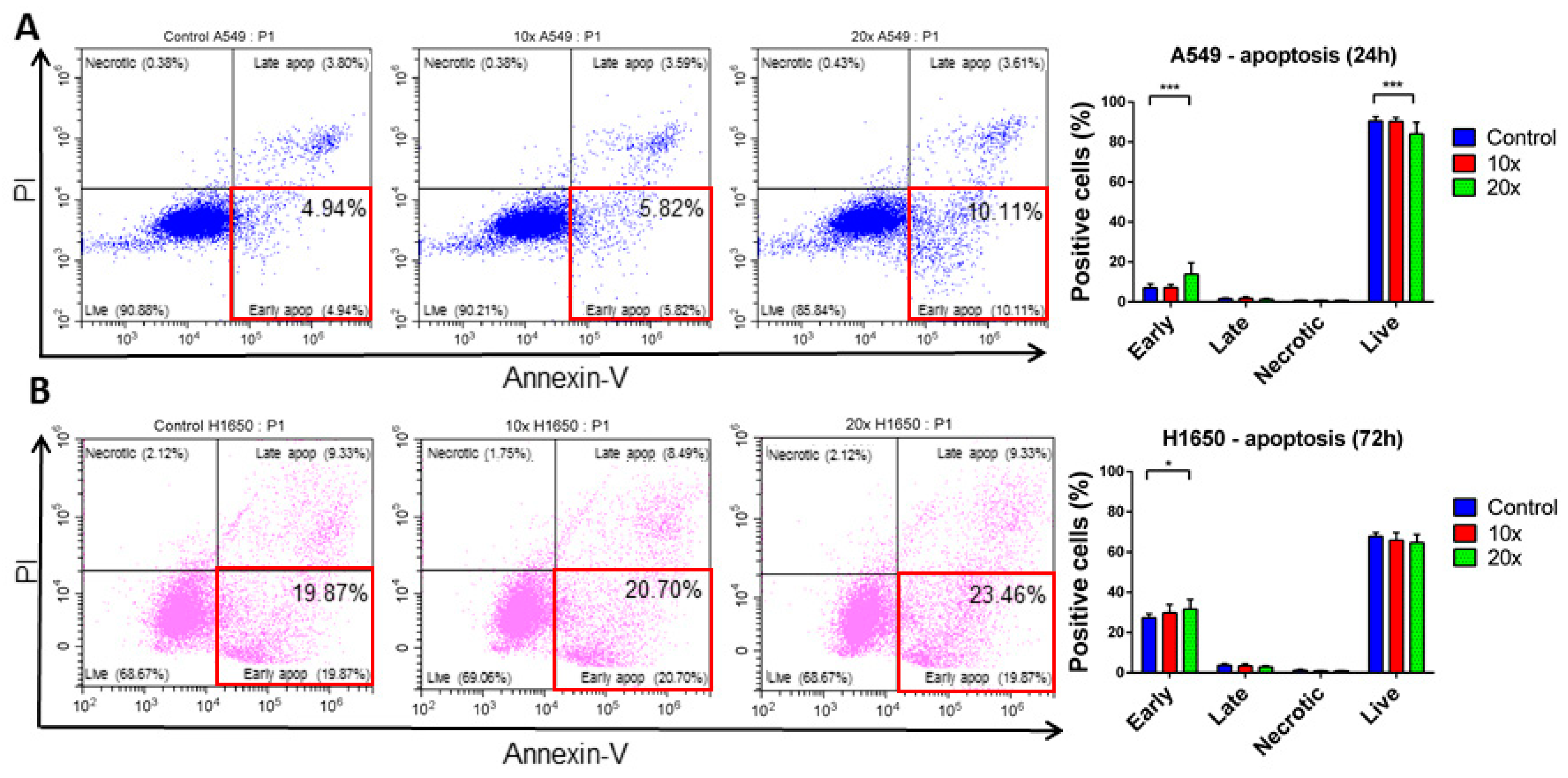
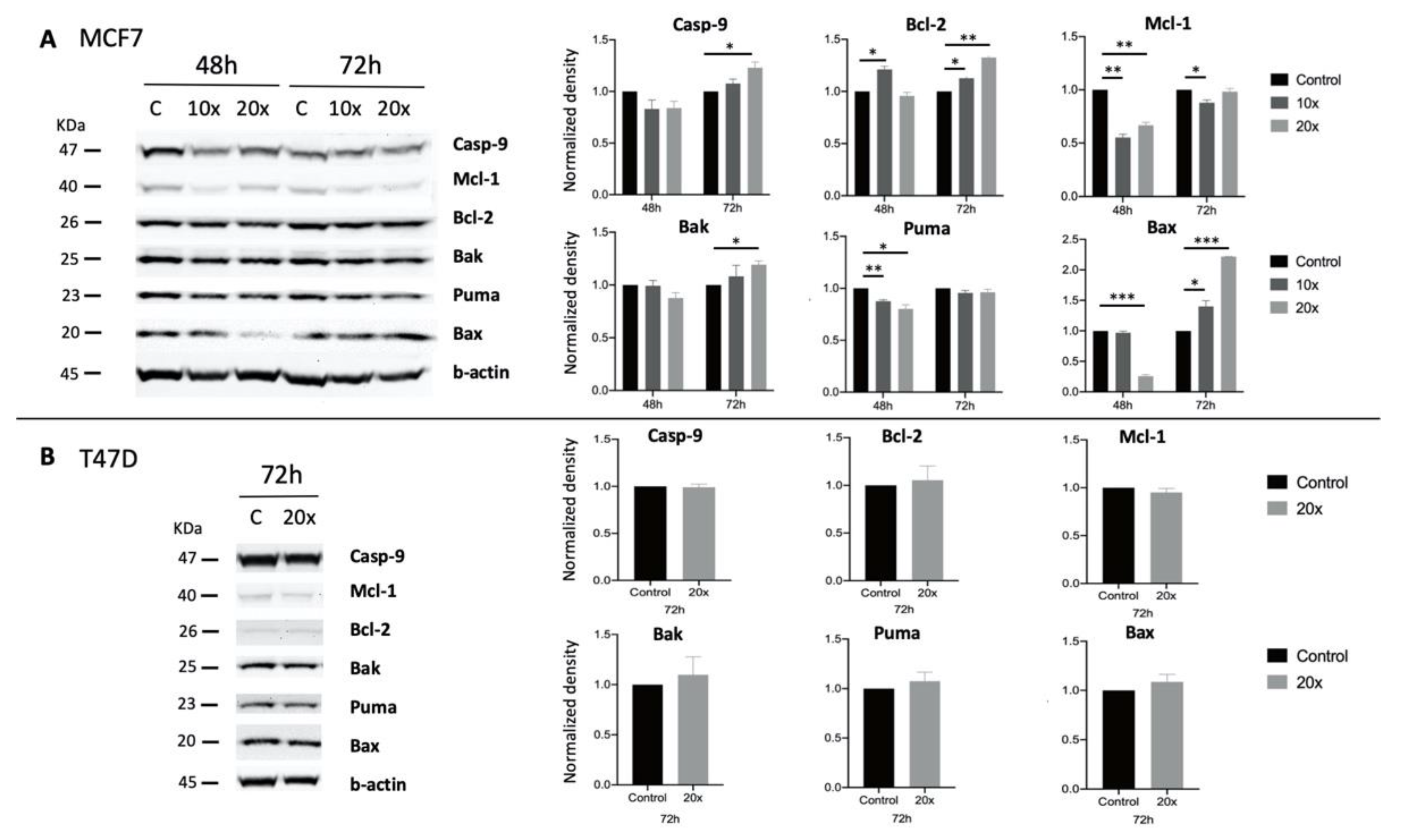
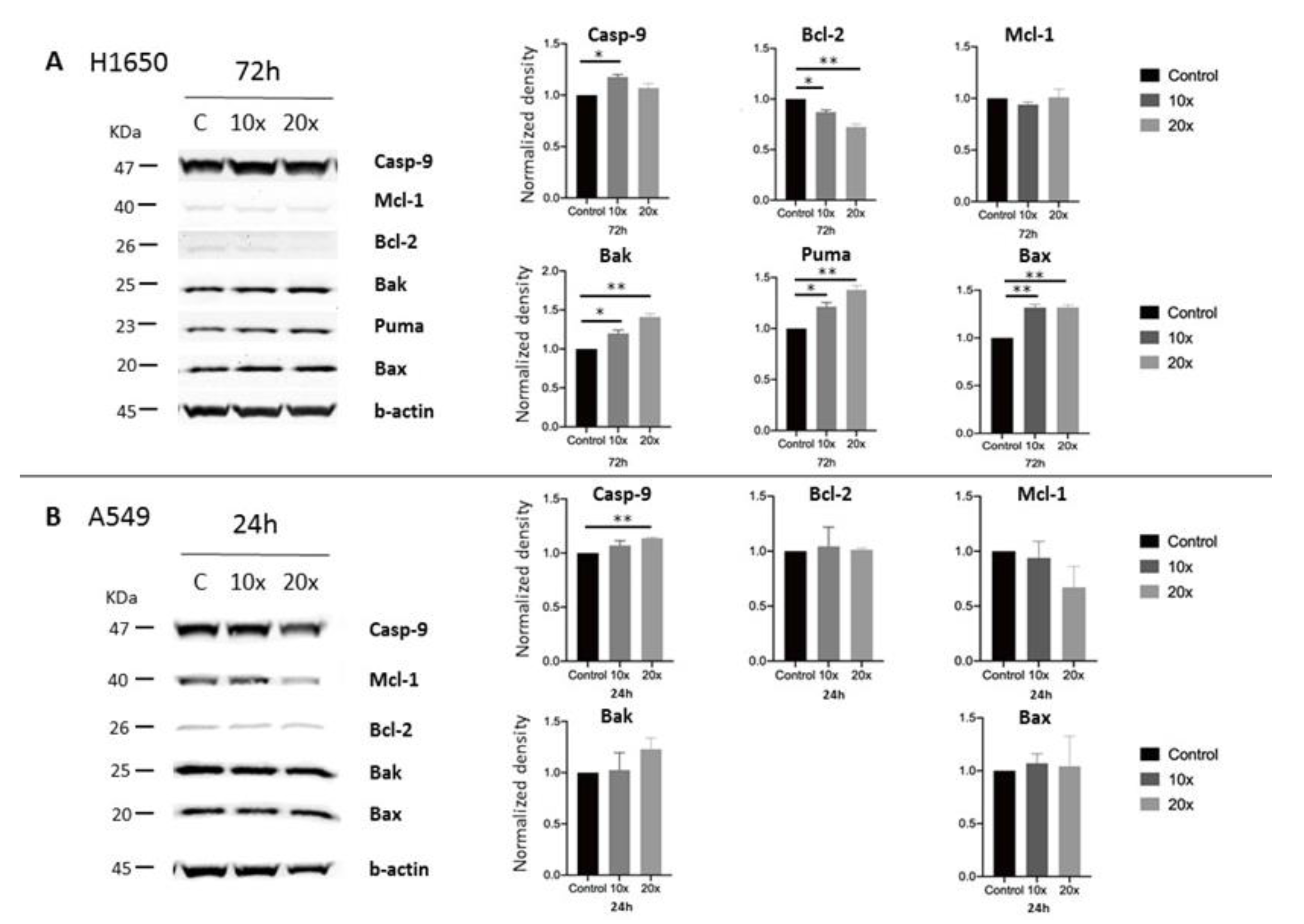
2.3. Detection of Apoptosis and Related Pathway Elements after Methyl-Donor Treatments
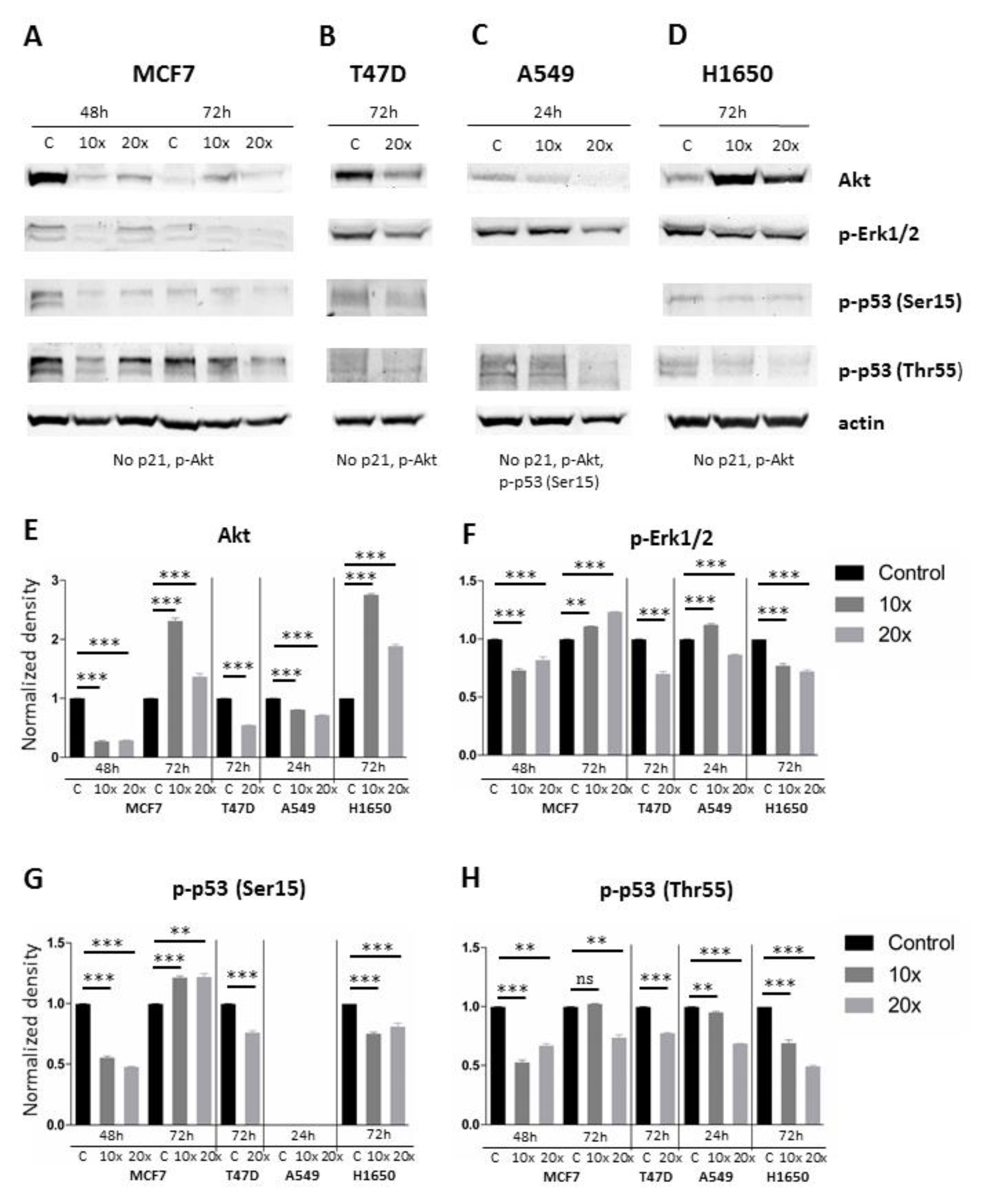
2.4. Investigating the MAPK/ERK and AKT Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Cell Culture Conditions
4.2. Methyl-Donor Treatments
4.3. Cell Proliferation Assay
4.4. Detection of Apoptosis
4.5. Cell Cycle Measurement
4.6. Western Blot
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- de Vogel, S.; Dindore, V.; van Engeland, M.; Goldbohm, R.A.; van den Brandt, P.A.; Weijenberg, M.P. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J. Nutr. 2008, 138, 2372–2378. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- NIDDK. Vitamin B. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; Updated 2016-May-9 ed.; National Institute of Diabetes and Digestive and Kidney Disease (NIDDK): Bethesda, MD, USA, 2012. [Google Scholar]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Trimboli, R.M.; Rossi, P.G.; Battisti, N.M.L.; Cozzi, A.; Magni, V.; Zanardo, M.; Sardanelli, F. Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging 2020, 11, 105. [Google Scholar] [CrossRef]
- Chen, P.; Li, C.; Li, X.; Li, J.; Chu, R.; Wang, H. Higher dietary folate intake reduces the breast cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2014, 110, 2327–2338. [Google Scholar] [CrossRef]
- Zeng, J.; Gu, Y.; Fu, H.; Liu, C.; Zou, Y.; Chang, H. Association between One-carbon Metabolism-related Vitamins and Risk of Breast Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Clin. Breast Cancer 2020, 20, e469–e480. [Google Scholar] [CrossRef]
- Wu, W.; Kang, S.; Zhang, D. Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: A dose-response meta-analysis. Br. J. Cancer 2013, 109, 1926–1944. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Deng, H.; Wang, Z. Association of One-Carbon Metabolism-Related Vitamins (Folate, B6, B12), Homocysteine and Methionine with the Risk of Lung Cancer: Systematic Review and Meta-Analysis. Front. Oncol. 2018, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Ben, S.; Du, M.; Ma, G.; Qu, J.; Zhu, L.; Chu, H.; Zhang, Z.; Wu, Y.; Gu, D.; Wang, M. Vitamin B2 intake reduces the risk for colorectal cancer: A dose-response analysis. Eur. J. Nutr. 2019, 58, 1591–1602. [Google Scholar] [CrossRef]
- Ben, S.; Du, M.; Ma, G.; Qu, J.; Zhu, L.; Chu, H.; Zhang, Z.; Wu, Y.; Gu, D.; Wang, M. A dose-response meta-analysis reveals an association between vitamin B12 and colorectal cancer risk. Public Health Nutr. 2016, 19, 1446–1456. [Google Scholar]
- Bo, Y.; Zhu, Y.; Tao, Y.; Li, X.; Zhai, D.; Bu, Y.; Wan, Z.; Wang, L.; Wang, Y.; Yu, Z. Association Between Folate and Health Outcomes: An Umbrella Review of Meta-Analyses. Front. Public Health 2020, 8, 550753. [Google Scholar] [CrossRef]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Zeisel, S.H. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002, 132 (Suppl. S8), 2333S–2335S. [Google Scholar] [CrossRef]
- Scotti, M.; Stella, L.; Shearer, E.J.; Stover, P.J. Modeling cellular compartmentation in one-carbon metabolism. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Q.; Zhang, L.; Shi, D.; Wang, H.; Wang, S.; Blan, B. Folate-targeted PTEN/AKT/P53 signaling pathway promotes apoptosis in breast cancer cells. Pteridines 2020, 31, 158–164. [Google Scholar] [CrossRef]
- Bhanumathi, R.; Manivannan, M.; Thangaraj, R.; Kannan, S. Drug-Carrying Capacity and Anticancer Effect of the Folic Acid- and Berberine-Loaded Silver Nanomaterial To Regulate the AKT-ERK Pathway in Breast Cancer. ACS Omega 2018, 3, 8317–8328. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.M.; Meinkoth, J.; Pittman, R.N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 2000, 151, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanism of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Castro-Piedras, I.; Vartak, D.; Sharma, M.; Pandey, S.; Casas, L.; Molehin, D.; Rasha, F.; Fokar, M.; Nichols, J.; Almodovar, S.; et al. Identification of Novel MeCP2 Cancer-Associated Target Genes and Post-Translational Modifications. Front. Oncol. 2020, 10, 576362. [Google Scholar] [CrossRef]
- Mahmood, N.; Rabbani, S.A. DNA Methylation Readers and Cancer: Mechanistic and Therapeutic Applications. Front. Oncol. 2019, 9, 489. [Google Scholar] [CrossRef]
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J. Breast Cancer 2013, 16, 23–31. [Google Scholar] [CrossRef]
- Pandey, M.; Shukla, S.; Gupta, S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int. J. Cancer 2010, 126, 2520–2533. [Google Scholar] [CrossRef]
- Mustachio, L.M.; Roszik, J. Current Targeted Therapies for the Fight against Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 347. [Google Scholar] [CrossRef]
- Singh, N.; Baldi, M.; Kaur, J.; Muthu, V.; Prasad, K.T.; Behera, D.; Bal, A.; Gupta, N.; Kapoor, R. Timing of folic acid/vitamin B12 supplementation and hematologic toxicity during first-line treatment of patients with nonsquamous non-small cell lung cancer using pemetrexed-based chemotherapy: The PEMVITASTART randomized trial. Cancer 2019, 125, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.; Davies, A.M.; Evans, W.K.; Haynes, A.E.; Lloyd, N.S. Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based C. The use of chemotherapy in patients with advanced malignant pleural mesothelioma: A systematic review and practice guideline. J. Thorac Oncol. 2006, 1, 591–601. [Google Scholar] [CrossRef][Green Version]
- Adjei, A.A. Pharmacology and mechanism of action of pemetrexed. Clin. Lung Cancer 2004, 5 (Suppl. S2), S51–S55. [Google Scholar] [CrossRef]
- Yang, T.Y.; Chang, G.C.; Hsu, S.L.; Huang, Y.R.; Chiu, L.Y.; Sheu, G.T. Effect of folic acid and vitamin B12 on pemetrexed antifolate chemotherapy in nutrient lung cancer cells. Biomed. Res. Int. 2013, 2013, 389046. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Park, C.S.; Cho, K.; Bae, D.R.; Joo, N.E.; Kim, H.H.; Mabasa, L.; Fowler, A.W. Methyl-donor nutrients inhibit breast cancer cell growth. Vitro Cell. Dev. Biol. Anim. 2008, 44, 268–272. [Google Scholar] [CrossRef]
- Galluzzi, L.; Marsili, S.; Vitale, I.; Senovilla, L.; Michels, J.; Garcia, P.; Vacchelli, E.; Chatelut, E.; Castedo, M.; Kroemer, G. Vitamin B6 metabolism influences the intracellular accumulation of cisplatin. Cell Cycle 2013, 12, 417–421. [Google Scholar] [CrossRef]
- Liu, X.; Montissol, S.; Uber, A.; Ganley, S.; Grossestreuer, A.V.; Berg, K.; Heydrick, S.; Donnino, M.W. The Effects of Thiamine on Breast Cancer Cells. Molecules 2018, 23, 1464. [Google Scholar] [CrossRef]
- Cao, Z.; Liao, Q.; Su, M.; Huang, K.; Jin, J.; Cao, D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019, 459, 30–40. [Google Scholar] [CrossRef]
- Allan, L.A.; Clarke, P.R. Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. FEBS J. 2009, 276, 6063–6073. [Google Scholar] [CrossRef]
- Hinz, N.; Jucker, M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell Commun. Signal. 2019, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Riggio, M.; Polo, M.L.; Blaustein, M.; Colman-Lerner, A.; Lüthy, I.; Lanari, C.; Novaro, V. PI3K/AKT pathway regulates phosphorylation of steroid receptors, hormone independence and tumor differentiation in breast cancer. Carcinogenesis 2012, 33, 509–518. [Google Scholar] [CrossRef]
- Liu, H.; Radisky, D.C.; Nelson, C.M.; Zhang, H.; Fata, J.E.; Roth, R.A.; Bissell, M.J. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc. Natl. Acad. Sci. USA 2006, 103, 4134–4139. [Google Scholar] [CrossRef]
- Gargini, R.; Cerliani, J.P.; Escoll, M.; Anton, I.M.; Wandosell, F. Cancer stem cell-like phenotype and survival are coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells. 2015, 33, 646–660. [Google Scholar] [CrossRef]
- Polytarchou, C.; Iliopoulos, D.; Hatziapostolou, M.; Kottakis, F.; Maroulakou, I.; Struhl, K.; Tsichlis, P.N. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res. 2011, 71, 4720–4731. [Google Scholar] [CrossRef]
- Santi, S.A.; Lee, H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS ONE 2011, 6, e14614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, G.; Chen, Y.; Fang, L.; Guan, C.; Bai, F.; Ma, M.; Lyu, J.; Meng, Q.H. Metformin Inhibits Tumorigenesis and Tumor Growth of Breast Cancer Cells by Upregulating miR-200c but Downregulating AKT2 Expression. J. Cancer 2017, 8, 1849–1864. [Google Scholar] [CrossRef]
- Pena-Blanco, A.; Garcia-Saez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Domina, A.M.; Smith, J.H.; Craig, R.W. Myeloid cell leukemia 1 is phosphorylated through two distinct pathways, one associated with extracellular signal-regulated kinase activation and the other with G2/M accumulation or protein phosphatase 1/2A inhibition. J. Biol. Chem. 2000, 275, 21688–21694. [Google Scholar] [CrossRef]
- Domina, A.M.; Vrana, J.A.; Gregory, M.A.; Hann, S.R.; Craig, R.W. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene 2004, 23, 5301–5315. [Google Scholar] [CrossRef]
- Yu, C.; Bruzek, L.M.; Meng, X.W.; Gores, G.J.; Carter, C.A.; Kaufmann, S.H.; Adjei, A.A. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene 2005, 24, 6861–6869. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Kanai, M.; Inoue-Yamauchi, A.; Tu, H.C.; Huang, Y.; Ren, D.; Kim, H.; Takeda, S.; Reyna, D.E.; Chan, P.M.; et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat. Cell Biol. 2015, 17, 1270–1281. [Google Scholar] [CrossRef]
- Cromie, M.M.; Liu, Z.; Gao, W. Epigallocatechin-3-gallate augments the therapeutic effects of benzo[a]pyrene-mediated lung carcinogenesis. Biofactors 2017, 43, 529–539. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X. Inhibition of Thr-55 phosphorylation restores p53 nuclear localization and sensitizes cancer cells to DNA damage. Proc. Natl. Acad. Sci. USA 2008, 105, 16958–16963. [Google Scholar] [CrossRef]
- Gao, W.; Lu, C.; Chen, L.; Keohavong, P. Overexpression of CRM1: A Characteristic Feature in a Transformed Phenotype of Lung Carcinogenesis and a Molecular Target for Lung Cancer Adjuvant Therapy. J. Thorac Oncol. 2015, 10, 815–825. [Google Scholar] [CrossRef]
- Shieh, S.Y.; Ikeda, M.; Taya, Y.; Prives, C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997, 91, 325–334. [Google Scholar] [CrossRef]
- Lu, C.; Shao, C.; Cobos, E.; Singh, K.P.; Gao, W. Chemotherapeutic sensitization of leptomycin B resistant lung cancer cells by pretreatment with doxorubicin. PLoS ONE 2012, 7, e32895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cromie, M.M.; Cai, Q.; Lv, T.; Singh, K.; Gao, W. Curcumin and vitamin E protect against adverse effects of benzo[a]pyrene in lung epithelial cells. PLoS ONE 2014, 9, e92992. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, J.H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of High-dose Vitamin C Combined with Anti-cancer Treatment on Breast Cancer Cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kurbacher, C.M.; Wagner, U.; Kolster, B.; Andreotti, P.E.; Krebs, D.; Bruckner, H.W. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 1996, 103, 183–189. [Google Scholar] [CrossRef]
- Chen, N.; Yin, S.; Song, X.; Fan, L.; Hu, H. Vitamin B(2) Sensitizes Cancer Cells to Vitamin-C-Induced Cell Death via Modulation of Akt and Bad Phosphorylation. J. Agric. Food Chem. 2015, 63, 6739–6748. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Gatter, K.C.; Harris, A.L.; Tumor and Angiogenesis Research Group. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia 2005, 7, 1–6. [Google Scholar] [CrossRef]
- Lin, H.J.; Hsieh, F.C.; Song, H.; Lin, J. Elevated phosphorylation and activation of PDK-1/AKT pathway in human breast cancer. Br. J. Cancer 2005, 93, 1372–1381. [Google Scholar] [CrossRef]
- Rubinstein, P. HLA and IDDM: Facts and speculations on the disease gene and its mode of inheritance. Hum. Immunol. 1991, 30, 270–277. [Google Scholar] [CrossRef]
- Pereira, P.T.V.T.; Reis, A.D.; Diniz, R.R.; Lima, F.A.; Leite, R.D.; da Silva, M.C.P.; Guerra, R.N.M.; de Moraes Vieira, É.B.; Garcia, J.B.S. Dietary supplements and fatigue in patients with breast cancer: A systematic review. Breast Cancer Res. Treat. 2018, 171, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Skinner, T.L.; Wright, O.R.L. Nutrition therapy for the management of cancer-related fatigue and quality of life: A systematic review and meta-analysis. Br. J. Nutr. 2019, 122, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Colacino, J.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Uphoff, C.C.; Drexler, H.G. Detection of mycoplasma in leukemia-lymphoma cell lines using polymerase chain reaction. Leukemia 2002, 16, 289–293. [Google Scholar] [CrossRef] [PubMed]
| Name | Manufacturer | Cat. Number | Dilution | Host |
|---|---|---|---|---|
| Caspase-9 | Cell Signaling | 9502S | 1:1000 | rabbit |
| Bak (D4E4) | Cell Signaling | 12105T | 1:1000 | rabbit |
| Puma | Cell Signaling | 12450T | 1:1000 | rabbit |
| Bax (D2E11) | Cell Signaling | 5023T | 1:1000 | rabbit |
| Bcl-2 (124) | Cell Signaling | 15071S | 1:1000 | mouse |
| Mcl-1 (D5V5L) | Cell Signaling | 39224S | 1:1000 | rabbit |
| p21 (SX118) | Santa Cruz Biotechnology | sc-53870 | 1:200 | mouse |
| p-Erk1/2 | Cell Signaling | 4370S | 1:2000 | rabbit |
| p-p53 (B-3) (Thr55) | Santa Cruz Biotechnology | sc-377553 | 1:200 | mouse |
| p-p53 (Ser15) | Cell Signaling | 9284T | 1:1000 | rabbit |
| p-Akt | Cell Signaling | 3787S | 1:1000 | rabbit |
| Akt (pan) (11E7) | Cell Signaling | 4685S | 1:1000 | rabbit |
| beta-Actin (13E5) | Cell Signaling | 4970S | 1:5000 | rabbit |
| Anti-mouse IgG, HRP-linked | Cell Signaling | 7076S | 1:1000 | horse |
| Anti-rabbit IgG, HRP-linked | Cell Signaling | 7074S | 1:1000 | goat |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, E.; Forika, G.; Mohacsi, R.; Nemeth, Z.; Krenacs, T.; Dank, M. Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 3598. https://doi.org/10.3390/ijms22073598
Kiss E, Forika G, Mohacsi R, Nemeth Z, Krenacs T, Dank M. Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines. International Journal of Molecular Sciences. 2021; 22(7):3598. https://doi.org/10.3390/ijms22073598
Chicago/Turabian StyleKiss, Eva, Gertrud Forika, Reka Mohacsi, Zsuzsanna Nemeth, Tibor Krenacs, and Magdolna Dank. 2021. "Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines" International Journal of Molecular Sciences 22, no. 7: 3598. https://doi.org/10.3390/ijms22073598
APA StyleKiss, E., Forika, G., Mohacsi, R., Nemeth, Z., Krenacs, T., & Dank, M. (2021). Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines. International Journal of Molecular Sciences, 22(7), 3598. https://doi.org/10.3390/ijms22073598







