The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review
Abstract
1. Introduction
2. Results
2.1. Soluble Matrix Metalloproteinases (MMPs)
2.2. Membrane-Tethered Matrix Metalloproteinases (MT-MMPs)
2.3. Tissue Inhibitors of Metalloproteinases (TIMPs)
2.4. A Disintegrin and Metalloproteinases (ADAMs)
2.5. A Disintegrin and Metalloproteinase with Thrombospondin Motifs (ADAMTSs)
3. Discussion
3.1. Overview
3.2. Positive Associations
3.3. Negative Associations
3.4. Mixed Associations
3.5. Understanding the Complexity
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion and Exclusion Criteria
4.3. Study Selection and Data Extraction
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020. [Google Scholar] [CrossRef]
- Saraon, P.; Drabovich, A.P.; Jarvi, K.A.; Diamandis, E.P. Mechanisms of androgen-independent prostate cancer. EJIFCC 2014, 25, 42–54. [Google Scholar]
- Descotes, J.-L. Diagnosis of prostate cancer. Asian J. Urol. 2019, 6, 129–136. [Google Scholar] [CrossRef]
- Gomis-Rüth, F.X. Structural aspects of the metzincin clan of metalloendopeptidases. Mol. Biotechnol. 2003, 24, 157–202. [Google Scholar] [CrossRef]
- Stöcker, W.; Bode, W. Structural features of a superfamily of zinc-endopeptidases: The metzincins. Curr. Opin. Struct. Biol. 1995, 5, 383–390. [Google Scholar] [CrossRef]
- Stöcker, W.; Grams, F.; Reinemer, P.; Bode, W.; Baumann, U.; Gomis-Rüth, F.-X.; Mckay, D.B. The metzincins—Topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a super family of zinc-peptidases. Protein Sci. 1995, 4, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Huxley-Jones, J.; Clarke, T.K.; Beck, C.; Toubaris, G.; Robertson, D.L.; Boot-Handford, R.P. The evolution of the vertebrate metzincins; insights from Ciona intestinalis and Danio rerio. BMC Evol. Biol. 2007, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef]
- Tang, B.L. ADAMTS: A novel family of extracellular matrix proteases. Int. J. Biochem. Cell Biol. 2001, 33, 33–44. [Google Scholar] [CrossRef]
- Binder, M.J.; McCoombe, S.; Williams, E.D.; McCulloch, D.R.; Ward, A.C. The extracellular matrix in cancer progression: Role of hyalectan proteoglycans and ADAMTS enzymes. Cancer Lett. 2017, 385, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.F.; Courtneidge, S.A. The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes Dev. 2003, 17, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Turunen, S.P.; Tatti-Bugaeva, O.; Lehti, K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1974–1988. [Google Scholar] [CrossRef]
- Baker, A.H.; Edwards, D.R.; Murphy, G. Metalloproteinase inhibitors: Biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chippada-Venkata, U.D.; Oh, W.K. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancer 2014, 6, 1298–1327. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Nair, M.P.N.; Sufrin, G.; Mahajan, S.D.; Chadha, K.C.; Chawda, R.P.; Schwartz, S.A. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004, 64, 5311–5321. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Nair, B.B.; Reynolds, J.L.; Sykes, D.E.; Mahajan, S.D.; Chadha, K.C.; Schwartz, S.A. Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunol. Invest. 2011, 40, 447–464. [Google Scholar] [CrossRef]
- Adabi, Z.; Mohsen Ziaei, S.A.; Imani, M.; Samzadeh, M.; Narouie, B.; Jamaldini, S.H.; Afshari, M.; Safavi, M.; Roshandel, M.R.; Hasanzad, M. Genetic polymorphism of MMP2 gene and susceptibility to prostate cancer. Arch. Med. Res. 2015, 46, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Cangüven, Ö.; Göktaş, C.; Aydemir, H.; Köksal, V. Role of MMP-1 1G/2G promoter gene polymorphism on the development of prostate cancer in the Turkish population. Urol. Int. 2007, 79, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Assikis, V.J.; Do, K.A.; Wen, S.; Wang, X.; Cho-Vega, J.H.; Brisbay, S.; Lopez, R.; Logothetis, C.J.; Troncoso, P.; Papandreou, C.N.; et al. Clinical and biomarker correlates of androgen-independent, locally aggressive prostate cancer with limited metastatic potential. Clin. Cancer Res. 2004, 10, 6770–6778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babichenko, I.I.; Andriukhin, M.I.; Pulbere, S.; Loktev, A. Immunohistochemical expression of matrix metalloproteinase-9 and inhibitor of matrix metalloproteinase-1 in prostate adenocarcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 9090–9098. [Google Scholar]
- Baspinar, S.; Bircan, S.; Ciris, M.; Karahan, N.; Bozkurt, K.K. Expression of NGF, GDNF and MMP-9 in prostate carcinoma. Pathol. Res. Pract. 2017, 213, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; Zajac, E.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 2011, 179, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Marciniak, W.; Muszyńska, M.; Baszuk, P.; Gupta, S.; Jaworska-Bieniek, K.; Sukiennicki, G.; Durda, K.; Gromowski, T.; Prajzendanc, K.; et al. Association of zinc level and polymorphism in MMP-7 gene with prostate cancer in Polish population. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Bok, R.A.; Hansell, E.J.; Nguyen, T.P.; Greenberg, N.M.; McKerrow, J.H.; Shuman, M.A. Patterns of protease production during prostate cancer progression: Proteomic evidence for cascades in a transgenic model. Prostate Cancer Prostatic Dis. 2003, 6, 272–280. [Google Scholar] [CrossRef][Green Version]
- Bonaldi, C.M.; Azzalis, L.A.; Junqueira, V.B.; de Oliveira, C.G.; Vilas Boas, V.A.; Gáscon, T.M.; Gehrke, F.S.; Kuniyoshi, R.K.; Alves, B.C.; Fonseca, F.L. Plasma levels of E-cadherin and MMP-13 in prostate cancer patients: Correlation with PSA, testosterone and pathological parameters. Tumori 2015, 101, 185–188. [Google Scholar] [CrossRef]
- Boxler, S.; Djonov, V.; Kessler, T.M.; Hlushchuk, R.; Bachmann, L.M.; Held, U.; Markwalder, R.; Thalmann, G.N. Matrix metalloproteinases and angiogenic factors: Predictors of survival after radical prostatectomy for clinically organ-confined prostate cancer? Am. J. Pathol. 2010, 177, 2216–2224. [Google Scholar] [CrossRef]
- Brehmer, B.; Biesterfeld, S.; Jakse, G. Expression of matrix metalloproteinases (MMP-2 and -9) and their inhibitors (TIMP-1 and -2) in prostate cancer tissue. Prostate Cancer Prostatic Dis. 2003, 6, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Bruni-Cardoso, A.; Johnson, L.C.; Vessella, R.L.; Peterson, T.E.; Lynch, C.C. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Mol. Cancer Res. 2010, 8, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, M.A.; Di Silverio, F.; Gentile, V. Quantitative immunohistochemical and in situ hybridization analysis of metalloproteinases in prostate cancer. Anticancer Res. 2006, 26, 973–982. [Google Scholar]
- Carozzi, F.; Tamburrino, L.; Bisanzi, S.; Marchiani, S.; Paglierani, M.; Di Lollo, S.; Crocetti, E.; Buzzoni, C.; Burroni, E.; Greco, L.; et al. Are biomarkers evaluated in biopsy specimens predictive of prostate cancer aggressiveness? J. Cancer Res. Clin. Oncol. 2016, 142, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Castellana, D.; Zobairi, F.; Martinez, M.C.; Panaro, M.A.; Mitolo, V.; Freyssinet, J.-M.; Kunzelmann, C. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: A role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009, 69, 785–793. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Gesteira, T.F.; Coulson-Thomas, Y.M.; Vicente, C.M.; Tersariol, I.L.; Nader, H.B.; Toma, L. Fibroblast and prostate tumor cell cross-talk: Fibroblast differentiation, TGF-β, and extracellular matrix down-regulation. Exp. Cell Res. 2010, 316, 3207–3226. [Google Scholar] [CrossRef]
- Daja, M.M.; Niu, X.; Zhao, Z.; Brown, J.M.; Russell, P.J. Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2003, 6, 15–26. [Google Scholar] [CrossRef][Green Version]
- De Cicco, C.; Ravasi, L.; Zorzino, L.; Sandri, M.T.; Botteri, E.; Verweij, F.; Granchi, D.; de Cobelli, O.; Paganelli, G. Circulating levels of VCAM and MMP-2 may help identify patients with more aggressive prostate cancer. Curr. Cancer Drug Targets 2008, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.; Mariano, A.; Terracciano, D.; Ferro, M.; Montanaro, V.; Marsicano, M.; Di Lorenzo, G.; Altieri, V.; Macchia, V. Matrix metalloproteinase-2 and -9 in the urine of prostate cancer patients. Oncol. Rep. 2010, 24, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Nemeth, J.A.; Cher, M.L.; Palmer, K.C.; Bright, R.C.; Fridman, R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 expression in co-cultures of prostate cancer and stromal cells. Int. J. Cancer 2001, 93, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.T.; Pontes, J.; Villanova, F.E.; Borra, P.M.d.A.; Antunes, A.A.; Dall’oglio, M.F.; Srougi, M.; Leite, K.R.M. Genetic polymorphisms of matrix metalloproteinases: Susceptibility and prognostic implications for prostate cancer. J. Urol. 2009, 181, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.T.; Villanova, F.E.; Andrade, P.M.; Pontes, J.; de Sousa-Canavez, J.M.; Sañudo, A.; Antunes, A.A.; Dall’Oglio, M.F.; Srougi, M.; Moreira Leite, K.R. Matrix metalloproteinase-2 polymorphism is associated with prognosis in prostate cancer. Urol. Oncol. 2010, 28, 624–627. [Google Scholar] [CrossRef]
- Reis, S.T.; Villanova, F.E.; de Andrade, P.M.; Pontes, J.; Silva, I.A.; Canavez, F.C.; Sañudo, A.; Srougi, M.; Leite, K.R.M. Polymorphisms of the matrix metalloproteinases associated with prostate cancer. Mol. Med. Rep. 2008, 1, 517–520. [Google Scholar] [CrossRef]
- Eiro, N.; Fernandez-Gomez, J.; Sacristán, R.; Fernandez-Garcia, B.; Lobo, B.; Gonzalez-Suarez, J.; Quintas, A.; Escaf, S.; Vizoso, F.J. Stromal factors involved in human prostate cancer development, progression and castration resistance. J. Cancer Res. Clin. Oncol. 2017, 143, 351–359. [Google Scholar] [CrossRef] [PubMed]
- El-Chaer, W.K.; Tonet-Furioso, A.C.; Morais Junior, G.S.; Souza, V.C.; Avelar, G.G.; Henriques, A.D.; Franco Moraes, C.; Nóbrega, O.T. Serum Levels of Matrix Metalloproteinase-1 in Brazilian Patients with Benign Prostatic Hyperplasia or Prostate Cancer. Curr. Gerontol. Geriatr. Res. 2020, 2020, 6012102. [Google Scholar] [CrossRef]
- Eryilmaz, I.E.; Aytac Vuruskan, B.; Kaygısız, O.; Egeli, U.; Tunca, B.; Kordan, Y.; Cecener, G. RNA-based markers in biopsy cores with atypical small acinar proliferation: Predictive effect of T2E fusion positivity and MMP-2 upregulation for a subsequent prostate cancer diagnosis. Prostate 2019, 79, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Escaff, S.; Fernández, J.M.; González, L.O.; Suárez, A.; González-Reyes, S.; González, J.M.; Vizoso, F.J. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br. J. Cancer 2010, 102, 922–929. [Google Scholar] [CrossRef]
- Escaff, S.; Fernández, J.M.; González, L.O.; Suárez, A.; González-Reyes, S.; González, J.M.; Vizoso, F.J. Comparative study of stromal metalloproteases expression in patients with benign hyperplasia and prostate cancer. J. Cancer Res. Clin. Oncol. 2011, 137, 551–555. [Google Scholar] [CrossRef]
- Escaff, S.; Fernández, J.M.; González, L.O.; Suárez, A.; González-Reyes, S.; González, J.M.; Vizoso, F.J. Collagenase-3 expression by tumor cells and gelatinase B expression by stromal fibroblast-like cells are associated with biochemical recurrence after radical prostatectomy in patients with prostate cancer. World J. Urol. 2011, 29, 657–663. [Google Scholar] [CrossRef]
- Fávaro, W.J.; Hetzl, A.C.; Reis, L.O.; Ferreira, U.; Billis, A.; Cagnon, V.H. Periacinar retraction clefting in nonneoplastic and neoplastic prostatic glands: Artifact or molecular involvement. Pathol. Oncol. Res. 2012, 18, 285–292. [Google Scholar] [CrossRef]
- Fernandez-Gomez, J.; Escaf, S.; Gonzalez, L.O.; Suarez, A.; Gonzalez-Reyes, S.; González, J.; Miranda, O.; Vizoso, F. Relationship between metalloprotease expression in tumour and stromal cells and aggressive behaviour in prostate carcinoma: Simultaneous high-throughput study of multiple metalloproteases and their inhibitors using tissue array analysis of radical prostatectomy samples. Scand. J. Urol. Nephrol. 2011, 45, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, C.; Bologna, M.; Vicentini, C.; Tacconelli, A.; Miano, R.; Violini, S.; Mackay, A.R. Increased matrix metalloproteinase-9 secretion in short-term tissue cultures of prostatic tumor cells. Int. J. Cancer 1996, 69, 386–393. [Google Scholar] [CrossRef]
- Gohji, K.; Fujimoto, N.; Hara, I.; Fujii, A.; Gotoh, A.; Okada, H.; Arakawa, S.; Kitazawa, S.; Miyake, H.; Kamidono, S.; et al. Serum matrix metalloproteinase-2 and its density in men with prostate cancer as a new predictor of disease extension. Int. J. Cancer 1998, 79, 96–101. [Google Scholar] [CrossRef]
- Gravina, G.L.; Mancini, A.; Ranieri, G.; Di Pasquale, B.; Marampon, F.; Di Clemente, L.; Ricevuto, E.; Festuccia, C. Phenotypic characterization of human prostatic stromal cells in primary cultures derived from human tissue samples. Int. J. Oncol. 2013, 42, 2116–2122. [Google Scholar] [CrossRef]
- Grindel, B.J.; Martinez, J.R.; Pennington, C.L.; Muldoon, M.; Stave, J.; Chung, L.W.; Farach-Carson, M.C. Matrilysin/matrix metalloproteinase-7 (MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol. 2014, 36, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Cao, W.; Sadashivaiah, K.; Chen, W.; Schneider, A.; Chellaiah, M.A. Promising noninvasive cellular phenotype in prostate cancer cells knockdown of matrix metalloproteinase 9. Sci. World J. 2013, 2013, 493689. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Fadlon, E.J.; Cottam, D.; Lawry, J.; Thurrell, W.; Silcocks, P.B.; Anderson, J.B.; Williams, J.L.; Rees, R.C. Matrix metalloproteinase 9 expression in primary human prostatic adenocarcinoma and benign prostatic hyperplasia. Br. J. Cancer 1994, 69, 177–182. [Google Scholar] [CrossRef]
- Hanqing, Z.; Yajun, X.; Gongchen, L.; Yong, C. Immunohistochemical studies of the expression of matrix metalloproteinase-2 and metalloproteinase-9 in human prostate cancer. J. Huazhong Univ. Sci. Med. 2003, 23, 373–374. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kihira, Y.; Matuo, Y.; Usui, T. Expression of matri metalloproteinase-7 and tissue inhibitor of metalloproteinase-1 in human prostate. J. Urol. 1998, 160, 1872–1876. [Google Scholar] [CrossRef]
- Hetzl, A.C.; Fávaro, W.J.; Billis, A.; Ferreira, U.; Cagnon, V.H.A. Steroid hormone receptors, matrix metalloproteinases, insulin-like growth factor, and dystroglycans interactions in prostatic diseases in the elderly men. Microsc. Res. Tech. 2012, 75, 1197–1205. [Google Scholar] [CrossRef]
- Incorvaia, L.; Badalamenti, G.; Rini, G.; Arcara, C.; Fricano, S.; Sferrazza, C.D.D.T.; Gebbia, N.; Leto, G. MMP-2, MMP-9 and activin A blood levels in patients with breast cancer or prostate cancer metastatic to the bone. Anticancer Res. 2007, 27, 1519–1525. [Google Scholar]
- Jaboin, J.J.; Hwang, M.; Lopater, Z.; Chen, H.; Ray, G.L.; Perez, C.; Cai, Q.; Wills, M.L.; Lu, B. The matrix metalloproteinase-7 polymorphism rs10895304 is associated with increased recurrence risk in patients with clinically localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Jędroszka, D.; Orzechowska, M.; Hamouz, R.; Górniak, K.; Bednarek, A.K. Markers of epithelial-to-mesenchymal transition reflect tumor biology according to patient age and Gleason score in prostate cancer. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Jennbacken, K.; Gustavsson, H.; Welén, K.; Vallbo, C.; Damber, J.-E. Prostate cancer progression into androgen independency is associated with alterations in cell adhesion and invasivity. Prostate 2006, 66, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Lein, M.; Ulbrich, N.; Rudolph, B.; Henke, W.; Schnorr, D.; Loening, S.A. Quantification of matrix metalloproteinases and tissue inhibitors of metalloproteinase in prostatic tissue: Analytical aspects. Prostate 1998, 34, 130–136. [Google Scholar] [CrossRef]
- Jung, K.; Krell, H.-W.; Ortel, B.; Hasan, T.; Römer, A.; Schnorr, D.; Loening, S.A.; Lein, M. Plasma matrix metalloproteinase 9 as biomarker of prostate cancer progression in Dunning (Copenhagen) rats. Prostate 2003, 54, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Nowak, L.; Lein, M.; Priem, F.; Schnorr, D.; Loening, S.A. Matrix metalloproteinases 1 and 3, tissue inhibitor of metalloproteinase-1 and the complex of metalloproteinase-1/tissue inhibitor in plasma of patients with prostate cancer. Int. J. Cancer 1997, 74, 220–223. [Google Scholar] [CrossRef]
- Jurasz, P.; North, S.; Venner, P.; Radomski, M.W. Matrix metalloproteinase-2 contributes to increased platelet reactivity in patients with metastatic prostate cancer: A preliminary study. Thromb. Res. 2003, 112, 59–64. [Google Scholar] [CrossRef]
- Kalantari, E.; Abolhasani, M.; Roudi, R.; Farajollahi, M.M.; Farhad, S.; Madjd, Z.; Askarian-Amiri, S.; Mohsenzadegan, M. Co-expression of TLR-9 and MMP-13 is associated with the degree of tumour differentiation in prostate cancer. Int. J. Exp. Pathol. 2019, 100, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, A.; Hahne, J.C.; Haddouti, E.-M.; Florin, A.; Wellmann, A.; Wernert, N. Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int. J. Mol. Med. 2006, 18, 941–950. [Google Scholar] [CrossRef]
- Kanoh, Y.; Akahoshi, T.; Ohara, T.; Ohtani, N.; Mashiko, T.; Ohtani, S.; Egawa, S.; Baba, S. Expression of matrix metalloproteinase-2 and prostate-specific antigen in localized and metastatic prostate cancer. Anticancer Res. 2002, 22, 1813–1817. [Google Scholar]
- Knox, J.D.; Wolf, C.; McDaniel, K.; Clark, V.; Loriot, M.; Bowden, G.T.; Nagle, R.B. Matrilysin expression in human prostate carcinoma. Mol. Carcinog. 1996, 15, 57–63. [Google Scholar] [CrossRef]
- Koshida, K.; Konaka, H.; Imao, T.; Egawa, M.; Mizokami, A.; Namiki, M. Comparison of two in vivo models for prostate cancer: Orthotopic and intratesticular inoculation of LNCaP or PC-3 cells. Int. J. Urol. 2004, 11, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Kuniyasu, H.; Troncoso, P.; Johnston, D.; Bucana, C.D.; Tahara, E.; Fidler, I.J.; Pettaway, C.A. Relative expression of type IV Collagenase, E-cadherin, and vascular endothelial growth factor/vascular permeability factor in prostatectomy specimens distinguishes organ-confined from pathologically advanced prostate cancers. Clin. Cancer Res. 2000, 6, 2295–2308. [Google Scholar] [PubMed]
- Kuniyasu, H.; Ukai, R.; Johnston, D.; Troncoso, P.; Fidler, I.J.; Pettaway, C.A. The relative mRNA expression levels of matrix metalloproteinase to E-cadherin in prostate biopsy specimens distinguishes organ-confined from advanced prostate cancer at radical prostatectomy. Clin. Cancer Res. 2003, 9, 2185–2194. [Google Scholar] [PubMed]
- Larsson, P.; Syed Khaja, A.S.; Semenas, J.; Wang, T.; Sarwar, M.; Dizeyi, N.; Simoulis, A.; Hedblom, A.; Wai, S.N.; Ødum, N.; et al. The functional interlink between AR and MMP9/VEGF signaling axis is mediated through PIP5K1α/pAKT in prostate cancer. Int. J. Cancer 2020, 146, 1686–1699. [Google Scholar] [CrossRef]
- Latil, A.; Bièche, I.; Chêne, L.; Laurendeau, I.; Berthon, P.; Cussenot, O.; Vidaud, M. Gene expression profiling in clinically localized prostate cancer: A four-gene expression model predicts clinical behavior. Clin. Cancer Res. 2003, 9, 5477–5485. [Google Scholar]
- Lein, M.; Nowak, L.; Jung, K.; Laube, C.; Ulbricht, N.; Schnorr, D.; Loening, S.A. Metalloproteinases and tissue inhibitors of matrix-metalloproteinases in plasma of patients with prostate cancer and in prostate cancer tissue. Ann. N. Y. Acad. Sci. 1999, 878, 544–546. [Google Scholar] [CrossRef]
- Leshner, M.; Devine, M.; Roloff, G.W.; True, L.D.; Misteli, T.; Meaburn, K.J. Locus-specific gene repositioning in prostate cancer. Mol. Biol. Cell 2016, 27, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Wu, H.C.; Hu, P.S.; Hsu, S.W.; Shen, T.C.; Hsia, T.C.; Chang, W.S.; Tsai, C.W.; Bau, D.T. The association of matrix metalloproteinase-1 promoter polymorphisms with prostate cancer in Taiwanese patients. Anticancer Res. 2018, 38, 3907–3911. [Google Scholar] [CrossRef]
- Lichtinghagen, R.; Musholt, P.B.; Lein, M.; Römer, A.; Rudolph, B.; Kristiansen, G.; Hauptmann, S.; Schnorr, D.; Loening, S.A.; Jung, K. Different mRNA and protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 in benign and malignant prostate tissue. Eur. Urol. 2002, 42, 398–406. [Google Scholar] [CrossRef]
- Lichtinghagen, R.; Musholt, P.B.; Stephan, C.; Lein, M.; Kristiansen, G.; Hauptmann, S.; Rudolph, B.; Schnorr, D.; Loening, S.A.; Jung, K. mRNA expression profile of matrix metalloproteinases and their tissue inhibitors in malignant and non-malignant prostatic tissue. Anticancer Res. 2003, 23, 2617–2624. [Google Scholar] [PubMed]
- Littlepage, L.E.; Sternlicht, M.D.; Rougier, N.; Phillips, J.; Gallo, E.; Yu, Y.; Williams, K.; Brenot, A.; Gordon, J.I.; Werb, Z. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010, 70, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gu, X.; Huang, T.; Luan, Y.; Ding, X. Identification of TMPRSS2-ERG mechanisms in prostate cancer invasiveness: Involvement of MMP-9 and plexin B1. Oncol. Rep. 2017, 37, 201–208. [Google Scholar] [CrossRef]
- Lokeshwar, B.L.; Selzer, M.G.; Block, N.L.; Gunja-Smith, Z. Secretion of matrix metalloproteinases and their inhibitors (tissue inhibitor of metalloproteinases) by human prostate in explant cultures: Reduced tissue inhibitor of metalloproteinase secretion by malignant tissues. Cancer Res. 1993, 53, 4493–4498. [Google Scholar] [PubMed]
- London, C.A.; Sekhon, H.S.; Arora, V.; Stein, D.A.; Iversen, P.L.; Devi, G.R. A novel antisense inhibitor of MMP-9 attenuates angiogenesis, human prostate cancer cell invasion and tumorigenicity. Cancer Gene Ther. 2003, 10, 823–832. [Google Scholar] [CrossRef][Green Version]
- Lynch, C.C.; Hikosaka, A.; Acuff, H.B.; Martin, M.D.; Kawai, N.; Singh, R.K.; Vargo-Gogola, T.C.; Begtrup, J.L.; Peterson, T.E.; Fingleton, B.; et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 2005, 7, 485–496. [Google Scholar] [CrossRef]
- Marín-Aguilera, M.; Reig, Ò.; Lozano, J.J.; Jiménez, N.; García-Recio, S.; Erill, N.; Gaba, L.; Tagliapietra, A.; Ortega, V.; Carrera, G.; et al. Molecular profiling of peripheral blood is associated with circulating tumor cells content and poor survival in metastatic castration-resistant prostate cancer. Oncotarget 2015, 6, 10604–10616. [Google Scholar] [CrossRef]
- Maruta, S.; Miyata, Y.; Sagara, Y.; Kanda, S.; Iwata, T.; Watanabe, S.-i.; Sakai, H.; Hayashi, T.; Kanetake, H. Expression of matrix metalloproteinase-10 in non-metastatic prostate cancer: Correlation with an imbalance in cell proliferation and apoptosis. Oncol. Lett. 2010, 1, 417–421. [Google Scholar] [CrossRef]
- Medina-González, A.; Eiró-Díaz, N.; Fernández-Gómez, J.M.; Ovidio-González, L.; Jalón-Monzón, A.; Casas-Nebra, J.; Escaf-Barmadah, S. Comparative analysis of the expression of metalloproteases (MMP-2, MMP-9, MMP-11 and MMP-13) and the tissue inhibitor of metalloprotease 3 (TIMP-3) between previous negative biopsies and radical prostatectomies. Actas Urol. Esp. 2020, 44, 78–85. [Google Scholar] [CrossRef]
- Miyake, H.; Muramaki, M.; Kurahashi, T.; Takenaka, A.; Fujisawa, M. Expression of potential molecular markers in prostate cancer: Correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol. Oncol. 2010, 28, 145–151. [Google Scholar] [CrossRef]
- Monfironi, R.; Fabris, G.; Lucarini, G.; Biagini, G. Location of 72-kd metalloproteinase (Type IV Collagenase) in untreated prostatic adenocarcinoma. Pathol. Res. Pract. 1995, 191, 1140–1146. [Google Scholar] [CrossRef]
- Montironi, R.; Lucarini, G.; Castaldini, C.; Galluzzi, C.M.; Biagini, G.; Fabris, G. Immunohistochemical evaluation of type IV collagenase (72-kd metalloproteinase) in prostatic intraepithelial neoplasia. Anticancer Res. 1996, 16, 2057–2062. [Google Scholar]
- Morgia, G.; Falsaperla, M.; Malaponte, G.; Madonia, M.; Indelicato, M.; Travali, S.; Mazzarino, M.C. Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2, MMP-9) markers of prostate cancer. Urol. Res. 2005, 33, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; Wiederschain, D.; Loughlin, K.R.; Zurakowski, D.; Lamb, C.C.; Freeman, M.R. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998, 58, 1395–1399. [Google Scholar] [PubMed]
- Muñoz, D.; Serrano, M.K.; Hernandez, M.E.; Haller, R.; Swanson, T.; Slaton, J.W.; Sinha, A.A.; Wilson, M.J. Matrix metalloproteinase and heparin-stimulated serine proteinase activities in post-prostate massage urine of men with prostate cancer. Exp. Mol. Pathol. 2017, 103, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Nabha, S.M.; Bonfil, R.D.; Yamamoto, H.A.; Belizi, A.; Wiesner, C.; Dong, Z.; Cher, M.L. Host matrix metalloproteinase-9 contributes to tumor vascularization without affecting tumor growth in a model of prostate cancer bone metastasis. Clin. Exp. Metastasis 2006, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Nagle, R.B.; Knox, J.D.; Wolf, C.; Bowden, G.T.; Cress, A.E. Adhesion molecules, extracellular matrix, and proteases in prostate carcinoma. J. Cell Biochem. Suppl. 1994, 19, 232–237. [Google Scholar]
- Nalla, A.K.; Gorantla, B.; Gondi, C.S.; Lakka, S.S.; Rao, J.S. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. 2010, 17, 599–613. [Google Scholar] [CrossRef]
- Neuhaus, J.; Schiffer, E.; Mannello, F.; Horn, L.-C.; Ganzer, R.; Stolzenburg, J.-U. Protease expression levels in prostate cancer tissue can explain prostate cancer-associated seminal biomarkers-an explorative concept study. Int. J. Mol. Sci. 2017, 18, 976. [Google Scholar] [CrossRef]
- Oguić, R.; Mozetič, V.; Cini Tešar, E.; Fučkar Čupić, D.; Mustać, E.; Dorđević, G. Matrix metalloproteinases 2 and 9 immunoexpression in prostate carcinoma at the positive margin of radical prostatectomy specimens. Pathol. Res. Int. 2014, 2014, 262195. [Google Scholar] [CrossRef][Green Version]
- Ok Atılgan, A.; Özdemir, B.H.; Yılmaz Akçay, E.; Tepeoğlu, M.; Börcek, P.; Dirim, A. Association between focal adhesion kinase and matrix metalloproteinase-9 expression in prostate adenocarcinoma and their influence on the progression of prostatic adenocarcinoma. Ann. Diagn. Pathol. 2020, 45, 151480. [Google Scholar] [CrossRef]
- Ouyang, X.S.; Wang, X.; Lee, D.T.; Tsao, S.W.; Wong, Y.C. Up-regulation of TRPM-2, MMP-7 and ID-1 during sex hormone-induced prostate carcinogenesis in the Noble rat. Carcinogenesis 2001, 22, 965–973. [Google Scholar] [CrossRef][Green Version]
- Ozden, F.; Saygin, C.; Uzunaslan, D.; Onal, B.; Durak, H.; Aki, H. Expression of MMP-1, MMP-9 and TIMP-2 in prostate carcinoma and their influence on prognosis and survival. J. Cancer Res. Clin. Oncol. 2013, 139, 1373–1382. [Google Scholar] [CrossRef]
- Pajouh, M.S.; Nagle, R.B.; Breathnach, R.; Finch, J.S.; Brawer, M.K.; Bowden, G.T. Expression of metalloproteinase genes in human prostate cancer. J. Cancer Res. Clin. Oncol. 1991, 117, 144–150. [Google Scholar] [CrossRef]
- Pang, S.T.; Flores-Morales, A.; Skoog, L.; Chuan, Y.C.; Nordstedt, G.; Pousette, A. Regulation of matrix metalloproteinase 13 expression by androgen in prostate cancer. Oncol. Rep. 2004, 11, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Pettaway, C.A.; Song, R.; Wang, X.; Sanchez-Ortiz, R.; Spiess, P.E.; Strom, S.; Troncoso, P. The ratio of matrix metalloproteinase to E-cadherin expression: A pilot study to assess mRNA and protein expression among African American prostate cancer patients. Prostate 2008, 68, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Pouyanfar, N.; Monabbati, A.; Sharifi, A.A.; Dianatpour, M. Expression levels of MMP9 and PIWIL2 in prostate cancer: A case-control study. Clin. Lab. 2016, 62, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.C.; Knox, J.D.; Navre, M.; Grogan, T.M.; Kittelson, J.; Nagle, R.B.; Bowden, G.T. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993, 53, 417–422. [Google Scholar] [PubMed]
- Prior, C.; Guillen-Grima, F.; Robles, J.E.; Rosell, D.; Fernandez-Montero, J.M.; Agirre, X.; Catena, R.; Calvo, A. Use of a combination of biomarkers in serum and urine to improve detection of prostate cancer. World J. Urol. 2010, 28, 681–686. [Google Scholar] [CrossRef]
- Reis, S.T.; Antunes, A.A.; Pontes-Junior, J.; Sousa-Canavez, J.M.d.; Dall’Oglio, M.F.; Piantino, C.B.; Cruz, J.A.S.d.; Morais, D.R.; Srougi, M.; Leite, K.R.M. Underexpression of MMP-2 and its regulators, TIMP2, MT1-MMP and IL-8, is associated with prostate cancer. Int. Braz. J. Urol. 2012, 38, 167–174. [Google Scholar] [CrossRef]
- Dos Reis, S.T.d.; Viana, N.I.; Iscaife, A.; Pontes-Junior, J.; Dip, N.; Antunes, A.A.; Guimarães, V.R.; Santana, I.; Nahas, W.C.; Srougi, M.; et al. Loss of TIMP-1 immune expression and tumor recurrence in localized prostate cancer. Int. Braz. J. Urol. 2015, 41, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.T.; Pontes-Junior, J.; Antunes, A.A.; de Sousa-Canavez, J.M.; Dall’Oglio, M.F.; Passerotti, C.C.; Abe, D.K.; Crippa, A.; da Cruz, J.A.; Timoszczuk, L.M.; et al. MMP-9 overexpression due to TIMP-1 and RECK underexpression is associated with prognosis in prostate cancer. Int. J. Biol. Markers 2011, 26, 255–261. [Google Scholar] [CrossRef]
- Riddick, A.C.; Shukla, C.J.; Pennington, C.J.; Bass, R.; Nuttall, R.K.; Hogan, A.; Sethia, K.K.; Ellis, V.; Collins, A.T.; Maitland, N.J.; et al. Identification of degradome components associated with prostate cancer progression by expression analysis of human prostatic tissues. Br. J. Cancer 2005, 92, 2171–2180. [Google Scholar] [CrossRef]
- Ross, J.S.; Kaur, P.; Sheehan, C.E.; Fisher, H.A.G.; Kaufman, R.A.; Kallakury, B.V.S. Prognostic significance of matrix metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expression in prostate cancer. Mod. Pathol. 2003, 16, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sakai, I.; Harada, K.-I.; Hara, I.; Eto, H.; Miyake, H. A comparison of the biological features between prostate cancers arising in the transition and peripheral zones. BJU Int. 2005, 96, 528–532. [Google Scholar] [CrossRef] [PubMed]
- San Francisco, I.F.; DeWolf, W.C.; Peehl, D.M.; Olumi, A.F. Expression of transforming growth factor-beta 1 and growth in soft agar differentiate prostate carcinoma-associated fibroblasts from normal prostate fibroblasts. Int. J. Cancer 2004, 112, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Sauer, C.G.; Kappeler, A.; Späth, M.; Kaden, J.J.; Michel, M.S.; Mayer, D.; Bleyl, U.; Grobholz, R. Expression and activity of matrix metalloproteinases-2 and -9 in serum, core needle biopsies and tissue specimens of prostate cancer patients. Virchows Archiv 2004, 444, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Weibel, S.; Donat, U.; Zhang, Q.; Aguilar, R.J.; Chen, N.G.; Szalay, A.A. Vaccinia virus-mediated intra-tumoral expression of matrix metalloproteinase 9 enhances oncolysis of PC-3 xenograft tumors. BMC Cancer 2012, 12, 366. [Google Scholar] [CrossRef]
- Schveigert, D.; Valuckas, K.P.; Kovalcis, V.; Ulys, A.; Chvatovic, G.; Didziapetriene, J. Significance of MMP-9 expression and MMP-9 polymorphism in prostate cancer. Tumori 2013, 99, 523–529. [Google Scholar] [CrossRef]
- Sehgal, G.; Hua, J.; Bernhard, E.J.; Sehgal, I.; Thompson, T.C.; Muschel, R.J. Requirement for matrix metalloproteinase-9 (gelatinase B) expression in metastasis by murine prostate carcinoma. Am. J. Pathol. 1998, 152, 591–596. [Google Scholar]
- Sehgal, I.; Forbes, K.; Webb, M.A. Reduced secretion of MMPs, plasminogen activators and TIMPS from prostate cancer cells derived by repeated metastasis. Anticancer Res. 2003, 23, 39–42. [Google Scholar] [PubMed]
- Serretta, V.; Abrate, A.; Siracusano, S.; Gesolfo, C.S.; Vella, M.; Di Maida, F.; Cangemi, A.; Cicero, G.; Barresi, E.; Sanfilippo, C. Clinical and biochemical markers of visceral adipose tissue activity: Body mass index, visceral adiposity index, leptin, adiponectin, and matrix metalloproteinase-3. Correlation with Gleason patterns 4 and 5 at prostate biopsy. Urol Ann. 2018, 10, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Sfar, S.; Saad, H.; Mosbah, F.; Gabbouj, S.; Chouchane, L. TSP1 and MMP9 genetic variants in sporadic prostate cancer. Cancer Genet. Cytogenet. 2007, 172, 38–44. [Google Scholar] [CrossRef]
- Sfar, S.; Saad, H.; Mosbah, F.; Chouchane, L. Combined effects of the angiogenic genes polymorphisms on prostate cancer susceptibility and aggressiveness. Mol. Biol. Rep. 2009, 36, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Wildes, F.; Kakkad, S.; Artemov, D.; Bhujwalla, Z.M. Lymphatic endothelial cells actively regulate prostate cancer cell invasion. NMR Biomed. 2016, 29, 904–911. [Google Scholar] [CrossRef]
- Shajarehpoor Salavati, L.; Tafvizi, F.; Manjili, H.K. The association between MMP2 −1306 C > T (rs243865) polymorphism and risk of prostate cancer. Ir. J. Med. Sci. 2017, 186, 103–111. [Google Scholar] [CrossRef]
- Shi, T.; Quek, S.I.; Gao, Y.; Nicora, C.D.; Nie, S.; Fillmore, T.L.; Liu, T.; Rodland, K.D.; Smith, R.D.; Leach, R.J.; et al. Multiplexed targeted mass spectrometry assays for prostate cancer-associated urinary proteins. Oncotarget 2017, 8, 101887–101898. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.d., Jr.; Matheus, W.E.; Garcia, P.V.; Stopiglia, R.M.; Billis, A.; Ferreira, U.; Fávaro, W.J. Characterization of reactive stroma in prostate cancer: Involvement of growth factors, metalloproteinase matrix, sexual hormones receptors and prostatic stem cells. Int. Braz. J. Urol. 2015, 41, 849–858. [Google Scholar] [CrossRef]
- Srivastava, P.; Lone, T.A.; Kapoor, R.; Mittal, R.D. Association of promoter polymorphisms in MMP2 and TIMP2 with prostate cancer susceptibility in North India. Arch. Med. Res. 2012, 43, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.; Stearns, M.E. Evidence for increased activated metalloproteinase 2 (MMP-2a) expression associated with human prostate cancer progression. Oncol. Res. 1996, 8, 69–75. [Google Scholar] [PubMed]
- Stearns, M.E.; Stearns, M. Immunohistochemical studies of activated matrix metalloproteinase-2 (MMP-2a)expression in human prostate cancer. Oncol. Res. 1996, 8, 63–67. [Google Scholar]
- Still, K.; Robson, C.N.; Autzen, P.; Robinson, M.C.; Hamdy, F.C. Localization and quantification of mRNA for matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in human benign and malignant prostatic tissue. Prostate 2000, 42, 18–25. [Google Scholar] [CrossRef]
- Szarvas, T.; Sevcenco, S.; Módos, O.; Keresztes, D.; Nyirády, P.; Csizmarik, A.; Ristl, R.; Puhr, M.; Hoffmann, M.J.; Niedworok, C.; et al. Matrix metalloproteinase 7, soluble Fas and Fas ligand serum levels for predicting docetaxel resistance and survival in castration-resistant prostate cancer. BJU Int. 2018, 122, 695–704. [Google Scholar] [CrossRef]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Membrane-type-1 matrix metalloproteinase, matrix metalloproteinase 2, and tissue inhibitor of matrix proteinase 2 in prostate cancer: Identification of patients with poor prognosis by immunohistochemistry. Hum. Pathol. 2008, 39, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Significance of MMP-2 expression in prostate cancer: An Immunohistochemical Study. Cancer Res. 2003, 63, 8511–8515. [Google Scholar]
- Trudel, D.; Fradet, Y.; Meyer, F.; Têtu, B. Matrix metalloproteinase 9 is associated with Gleason score in prostate cancer but not with prognosis. Hum. Pathol. 2010, 41, 1694–1701. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Narita, S.; Kumazawa, T.; Inoue, T.; Ma, Z.; Tsuruta, H.; Saito, M.; Horikawa, Y.; Yuasa, T.; Satoh, S.; et al. Clinical significance of a single nucleotide polymorphism and allelic imbalance of matrix metalloproteinase-1 promoter region in prostate cancer. Oncol. Rep. 2009, 22, 493–499. [Google Scholar] [CrossRef]
- Upadhyay, J.; Shekarriz, B.; Nemeth, J.A.; Dong, Z.; Cummings, G.D.; Fridman, R.; Sakr, W.; Grignon, D.J.; Cher, M.L. Membrane type 1-matrix metalloproteinase (MT1-MMP) and MMP-2 immunolocalization in human prostate: Change in cellular localization associated with high-grade prostatic intraepithelial neoplasia. Clin. Cancer Res. 1999, 5, 4105–4110. [Google Scholar]
- Vallbo, C.; Damber, J.-E. Thrombospondins, metallo proteases and thrombospondin receptors messenger RNA and protein expression in different tumour sublines of the Dunning prostate cancer model. Acta Oncol. 2005, 44, 293–298. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Zhou, W.; Wang, M.; Xia, W.; Tang, Q. Prognostic value of matrix metalloprotease-1/protease-activated receptor-1 axis in patients with prostate cancer. Med. Oncol. 2014, 31, 968. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, C.; Bonfil, R.D.; Dong, Z.; Yamamoto, H.; Nabha, S.M.; Meng, H.; Saliganan, A.; Sabbota, A.; Cher, M.L. Heterogeneous activation of MMP-9 due to prostate cancer-bone interaction. Urology 2007, 69, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Sellers, R.G.; Wiehr, C.; Melamud, O.; Pei, D.; Peehl, D.M. Expression of matrix metalloproteinase-2 and -9 and their inhibitors, tissue inhibitor of metalloproteinase-1 and -2, in primary cultures of human prostatic stromal and epithelial cells. J. Cell Physiol. 2002, 191, 208–216. [Google Scholar] [CrossRef]
- Wilson, M.J.; Sinha, A.A. Plasminogen activator and metalloprotease activities of Du-145, PC-3, and 1-LN-PC-3-1A human prostate tumors grown in nude mice: Correlation with tumor invasive behavior. Cell Mol. Biol. Res. 1993, 39, 751–760. [Google Scholar] [PubMed]
- Wood, M.; Fudge, K.; Mohler, J.L.; Frost, A.R.; Garcia, F.; Wang, M.; Stearns, M.E. In situ hybridization studies of metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in human prostate cancer. Clin. Exp. Metastasis 1997, 15, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lu, W.; Liu, S.; Yang, Q.; Goodwin, J.S.; Sathyanarayana, S.A.; Pratap, S.; Chen, Z. MMP7 interacts with ARF in nucleus to potentiate tumor microenvironments for prostate cancer progression in vivo. Oncotarget 2016, 7, 47609–47619. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; McKee, C.M.; Cao, Y.; Ding, Y.; Kessler, B.M.; Muschel, R.J. Matrix metalloproteinase-9 regulates tumor cell invasion through cleavage of protease nexin-1. Cancer Res. 2010, 70, 6988–6998. [Google Scholar] [CrossRef]
- Yaykaşli, K.O.; Kayikçi, M.A.; Yamak, N.; Soğuktaş, H.; Düzenli, S.; Arslan, A.O.; Metın, A.; Kaya, E.; Hatıpoğlu Ö, F. Polymorphisms in MMP-2 and TIMP-2 in Turkish patients with prostate cancer. Turk. J. Med. Sci. 2014, 44, 839–843. [Google Scholar] [CrossRef]
- Zellweger, T.; Ninck, C.; Bloch, M.; Mirlacher, M.; Koivisto, P.A.; Helin, H.J.; Mihatsch, M.J.; Gasser, T.C.; Bubendorf, L. Expression patterns of potential therapeutic targets in prostate cancer. Int J. Cancer 2005, 113, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jung, K.; Lein, M.; Kristiansen, G.; Rudolph, B.; Hauptmann, S.; Schnorr, D.; Loening, S.A.; Lichtinghagen, R. Differential expression of matrix metalloproteinases and their tissue inhibitors in human primary cultured prostatic cells and malignant prostate cell lines. Prostate 2002, 50, 38–45. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, J.; Feng, J.; Klocker, H.; Lee, C.; Zhang, J. Type IV collagenase (matrix metalloproteinase-2 and -9) in prostate cancer. Prostate Cancer Prostatic Dis. 2004, 7, 327–332. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, L.; Li, M.; Zhang, D.; Xu, S.; Wang, N.; Sun, B. Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J. Exp. Clin. Cancer Res. 2008, 27, 62. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, C.; Yao, S.; Wang, Z.; Xu, L.; Yang, R.; Meng, X.; Wu, J.; Zhou, L.; Sun, Z. Proteomic analysis of human prostate cancer PC-3M-1E8 cells and PC-3M-2B4 cells of same origin but with different metastatic potential. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Xiao, A.Z.; Newcomer, R.G.; Park, H.I.; Kang, T.; Chung, L.W.; Swanson, M.G.; Zhau, H.E.; Kurhanewicz, J.; Sang, Q.X. Activation of pro-gelatinase B by endometase/matrilysin-2 promotes invasion of human prostate cancer cells. J. Biol. Chem. 2003, 278, 15056–15064. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.D.; Han, Z.D.; He, H.C.; Bi, X.C.; Dai, Q.S.; Zhu, G.; Ye, Y.K.; Liang, Y.X.; Qin, W.J.; Zhang, Z.; et al. CD147, MMP-1, MMP-2 and MMP-9 protein expression as significant prognostic factors in human prostate cancer. Oncology 2008, 75, 230–236. [Google Scholar] [CrossRef]
- Zhu, B.; Block, N.L.; Lokeshwar, B.L. Interaction between stromal cells and tumor cells induces chemoresistance and matrix metalloproteinase secretion. Ann. N. Y. Acad. Sci. 1999, 878, 642–646. [Google Scholar] [CrossRef]
- Cao, J.; Chiarelli, C.; Richman, O.; Zarrabi, K.; Kozarekar, P.; Zucker, S. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J. Biol Chem 2008, 283, 6232–6240. [Google Scholar] [CrossRef] [PubMed]
- Bair, E.L.; Chen, M.L.; McDaniel, K.; Sekiguchi, K.; Cress, A.E.; Nagle, R.B.; Bowden, G.T. Membrane type 1 matrix metalloprotease cleaves laminin-10 and promotes prostate cancer cell migration. Neoplasia 2005, 7, 380–389. [Google Scholar] [CrossRef]
- Sabbota, A.L.; Kim, H.R.; Zhe, X.; Fridman, R.; Bonfil, R.D.; Cher, M.L. Shedding of RANKL by tumor-associated MT1-MMP activates Src-dependent prostate cancer cell migration. Cancer Res. 2010, 70, 5558–5566. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Zucker, S.; Zarrabi, K.; Kadam, P.; Schmidt, C.; Cao, J. Oxidative stress and prostate cancer progression are elicited by membrane-type 1 matrix metalloproteinase. Mol. Cancer Res. 2011, 9, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, K.; Dufour, A.; Li, J.; Kuscu, C.; Pulkoski-Gross, A.; Zhi, J.; Hu, Y.; Sampson, N.S.; Zucker, S.; Cao, J. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J. Biol. Chem. 2011, 286, 33167–33177. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, R.D.; Dong, Z.; Trindade Filho, J.C.; Sabbota, A.; Osenkowski, P.; Nabha, S.; Yamamoto, H.; Chinni, S.R.; Zhao, H.; Mobashery, S.; et al. Prostate cancer-associated membrane type 1-matrix metalloproteinase: A pivotal role in bone response and intraosseous tumor growth. Am. J. Pathol. 2007, 170, 2100–2111. [Google Scholar] [CrossRef]
- Wang, X.; Wilson, M.J.; Slaton, J.W.; Sinha, A.A.; Ewing, S.L.; Pei, D. Increased aggressiveness of human prostate PC-3 tumor cells expressing cell surface localized membrane type-1 matrix metalloproteinase (MT1-MMP). J. Androl. 2009, 30, 259–274. [Google Scholar] [CrossRef]
- Cheng, T.; Li, F.; Wei, R.; Lv, M.Q.; Zhou, Y.; Dai, Y.; Yuan, Y.; Jiang, G.Y.; Ma, D.; Gao, Q.L. MMP26: A potential biomarker for prostate cancer. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Sun, Z.Y.; Meng, X.L.; Wu, J.H.; He, G.L.; Liu, G.M.; Jiang, X.R. Differential metastasis-associated gene analysis of prostate carcinoma cells derived from primary tumor and spontaneous lymphatic metastasis in nude mice with orthotopic implantation of PC-3M cells. Cancer Lett. 2006, 233, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, J.; Dong, C.; Wei, W.; Li, J.; Li, X. Membranous type matrix metalloproteinase 16 induces human prostate cancer metastasis. Oncol. Lett. 2017, 14, 3096–3102. [Google Scholar] [CrossRef]
- Jung, M.; Römer, A.; Keyszer, G.; Lein, M.; Kristiansen, G.; Schnorr, D.; Loening, S.A.; Jung, K. mRNA expression of the five membrane-type matrix metalloproteinases MT1–MT5 in human prostatic cell lines and their down-regulation in human malignant prostatic tissue. Prostate 2003, 55, 89–98. [Google Scholar] [CrossRef]
- Khamis, Z.I.; Iczkowski, K.A.; Man, Y.-G.; Bou-Dargham, M.J.; Sang, Q.-X.A. Evidence for a proapoptotic role of matrix metalloproteinase-26 in human prostate cancer cells and tissues. J. Cancer 2016, 7, 80–87. [Google Scholar] [CrossRef]
- Lee, S.; Desai, K.K.; Iczkowski, K.A.; Newcomer, R.G.; Wu, K.J.; Zhao, Y.-G.; Tan, W.W.; Roycik, M.D.; Sang, Q.-X.A. Coordinated peak expression of MMP-26 and TIMP-4 in preinvasive human prostate tumor. Cell Res. 2006, 16, 750–758. [Google Scholar] [CrossRef]
- Lin, H.Y.; Amankwah, E.K.; Tseng, T.-S.; Qu, X.; Chen, D.-T.; Park, J.Y. SNP-SNP interaction network in angiogenesis genes associated with prostate cancer aggressiveness. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Cheng, C.H.; Chen, D.T.; Chen, Y.A.; Park, J.Y. Coexpression and expression quantitative trait loci analyses of the angiogenesis gene-gene interaction network in prostate cancer. Transl. Cancer Res. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Atteridge, C.L.; Wang, X.; Lundgren, A.D.; Wu, J.D. Cutting edge: The membrane type matrix metalloproteinase MMP14 mediates constitutive shedding of MHC Class I chain-related molecule a independent of a disintegrin and metalloproteinases. J. Immunol. 2010, 184, 3346–3350. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, O.; Murakami, K.; Yamaura, T.; Fujiuchi, Y.; Murata, J.; Fuse, H.; Saiki, I. Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) on prostate cancer cell lines. Cancer Lett. 2000, 155, 173–179. [Google Scholar] [CrossRef]
- Sroka, I.C.; McDaniel, K.; Nagle, R.B.; Bowden, G.T. Differential localization of MT1-MMP in human prostate cancer tissue: Role of IGF-1R in MT1-MMP expression. Prostate 2008, 68, 463–476. [Google Scholar] [CrossRef]
- Udayakumar, T.S.; Chen, M.L.; Bair, E.L.; Von Bredow, D.C.; Cress, A.E.; Nagle, R.B.; Bowden, G.T. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003, 63, 2292–2299. [Google Scholar] [PubMed]
- Liu, A.Y.; Zhang, H.U.I.; Sorensen, C.M.; Diamond, D.L. Analysis of prostate cancer by proteomics using tissue specimens. J. Urol. 2005, 173, 73–78. [Google Scholar] [CrossRef]
- Ashida, S.; Nakagawa, H.; Katagiri, T.; Furihata, M.; Iiizumi, M.; Anazawa, Y.; Tsunoda, T.; Takata, R.; Kasahara, K.; Miki, T.; et al. Molecular features of the transition from prostatic intraepithelial neoplasia (PIN) to prostate cancer: Genome-wide gene-expression profiles of prostate cancers and PINs. Cancer Res. 2004, 64, 5963–5972. [Google Scholar] [CrossRef]
- Kim, Y.; Ignatchenko, V.; Yao, C.Q.; Kalatskaya, I.; Nyalwidhe, J.O.; Lance, R.S.; Gramolini, A.O.; Troyer, D.A.; Stein, L.D.; Boutros, P.C.; et al. Identification of differentially expressed proteins in direct expressed prostatic secretions of men with organ-confined versus extracapsular prostate cancer. Mol. Cell Proteomics 2012, 11, 1870–1884. [Google Scholar] [CrossRef]
- Stearns, M.E.; Wang, M.; Stearns, M. IL-10 blocks collagen IV invasion by "invasion stimulating factor" activated PC-3 ML cells: Upregulation of TIMP-1 expression. Oncol. Res. 1995, 7, 157–163. [Google Scholar]
- Adissu, H.A.; McKerlie, C.; Di Grappa, M.; Waterhouse, P.; Xu, Q.; Fang, H.; Khokha, R.; Wood, G.A. Timp3 loss accelerates tumour invasion and increases prostate inflammation in a mouse model of prostate cancer. Prostate 2015, 75, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.; Tickle, S.; Wasan, H.; Docherty, A.; Isenberg, D.; Waxman, J. Serum metalloproteinases and their inhibitors: Markers for malignant potential. Br. J. Cancer 1994, 70, 506–512. [Google Scholar] [CrossRef]
- Deng, X.; Bhagat, S.; Dong, Z.; Mullins, C.; Chinni, S.R.; Cher, M. Tissue inhibitor of metalloproteinase-3 induces apoptosis in prostate cancer cells and confers increased sensitivity to paclitaxel. Eur. J. Cancer 2006, 42, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chippada-Venkata, U.D.; Galsky, M.D.; Huang, J.; Oh, W.K. Elevated circulating tissue inhibitor of metalloproteinase 1 (TIMP-1) levels are associated with neuroendocrine differentiation in castration resistant prostate cancer. Prostate 2015, 75, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, H.; Jennbacken, K.; Welén, K.; Damber, J.-E. Altered expression of genes regulating angiogenesis in experimental androgen-independent prostate cancer. Prostate 2008, 68, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.O.; Topaloglu, O.; Begum, S.; Henrique, R.; Rosenbaum, E.; Criekinge, W.V.; Westra, W.H.; Sidransky, D. Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects. J. Clin. Oncol. 2005, 23, 6569–6575. [Google Scholar] [CrossRef]
- Jerónimo, C.; Henrique, R.; Hoque, M.O.; Mambo, E.; Ribeiro, F.R.; Varzim, G.; Oliveira, J.; Teixeira, M.R.; Lopes, C.; Sidransky, D. A quantitative promoter methylation profile of prostate cancer. Clin. Cancer Res. 2004, 10, 8472–8478. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, K.; Białkowska, A.; Kowalewski, J.; Huang, S.; Lewandowska, M.A. Differential gene methylation patterns in cancerous and non-cancerous cells. Oncol. Rep. 2019, 42, 43–54. [Google Scholar] [CrossRef]
- Karan, D.; Lin, F.C.; Bryan, M.; Ringel, J.; Moniaux, N.; Lin, M.F.; Batra, S.K. Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int. J. Oncol. 2003, 23, 1365–1371. [Google Scholar] [CrossRef]
- Kuefer, R.; Day, K.C.; Kleer, C.G.; Sabel, M.S.; Hofer, M.D.; Varambally, S.; Zorn, C.S.; Chinnaiyan, A.M.; Rubin, M.A.; Day, M.L. ADAM15 disintegrin is associated with aggressive prostate and breast cancer disease. Neoplasia 2006, 8, 319–329. [Google Scholar] [CrossRef]
- Kwabi-Addo, B.; Wang, S.; Chung, W.; Jelinek, J.; Patierno, S.R.; Wang, B.-D.; Andrawis, R.; Lee, N.H.; Apprey, V.; Issa, J.-P.; et al. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin. Cancer Res. 2010, 16, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Jung, J.-I.; Kwon, S.-H.; Lee, S.-M.; Morita, K.; Her, S. TIMP-2 fusion protein with human serum albumin potentiates anti-angiogenesis-mediated inhibition of tumor growth by suppressing MMP-2 expression. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Vargas, R.; Jacobus, S.; Leitzel, K.; Regan, M.M.; Hamer, P.; Pierce, K.; Brown-Shimer, S.; Carney, W.; Ali, S.M.; et al. Elevated plasma tissue inhibitor of metalloproteinase-1 levels predict decreased survival in castration-resistant prostate cancer patients. Cancer 2011, 117, 517–525. [Google Scholar] [CrossRef]
- Pulukuri, S.M.; Patibandla, S.; Patel, J.; Estes, N.; Rao, J.S. Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors. Oncogene 2007, 26, 5229–5237. [Google Scholar] [CrossRef]
- Ross, R.W.; Galsky, M.D.; Scher, H.I.; Magidson, J.; Wassmann, K.; Lee, G.S.; Katz, L.; Subudhi, S.K.; Anand, A.; Fleisher, M.; et al. A whole-blood RNA transcript-based prognostic model in men with castration-resistant prostate cancer: A prospective study. Lancet Oncol. 2012, 13, 1105–1113. [Google Scholar] [CrossRef]
- Shinojima, T.; Yu, Q.; Huang, S.K.; Li, M.; Mizuno, R.; Liu, E.T.; Hoon, D.S.; Lessard, L. Heterogeneous epigenetic regulation of TIMP3 in prostate cancer. Epigenetics 2012, 7, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Watanabe, M.; Yamada, Y.; Takagi, A.; Murata, T.; Takahashi, H.; Suzuki, H.; Ito, H.; Tsukino, H.; Katoh, T.; et al. Altered methylation of multiple genes in carcinogenesis of the prostate. Int. J. Cancer 2003, 106, 382–387. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Zhao, D.; Lin, G.; Guo, B.; Li, Y.; Liang, Z.; Zhao, X.J.; Fang, X. Inhibition of tumor growth and induction of apoptosis in prostate cancer cell lines by overexpression of tissue inhibitor of matrix metalloproteinase-3. Cancer Gene Ther. 2010, 17, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Burdelski, C.; Fitzner, M.; Hube-Magg, C.; Kluth, M.; Heumann, A.; Simon, R.; Krech, T.; Clauditz, T.; Büscheck, F.; Steurer, S.; et al. Overexpression of the a disintegrin and metalloproteinase ADAM15 is linked to a small but highly aggressive subset of prostate cancers. Neoplasia 2017, 19, 279–287. [Google Scholar] [CrossRef]
- Najy, A.J.; Day, K.C.; Day, M.L. ADAM15 supports prostate cancer metastasis by modulating tumor cell–endothelial cell interaction. Cancer Res. 2008, 68, 1092–1099. [Google Scholar] [CrossRef]
- Lin, P.; Sun, X.; Feng, T.; Zou, H.; Jiang, Y.; Liu, Z.; Zhao, D.; Yu, X. ADAM17 regulates prostate cancer cell proliferation through mediating cell cycle progression by EGFR/PI3K/AKT pathway. Mol. Cell Biochem. 2012, 359, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-J.; Lin, P.; Lin, F.; Liu, X.; Qin, W.; Zou, H.-F.; Guo, L.; Liu, W.; Wang, S.-J.; Yu, X.-G. ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int. J. Oncol. 2012, 40, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, C.; Mochizuki, S.; Okada, Y.; McLaughlin, C.; Leedman, P.J.; Stuart, L.; Epis, M.; Hoyne, G.; Boulos, S.; Johnson, L.; et al. Overexpression and knock-down studies highlight that a disintegrin and metalloproteinase 28 controls proliferation and migration in human prostate cancer. Medicine 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Enokida, H.; Kubo, H.; Kagara, I.; Matsuda, R.; Toki, K.; Nishimura, H.; Chiyomaru, T.; Tatarano, S.; Idesako, T.; et al. Nuclear translocation of ADAM-10 contributes to the pathogenesis and progression of human prostate cancer. Cancer Sci. 2007, 98, 1720–1726. [Google Scholar] [CrossRef]
- Bilgin Doğru, E.; Dizdar, Y.; Akşit, E.; Ural, F.; Şanlı, Ö.; Yasasever, V. EMMPRIN and ADAM12 in prostate cancer: Preliminary results of a prospective study. Tumour Biol. 2014, 35, 11647–11653. [Google Scholar] [CrossRef]
- Fritzsche, F.R.; Jung, M.; Xu, C.; Rabien, A.; Schicktanz, H.; Stephan, C.; Dietel, M.; Jung, K.; Kristiansen, G. ADAM8 expression in prostate cancer is associated with parameters of unfavorable prognosis. Virchows Archiv. 2006, 449, 628–636. [Google Scholar] [CrossRef]
- Fritzsche, F.R.; Jung, M.; Tölle, A.; Wild, P.; Hartmann, A.; Wassermann, K.; Rabien, A.; Lein, M.; Dietel, M.; Pilarsky, C.; et al. ADAM9 expression is a significant and independent prognostic marker of PSA relapse in prostate cancer. Eur. Urol. 2008, 54, 1097–1108. [Google Scholar] [CrossRef]
- Hoyne, G.; Rudnicka, C.; Sang, Q.-X.; Roycik, M.; Howarth, S.; Leedman, P.; Schlaich, M.; Candy, P.; Matthews, V. Genetic and cellular studies highlight that a disintegrin and metalloproteinase 19 is a protective biomarker in human prostate cancer. BMC Cancer 2016, 16, 151. [Google Scholar] [CrossRef]
- Josson, S.; Anderson, C.S.; Sung, S.Y.; Johnstone, P.A.; Kubo, H.; Hsieh, C.L.; Arnold, R.; Gururajan, M.; Yates, C.; Chung, L.W. Inhibition of ADAM9 expression induces epithelial phenotypic alterations and sensitizes human prostate cancer cells to radiation and chemotherapy. Prostate 2011, 71, 232–240. [Google Scholar] [CrossRef]
- Lin, G.-W.; Yao, X.-D.; Ye, D.-W.; Zhang, S.-L.; Dai, B.; Zhang, H.-L.; Ma, C.-G. ADAM9 decreases in castration resistant prostate cancer and is a prognostic factor for overall survival. Chin. Med. J. 2012, 125. [Google Scholar]
- Liu, C.-M.; Hsieh, C.-L.; He, Y.-C.; Lo, S.-J.; Liang, J.-A.; Hsieh, T.-F.; Josson, S.; Chung, L.W.K.; Hung, M.-C.; Sung, S.-Y. In vivo targeting of ADAM9 gene expression using lentivirus-delivered shRNA suppresses prostate cancer growth by regulating REG4 dependent cell cycle progression. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.R.; Harvey, M.; Herington, A.C. The expression of the ADAMs proteases in prostate cancer cell lines and their regulation by dihydrotestosterone. Mol. Cell Endocrinol. 2000, 167, 11–21. [Google Scholar] [CrossRef]
- McCulloch, D.R.; Akl, P.; Samaratunga, H.; Herington, A.C.; Odorico, D.M. Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin. Cancer Res. 2004, 10, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Peduto, L.; Reuter, V.E.; Sehara-Fujisawa, A.; Shaffer, D.R.; Scher, H.I.; Blobel, C.P. ADAM12 is highly expressed in carcinoma-associated stroma and is required for mouse prostate tumor progression. Oncogene 2006, 25, 5462–5466. [Google Scholar] [CrossRef] [PubMed]
- Peduto, L.; Reuter, V.E.; Shaffer, D.R.; Scher, H.I.; Blobel, C.P. Critical function for ADAM9 in mouse prostate cancer. Cancer Res. 2005, 65, 9312–9319. [Google Scholar] [CrossRef] [PubMed]
- Pen, C.C.; Liu, C.M.; Lin, C.C.; Lin, C.C.; Hsieh, T.F.; Josson, S.; He, Y.C.; Chung, L.W.; Lin, K.L.; Sung, S.Y. Combined dynamic alterations in urinary VEGF levels and tissue ADAM9 expression as markers for lethal phenotypic progression of prostate cancer. Chin. J. Physiol. 2012, 55, 390–397. [Google Scholar] [CrossRef]
- Shigemura, K.; Sung, S.-Y.; Kubo, H.; Arnold, R.S.; Fujisawa, M.; Gotoh, A.; Zhau, H.E.; Chung, L.W.K. Reactive oxygen species mediate androgen receptor- and serum starvation-elicited downstream signaling of ADAM9 expression in human prostate cancer cells. Prostate 2007, 67, 722–731. [Google Scholar] [CrossRef]
- Sung, S.Y.; Kubo, H.; Shigemura, K.; Arnold, R.S.; Logani, S.; Wang, R.; Konaka, H.; Nakagawa, M.; Mousses, S.; Amin, M.; et al. Oxidative stress induces ADAM9 protein expression in human prostate cancer cells. Cancer Res. 2006, 66, 9519–9526. [Google Scholar] [CrossRef]
- Gustavsson, H.; Tesan, T.; Jennbacken, K.; Kuno, K.; Damber, J.-E.; Welén, K. ADAMTS1 alters blood vessel morphology and TSP1 levels in LNCaP and LNCaP-19 prostate tumors. BMC Cancer 2010, 10, 288. [Google Scholar] [CrossRef]
- Gustavsson, H.; Wang, W.; Jennbacken, K.; Welén, K.; Damber, J.-E. ADAMTS1, a putative anti-angiogenic factor, is decreased in human prostate cancer. BJU Int. 2009, 104, 1786–1790. [Google Scholar] [CrossRef]
- Binder, M.J.; McCoombe, S.; Williams, E.D.; McCulloch, D.R.; Ward, A.C. ADAMTS-15 has a tumor suppressor role in prostate cancer. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.A.; Chandrasekharan, S.; Jokonya, N.; Fowles, A.; Hamdy, F.C.; Buttle, D.J.; Eaton, C.L. The expression and regulation of ADAMTS-1, -4, -5, -9, and -15, and TIMP-3 by TGFβ1 in prostate cells: Relevance to the accumulation of versican. Prostate 2005, 63, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Molokwu, C.N.; Adeniji, O.O.; Chandrasekharan, S.; Hamdy, F.C.; Buttle, D.J. Androgen regulates ADAMTS15 gene expression in prostate cancer cells. Cancer Invest. 2010, 28, 698–710. [Google Scholar] [CrossRef]
- Rienks, M.; Barallobre-Barreiro, J.; Mayr, M. The emerging role of the ADAMTS family in vascular diseases. Circ. Res. 2018, 123, 1279–1281. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Jiang, A.; Wiehr, C.; Wang, X.; Sinha, A.A.; Pei, D. Limited processing of pro-matrix metalloprotease-2 (Gelatinase A) overexpressed by transfection in PC-3 human prostate tumor cells: Association with restricted cell surface localization of membrane-type matrix metalloproteinase-1. J. Androl. 2004, 25, 274–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, X.; Thrasher, J.B.; Pelling, J.; Holzbeierlein, J.; Sang, Q.X.; Li, B. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology 2003, 144, 1656–1663. [Google Scholar] [CrossRef]
- Li, B.Y.; Liao, X.B.; Fujito, A.; Thrasher, J.B.; Shen, F.Y.; Xu, P.Y. Dual androgen-response elements mediate androgen regulation of MMP-2 expression in prostate cancer cells. Asian J. Androl. 2007, 9, 41–50. [Google Scholar] [CrossRef]
- Montico, F.; Kido, L.A.; Hetzl, A.C.; Lorencini, R.M.; Cândido, E.M.; Cagnon, V.H.A. Antiangiogenic therapy effects on age-associated matrix metalloproteinase-9 (MMP-9) and insulin-like growth factor receptor-1 (IGFR-1) responses: A comparative study of prostate disorders in aged and TRAMP mice. Histochem. Cell Biol. 2014, 142, 269–284. [Google Scholar] [CrossRef]
- Von Bredow, D.C.; Nagle, R.B.; Bowden, G.T.; Cress, A.E. Cleavage of β4 Integrin by Matrilysin. Exp. Cell Res. 1997, 236, 341–345. [Google Scholar] [CrossRef]
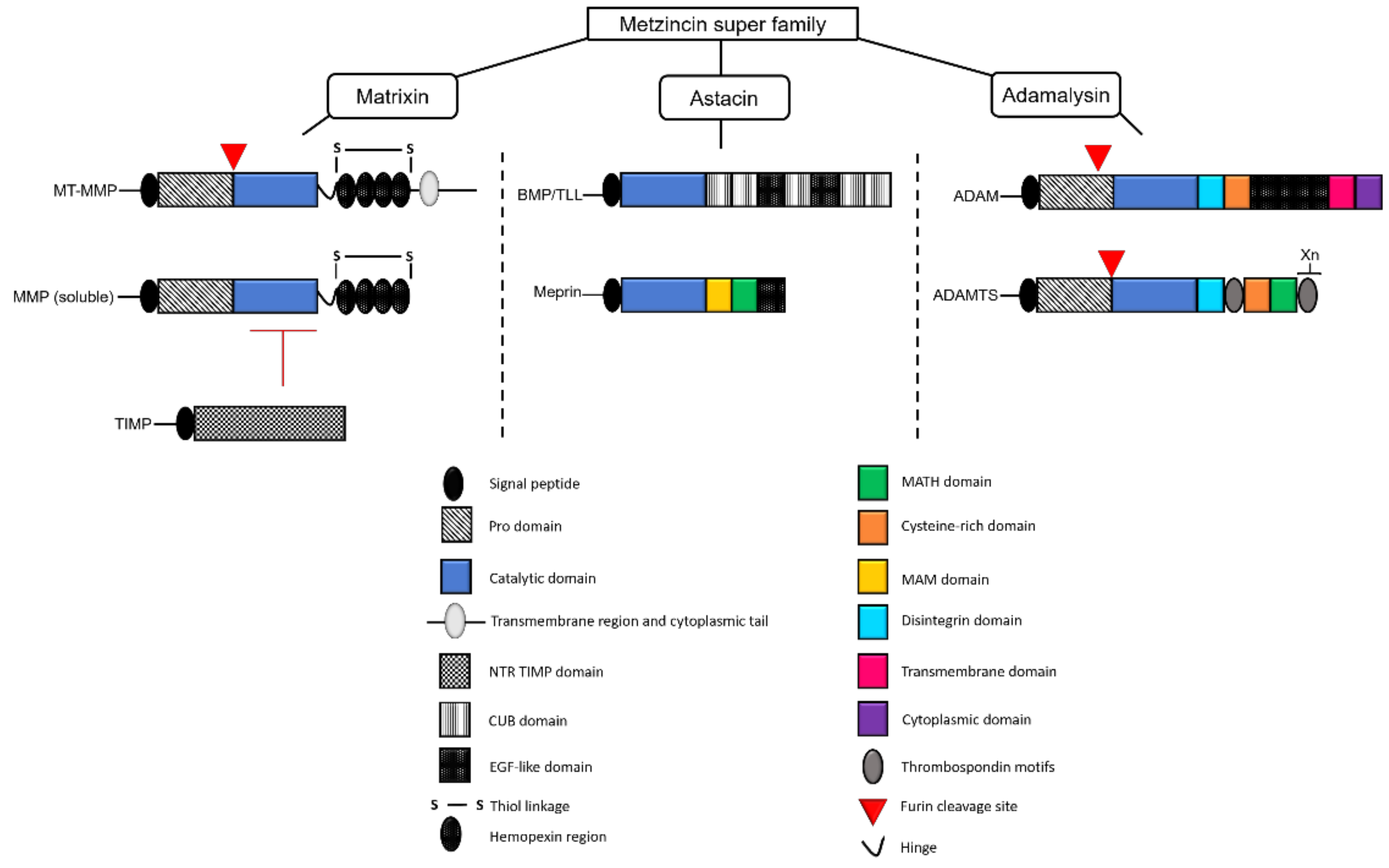
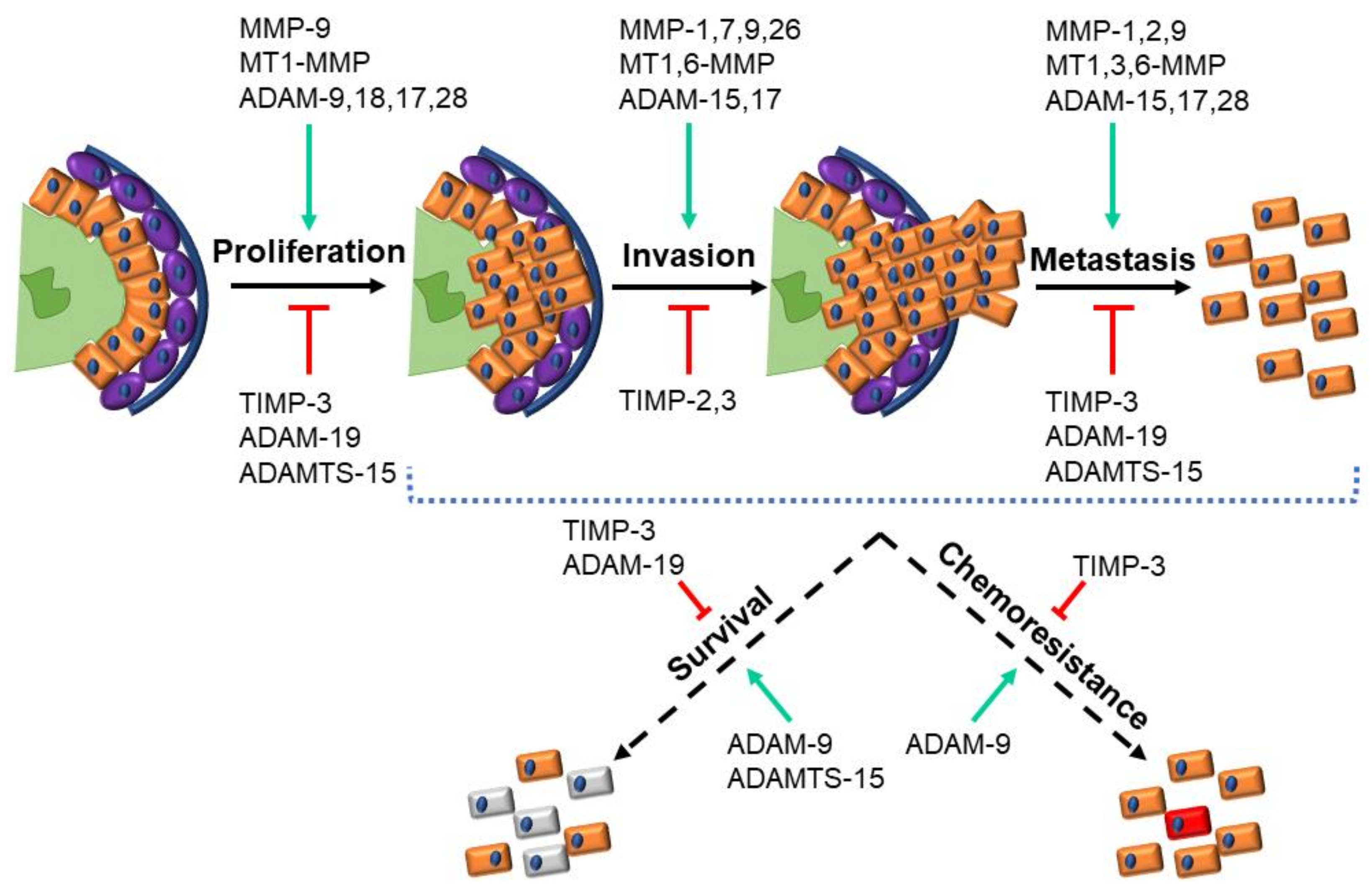
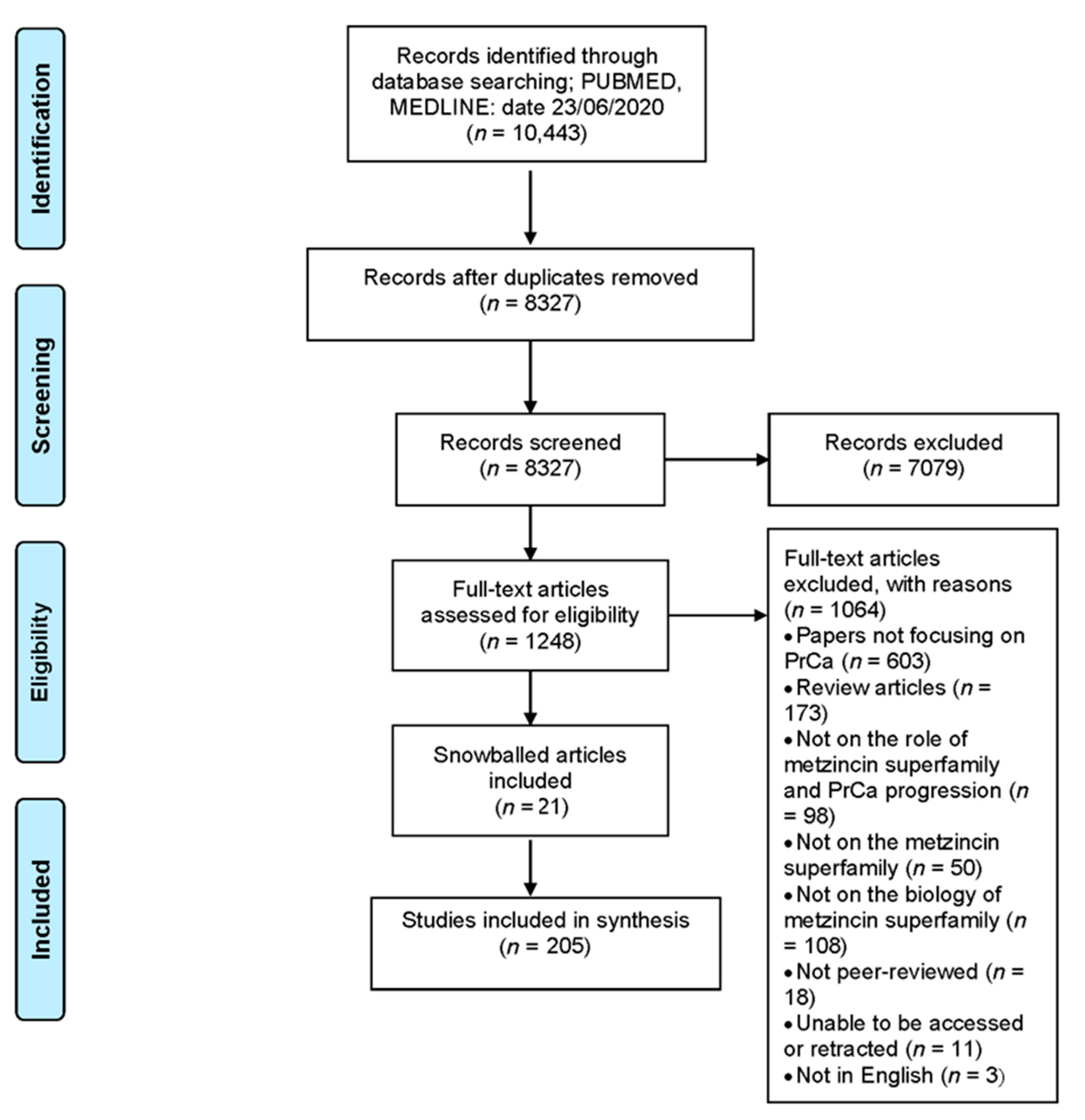
| Authors | Year | MMP | PrCa Platform | Role |
|---|---|---|---|---|
| Aalinkeel et al. [20] | 2004 | MMP-9 * | Yes—Prostate cell lines | MMP-9 expression increased in metastatic PrCa lines. Enforced expression of MMP-9 increased invasiveness, whereas ablation of expression decreased invasiveness. |
| Aalinkeel et al. [21] | 2011 | MMP-9 | Yes—Prostate cell lines | Enforced expression of MMP-9 increased invasiveness, whereas ablation decreased invasiveness, with no change in migration. |
| Adabi et al. [22] | 2015 | MMP-2 | Yes—Prostate samples | MMP-2 polymorphism not associated with PrCa risk or degree of metastasis. |
| Albayrak et al. [23] | 2007 | MMP-1 | Yes—Prostate samples | MMP-1 polymorphism not associated with PrCa risk. |
| Assikis et al. [24] | 2004 | MMP-9 | Yes—Prostate samples | MMP-9 expression low in PrCa. |
| Babichenko et al. [25] | 2014 | MMP-9* | Yes—Prostate samples | MMP-9 expression negatively correlated with Gleason score and proliferation index. |
| Baspinar et al. [26] | 2017 | MMP-9 | Yes—Prostate samples | MMP-9 expression increased in samples with high metastatic potential scores and staging. |
| Bekes et al. [27] | 2011 | MMP-9 | Yes—Prostate cell lines | Increased MMP-9-positive neutrophils in highly disseminated PrCa correlated with angiogenic potential. |
| Białkowska et al. [28] | 2018 | MMP-1, 2, 7 and 13 | Yes—Prostate samples | Polymorphism in MMP-7 (but not MMP-1, 2 and 13) correlated with increased PrCa risk. |
| Bok et al. [29] | 2003 | MMP-2, 3, 7& 9 | Yes-Mice | Active forms of MMP-2 and 9 present in late stage PrCa in mouse model, but MMP-3 not expressed and MMP-7 only focal expression. |
| Bonaldi et al. [30] | 2015 | MMP-13 | Yes—Prostate samples | MMP-13 expression not changed in PrCa compared to healthy controls. |
| Boxler et al. [31] | 2010 | MMP-2, 3, 7, 9, 13 and 19 | Yes—Prostate samples | Expression of MMP-9 (but not MMP-2, 3, 7, 13 or 19) negatively correlated with overall, recurrence-free and disease-specific survival. |
| Brehmer et al. [32] | 2003 | MMP-2 and 9 * | Yes—Prostate samples | MMP-2 expressed significantly in more advanced PrCa tumors, and MMP-9 significantly less. |
| Bruni-Cardoso et al. [33] | 2010 | MMP-9 | Yes—Mice and rats | MMP-9 expression in osteoclasts contributed to PrCa tumor growth in the bone through increased angiogenesis. |
| Cardillo et al. [34] | 2006 | MMP-1, 7 and 9 * | Yes—Prostate samples | Expression of MMPs significantly increased in the epithelium than the stroma, and of MMP-7 and 9 (but not MMP-1) with Gleason score. |
| Carozzi et al. [35] | 2016 | MMP-2 and 9 | Yes—Prostate samples | Expression of MMP-2 and 9 negatively correlated with survival. |
| Castellana et al. [36] | 2009 | MMP-2, 3, 7, 9 and 13 * | Yes—Prostate cell lines | Tumor-derived microvesicles induced MMP-9 expression that correlated with increased migration and resistance to apoptosis. |
| Coulson-Thomas et al. [37] | 2010 | MMP-1 and 9 * | Yes—Prostate cell lines | MMP-1 and 9 differentially expressed following co-culture with metastatic PrCa. |
| Daja et al. [38] | 2003 | MMP-1 and 13 * | Yes—Prostate cell lines | MMP-1 and 13 expression higher in more aggressive sublines |
| De Cicco et al. [39] | 2008 | MMP-2 and 9 * | Yes—Prostate samples | Low serum MMP-2 (but not MMP-9) associated with increased risk of disease progression. |
| Di Carlo et al. [40] | 2010 | MMP-2 and 9 | Yes—Prostate samples/urine | Active MMP-9 in urine (but not MMP-2) decreased in PrCa versus benign prostatic hyperplasia. |
| Dong et al. [41] | 2001 | MMP-9 | Yes—Prostate cell lines and mice | Pro-MMP-9 expression levels enhanced during PrCa co-culture, including in bone implants in mice. |
| dos Reis et al. [42] | 2009 | MMP-1, 2, 7 and 9 | Yes—Prostate samples | Polymorphisms in MMP-1, 2 and 9 (but not MMP-7) lower in PrCa versus controls. |
| dos Reis et al. [43] | 2010 | MMP-2 | Yes—Prostate samples | Polymorphism in MMP-2 more frequent in PrCa, including in higher Gleason scores, compared to those in MMP-9 that were associated with lower scores. |
| dos Reis et al. [44] | 2008 | MMP-1, 2, 7 and 9 | Yes—Prostate samples | Polymorphisms in MMP-1, 2 and 9 (but not MMP-7) lower in PrCa versus controls. |
| Eiro et al. [45] | 2017 | MMP-2, 9 and 11 | Yes—Prostate samples | MMP-2 expression lower and MMP-11 higher in cancer-associated fibroblasts in PrCa. |
| El-Chaer et al. [46] | 2020 | MMP-1 | Yes—Prostate samples/Serum | Genotype adjusted MMP-1 expression higher in PrCa compared to benign prostatic hyperplasia. |
| Eryilmaz et al. [47] | 2019 | MMP-2 and 9 | Yes—Prostate samples | MMP-2 expression associated with increased PrCa risk. |
| Escaff et al. [48] | 2010 | MMP-1, 2, 7, 9, 11 and 13 * | Yes—Prostate samples | Increased expression of MMP-11 and 13 associated with significant probability of biochemical recurrence. |
| Escaff et al. [49] | 2011 | MMP-1, 2, 7, 9, 11 and 13 * | Yes—Prostate samples | Expression of MMP-2 in fibroblasts and MMP-9 in mononuclear inflammatory cells associated with PrCa. |
| Escaff et al. [50] | 2011 | MMP-1, 2, 7, 9, 11 and 13 * | Yes—Prostate samples | Expression of MMP-9 and 13 in fibroblasts. MMP-13 in tumor cells associated with biological recurrence. |
| Favaro et al. [51] | 2012 | MMP-2 | Yes—Prostate samples | MMP-2 expression increased in periacinar retraction during PrCa. |
| Fernandez-Gomez et al. [52] | 2011 | MMP-1, 2, 7, 9, 11 and 13 * | Yes—prostate samples | Expression of MMP-2 negatively associated with high tumor grade, MMP-7 expression negatively associated with Prostate-Specific Antigen (PSA), whereas MMP-13 expression positively associated with PSA. |
| Festuccia et al. [53] | 1996 | MMP-2 and 9 | Yes—Prostate samples and prostate cell lines | MMP-2 and 9 highly expressed in PrCa. High MMP-9 expression and activity relative to MMP-2 associated with high Gleason grade. |
| Gohji et al. [54] | 1998 | MMP-2 | Yes—Prostate samples/Serum | Serum MMP-2 higher in patients with PrCa and higher in those with metastasis. |
| Gravina et al. [55] | 2013 | MMP-2 and 9 * | Yes—Prostate cell lines | MMP-2 consistently secreted by PrCa, whereas MMP-9 secretion sporadic. |
| Grindel et al. [56] | 2014 | MMP-7 | Yes—Prostate cell lines | MMP-7 expression associated with increased invasiveness. |
| Gupta et al. [57] | 2013 | MMP-9 | Yes—Prostate cell lines | MMP-9 knockdown resulted in increased adhesion and cell spreading. |
| Hamdy et al. [58] | 1994 | MMP-9 | Yes—Prostate samples | MMP-9 activity increased in malignant PrCa tissue compared to benign. |
| Hanqing et al. [59] | 2003 | MMP-2 and 9 | Yes—Prostate samples | MMP-2 and 9 expression higher in PrCa tissue. |
| Hashimoto et al. [60] | 1998 | MMP-7 (matrilysin) * | Yes—Prostate samples | MMP-7 levels and MMP-7/TIMP-1 ratio higher in advanced PrCa, and correlated with pathological stage, lymph node metastasis, histological differentiation, as well as vascular and lymphatic invasion. |
| Hetzl et al. [61] | 2012 | MMP-2 and 9 | Yes—Prostate samples | MMP-2 and 9 expression increased in PrCa versus controls. |
| Incorvaia et al. [62] | 2007 | MMP-2 and 9 | Yes—Prostate samples | Circulating MMP-9 (but not MMP-2) showed significant correlation with PSA. |
| Jaboin et al. [63] | 2011 | MMP-7 (matrilysin) | Yes—Prostate samples | MMP-7 polymorphism associated with PrCa recurrence. |
| Jędroszka et al. [64] | 2017 | MMP-2, 3 and 9 | Yes—Prostate samples | Expression of MMP-2, 3 and 9 increased in Gleason grade 8 and 9 tissues. |
| Jennbacken et al. [65] | 2006 | MMP-2 and 9 * | Yes—Prostate cell lines | MMP-9 expression increased in PrCa, but MMP-2 expression not detected. |
| Jung et al. [66] | 1998 | MMP * | Yes—Prostate samples | MMP levels decreased but MMP/TIMP ratio increased in PrCa. |
| Jung et al. [67] | 2003 | MMP-2 and 9 | Yes—Rats | Expression of MMP-9 (but not MMP-2) increased in advanced PrCa. |
| Jung et al. [68] | 1997 | MMP-1 and 3 * | Yes—Prostate samples | MMP-3 (but not MMP-1) highly expressed in PrCa patients with metastasis. |
| Jurasz et al. [69] | 2003 | MMP-2 | Yes—Prostate samples/Serum | Platelet MMP-2 levels increased in metastatic versus localized PrCa. |
| Kalantari et al. [70] | 2019 | MMP-13 | Yes—Prostate samples | MMP-13 highly expressed in PrCa tissue and associated with Gleason score. |
| Kaminski et al. [71] | 2006 | MMP-1 | Yes—Prostate cell lines | PrCa conditioned medium increased MMP-1 expression in fibroblasts. |
| Kanoh et al. [72] | 2002 | MMP-2 | Yes—Prostate samples/Serum | Serum MMP-2 increased in PrCa and bone metastasis, but not correlated with PSA. |
| Knox et al. [73] | 1996 | MMP-7 (matrilysin) | Yes—Prostate samples | MMP-7 expressed in PrCa. |
| Koshida et al. [74] | 2004 | MMP-1 and 2 | Yes—Prostate cell lines | MMP-1 and 2 expressed in PrCa, but only MMP-2 expression increased following implantation. |
| Kuniyasu et al. [75] | 2000 | MMP-2 and 9 | Yes—Prostate samples | Expression of MMP-2 and 9 in high grade tumors and associated with Gleason score. |
| Kuniyasu et al. [76] | 2003 | MMP-2 and 9 | Yes—Prostate samples | Increased MMP/E-cadherin ratio correlated with increased stage. |
| Larsson et al. [77] | 2020 | MMP-9 | Yes—Prostate samples, Prostate cell lines and mice | High MMP-9 expression associated with poor prognosis. |
| Latil et al. [78] | 2003 | MMP-9 * | Yes—Patient samples | MMP-9 expressed in PrCa tissue. |
| Lein et al. [79] | 1999 | MMP-2 and 3 * | Yes—Prostate samples/Serum | Plasma MMP-3 (but not MMP-2) increased in PrCa. |
| Leshner et al. [80] | 2016 | MMP-2 and 9 * | Yes—Prostate samples | MMP-9 gene repositioned in PrCa and MMP-2 in both PrCa and hyperplasia. |
| Liao et al. [81] | 2018 | MMP-1 | Yes—Prostate samples/Serum | MMP-1 promotor polymorphisms not a risk factor for PrCa. |
| Lichtinghagen et al. [82] | 2002 | MMP-2 and 9 * | Yes—Prostate samples | Expression of MMP-2 gene decreased and MMP-9 unchanged, but MMP-9 protein higher in cancerous tissue, with no change in MMP-2 protein. |
| Lichtinghagen et al. [83] | 2003 | MMP-1, 2, 7, 9 and 11 * | Yes—Prostate samples | Expression of MMP-2 and 11 decreased, and MMP-9 increased in PrCa, but no correlations with grade, stage or PSA. |
| Littlepage et al. [84] | 2010 | MMP-2, 7, 9 and 13 * | Yes—Mice | Expression of MMP-2, 7 and 9 increased with PrCa progression. MMP-2 knockout mice showed reduced tumor burden, prolonged survival, decreased lung metastasis, and decreased blood vessel density. Knockout of MMP-7 or MMP-9 did not impact tumor growth or survival but affected blood vessel formation. |
| Liu et al. [85] | 2017 | MMP-9 | Yes—Prostate samples and prostate cell lines | MMP-9 expression increased in metastatic cancer. |
| Lokeshwar et al. [86] | 1993 | MMP-2 and 9 * | Yes—Prostate samples/Serum | MMP-2 and 9 secretion, including of the active form of MMP-2, increased in neoplastic tissue. |
| London et al. [87] | 2003 | MMP-9 | Yes—Prostate cell lines | Ablation of MMP-9 caused decreased tumor invasion, migration, and growth. |
| Lynch et al. [88] | 2005 | MMP-7 (matrilysin) | Yes—Rat | MMP-7 expression increased at tumor/bone interface. MMP-7 knockout mice showed reduced tumor-induced osteolysis. |
| Marin-Aguilera et al. [89] | 2015 | MMP-9 | Yes—Prostate samples | MMP-9 upregulated in PrCa and correlated with poorer overall survival. |
| Maruta et al. [90] | 2010 | MMP-10 | Yes—Prostate samples | MMP-10 expression correlated with stage, cell renewal and vascular invasion. |
| Medina-González et al. [91] | 2020 | MMP-2, 9, 11 and 13 * | Yes—Prostate samples | Expression of MMP-2 and 9 increased (but MMP-11 and 13 unchanged) in PrCa. |
| Miyake et al. [92] | 2010 | MMP-2 and 9 | Yes—Prostate samples | MMP-2 and 9 expression correlated with stage, recurrence, proliferation, and invasion. |
| Montironi et al. [93] | 1995 | MMP-2 (type IV collagenase) * | Yes—Prostate samples | MMP-2 protein expression identified in cells in contact with the stroma |
| Montironi et al. [94] | 1996 | MMP-2 (type IV collagenase) | Yes—Prostate samples | MMP-2 expression correlated with progression. |
| Morgia et al. [95] | 2005 | MMP-2, 3 and 13 * | Yes—Prostate samples | Plasma levels of MMP-2 and 9 (but not MMP-13) increased in metastatic PrCa. |
| Moses et al. [96] | 1998 | MMP-2 and 9 | Yes—Prostate samples/Urine | Active MMP-2 and 9 in urine were independent predictor of organ-confined PrCa. |
| Muñoz et al. [97] | 2017 | MMP-2 and 9 | Yes—Prostate samples/Urine | No difference in urine levels of MMP-2 or MMP-9 species in PrCa. |
| Nabha et al. [98] | 2006 | MMP-9 | Yes—Mice | MMP-9 knockout resulted in no difference in tumor incidence, growth or microvascularity. |
| Nagle et al. [99] | 1994 | MMP-7 (matrilysin) | Yes—Prostate samples | MMP-7 expression in PrCa located in dilated ducts when inflamed and atrophic glands. |
| Nalla et al. [100] | 2010 | MMP-9 | Yes—Prostate cell lines | Ablation of MMP-9 reduced migration and invasion and induced apoptosis. |
| Neuhaus et al. [101] | 2017 | MMP-3, 7, 13 and 20 * | Yes—Prostate samples | Decreased MMP-3/TIMP ratio in PrCa, but other MMPs not altered. |
| Oguić et al. [102] | 2014 | MMP-2 and 9 | Yes—Prostate samples | Higher MMP-2 and 9 expression in positive surgical margins. MMP-9 expression associated with biochemical recurrence. |
| Ok Atılgan et al. [103] | 2020 | MMP-9 | Yes—Patient samples | MMP-9 expression positively associated with WHO grade, tumor stage, extracapsular extension, positive surgical margin lymphovascular, perineural invasion and decreased disease-free survival. |
| Ouyang et al. [104] | 2001 | MMP-7 (matrilysin) | Yes—Rats | MMP-7 expressed in premalignant and malignant tissue. |
| Ozden et al. [105] | 2013 | MMP-1 and 9 * | Yes—Prostate samples | MMP-1 expression in tumors correlated with higher grades and Gleason scores. MMP-9 expression in normal glands correlated with low PSA and Gleason scores. |
| Pajouh et al. [106] | 1991 | MMP-7 (matrilysin) | Yes—Prostate cell lines | MMP-7 expressed in invasive metastatic primary human PrCa. |
| Pang et al. [107] | 2004 | MMP-13 | Yes—Prostate samples and prostate cell lines | MMP-13 expressed in PrCa. |
| Pettaway et al. [108] | 2008 | MMP-2 and 9 | Yes—Prostate samples | MMP-2 and MMP-9/E-cadherin ratio increased at tumor edge and correlated with disease, biochemical recurrence, and pathological stage. |
| Pouyanfar et al. [109] | 2016 | MMP-9 | Yes—Prostate samples | MMP-9 expression higher in PrCa patients related to Gleason score and age, but not PSA, metastasis, or survival. |
| Powell et al. [110] | 1993 | MMP-7 (matrilysin) | Yes—Prostate cell lines and mice | Enforced MMP-7 expression led to increased invasion. |
| Prior et al. [111] | 2010 | MMP-2 | Yes—Prostate samples | Increased MMP-2 levels in urine/blood associated with PrCa progression. |
| Reis et al. [112] | 2012 | MMP-2 * | Yes—Prostate samples | MMP-2 expression reduced in PrCa samples but increased in higher grades. |
| Reis et al. [113] | 2015 | MMP-2&9 * | Yes—Prostate samples | MMP-2 and 9 expressed in most PrCa but no prognostic value. |
| Reis et al. [114] | 2011 | MMP-9 * | Yes—Prostate samples | Higher MMP-9 expression associated with increased PSA and recurrence, but not Gleason score. |
| Riddick et al. [115] | 2005 | MMP-2, 10, 23 and 25 * | Yes—Prostate samples | Increased expression of MMP-10 and 25, but MMP-2 and 23 decreased in PrCa. |
| Ross et al. [116] | 2003 | MMP-2 * | Yes—Prostate samples | MMP-2 expressed in more advanced PrCa and correlated with prognostic variables. |
| Sakai et al. [117] | 2005 | MMP-2 and 9 | Yes—Prostate samples | Increased expression of MMP-2 and 9 in peripheral zone cancers compared to transitional zone. |
| San Francisco et al. [118] | 2004 | MMP-9 | Yes—Prostate cell lines | MMP-9 expression not changed in PrCa. |
| Sauer et al. [119] | 2004 | MMP-2 and 9 | Yes—Prostate samples/Serum | MMP-9 serum levels increased in PrCa patients and correlated with grade, but tissue MMP-9 activity not related to stage or grade. MMP-2 activity correlated with disease progression. |
| Schäfer et al. [120] | 2012 | MMP-9 | Yes—Prostate cell lines and mice | Enforced MMP-9 expression enhanced tumor regression and impacted metastasis. |
| Schveigert et al. [121] | 2013 | MMP-9 | Yes—Prostate samples | MMP-9 polymorphism and increased expression associated with PrCa, with polymorphism related to pathological stage and prognostic group, and expression with survival. |
| Sehgal et al. [122] | 1998 | MMP-9 | Yes—Mice | Ablation of MMP-9 reduced metastatic potential. |
| Sehgal et al. [123] | 2003 | MMP-1, 2 and 9 * | Yes—Prostate cell lines | Expression of MMP-1 (but not MMP-2 or MMP-9) decreased in more metastatic PrCa. |
| Serretta et al. [124] | 2018 | MMP-3 | Yes—Prostate samples | MMP-3 expression not associated with Gleason score 4 and 5. |
| Sfar et al. [125] | 2007 | MMP-9 | Yes—Prostate samples | MMP-9 polymorphism associated with increased risk of advanced PrCa. |
| Sfar et al. [126] | 2009 | MMP-9 | Yes—Prostate samples | MMP-9 polymorphism associated with increased risk of developing PrCa. |
| Shah et al. [127] | 2016 | MMP-9 | Yes—Prostate cell lines | Increased MMP-9 expression in PrCa cells co-cultured with dermal lymphatic microvascular endothelial cells. |
| Shajarehpoor Salavati et al. [128] | 2017 | MMP-2 | Yes—Prostate sample | MMP-2 polymorphism not associated with PrCa risk. |
| Shi et al. [129] | 2017 | MMP-9 | Yes—Prostate samples/Urine | MMP-9 detected in urine of PrCa patients. |
| Silva et al. [130] | 2015 | MMP-2 | Yes—Prostate samples | Increased MMP-2 expression in reactive stroma. |
| Srivastava et al. [131] | 2012 | MMP-2 * | Yes—Prostate samples | MMP-2 polymorphism associated with PrCa risk but not staging. |
| Stearns et al. [132] | 1996 | MMP-2 | Yes—Prostate samples and prostate cell lines | MMP-2 expression increased in higher PrCa grades. |
| Stearns et al. [133] | 1996 | MMP-2 | Yes—Prostate samples | MMP-2a expressed in glandular epithelial cells and increased in PrCa samples with high Gleason score. |
| Still et al. [134] | 2000 | MMP-2 * | Yes—Prostate samples | MMP-2 localized to malignant cells, with increased MMP-2/TIMP-2 ratio associated with high grade and stage. |
| Szarvas et al. [135] | 2018 | MMP-7 (matrilysin) | Yes—Prostate samples | Serum MMP-7 level significantly higher in docetaxel-resistant PrCa and associated with poor survival. |
| Trudel et al. [136] | 2008 | MMP-2 * | Yes—Prostate samples | MMP-2 expression in basal epithelial cells and stromal cells associated with shorter disease-free survival. |
| Trudel et al. [137] | 2003 | MMP-2 | Yes—Prostate samples | MMP-2 expression in malignant cells associated with disease-free survival. |
| Trudel et al. [138] | 2010 | MMP-9 | Yes—Prostate samples | MMP-9 expression correlated with Gleason score but not disease-free survival. |
| Tsuchiya et al. [139] | 2009 | MMP-1 | Yes—Prostate samples | MMP-1 promotor polymorphisms (and increased expression) associated with pathological stage but not PrCa susceptibility or progression. |
| Upadhyay et al. [140] | 1999 | MMP-2 * | Yes—Prostate samples | MMP-2 localization altered in PrCa. |
| Vallbo et al. [141] | 2005 | MMP-2 and 9 | Yes-Rat | MMP-2 (but not MMP-9) expressed in malignant cells. |
| Wang et al. [142] | 2014 | MMP-1 | Yes—Prostate samples | MMP-1 expression significantly increased in PrCa and associated with higher Gleason score, metastasis and pathological stage, as well as reduced overall and recurrence-free survival. |
| Wiesner C [143] | 2007 | MMP-9 | Yes—Prostate cell lines and mice | Increased MMP-9 activity when PrCa cells interact with bone. |
| Wilson et al. [144] | 2002 | MMP-2 and 9 | Yes—Prostate samples and prostate cell lines | Epithelial cells secreted little MMP-2 or MMP-9, whereas pro-MMP-2 (but not MMP-9) secreted by stromal cells. |
| Wilson et al. [145] | 1993 | MMP | Yes—Prostate cell lines and mice | MMP isoforms differentially altered in PrCa. |
| Wood et al. [146] | 1997 | MMP-2 and 9 * | Yes—Prostate samples | Increased expression of MMP-2&9 in high grade samples. |
| Xie et al. [147] | 2016 | MMP-7 (matrilysin) | Yes-Mice | MMP-7 expression elevated in PrCa. |
| Xu et al. [148] | 2010 | MMP-9 | Yes—Prostate cell lines and mice | Ablation of MMP-9 led to decreased cell invasion. |
| Yaykaşli et al. [149] | 2014 | MMP-2 * | Yes—Prostate samples | MMP-2 polymorphisms increased in PrCa patients. |
| Zellweger et al. [150] | 2005 | MMP-2 | Yes—Prostate samples | MMP-2 expressed in PrCa, but not altered across different types. |
| Zhang et al. [151] | 2002 | MMP-2 and 7 * | Yes—Prostate samples and prostate cell lines | MMP-2 expressed in stromal cells and MMP-7 expressed in epithelial cells. Differential expression between cell lines. |
| Zhang et al. [152] | 2004 | MMP-2 and 9 | Yes—Prostate samples and Prostate cell lines | Expression of MMP-9 (but not MMP-2) increased in malignant samples. |
| Zhang et al. [153] | 2008 | MMP-2 and 9 | Yes—Prostate samples and prostate cell lines | Expression of MMP-2 and 9 increased in PrCa. |
| Zhang et al. [154] | 2018 | MMP-1 | Yes—Prostate cell lines | Expression of MMP-1 increased in more metastatic lines. Ablation of MMP-1 decreased invasion and migration. |
| Zhao et al. [155] | 2003 | MMP-9 and 26 | Yes—Prostate samples and prostate cell lines | Expression of MMP-26 (but not MMP-9) increased in PrCa. Blocking of either reduced invasion. |
| Zhong et al. [156] | 2008 | MMP-1, 2 and 9 | Yes—Prostate samples | MMP-1, 2 and 9 expression significantly higher in PrCa. MMP-2 expression correlated with TMN grade and Gleason score. |
| Zhu et al. [157] | 1999 | MMP-2 and 9 | Yes—Prostate cell lines | MMP-2 expression enhanced when PrCa co-cultured. |
| Authors | Year | MT-MMP | PrCa Platform | Role |
|---|---|---|---|---|
| Aalinkeel et al. [20] | 2004 | MT1- and MT4-MMP * | Yes—Prostate cell lines | MT4-MMP expression higher in metastatic PrCa cells lines. |
| Bair et al. [159] | 2005 | MT1-MMP | Yes—Prostate samples and prostate cell lines | Ablation of MT1-MMP decreased migration and invasion. |
| Bonfil et al. [163] | 2007 | MT1-MMP | Yes—Prostate samples and prostate cell lines | MT1-MMP expressed in PrCa bone metastasis. Enforced expression of MT1-MMP enhanced tumor growth and osteolysis. |
| Cao et al. [158] | 2008 | MT1-MMP | Yes—Prostate cell lines | MT1-MMP expression increased in PrCa. Enforced expression of MT1-MMP induced epithelial to mesenchymal transition associated with metastatic ability. |
| Cardillo et al. [34] | 2006 | MT1-MMP * | Yes—Prostate samples | MT1-MMP expression increased in epithelial and stromal tissues in PrCa. |
| Castellana et al. [36] | 2009 | MT1-MMP * | Yes—Prostate cell lines | MT1-MMP protein levels high in PrCa microvesicles. |
| Cheng et al. [165] | 2017 | MT6-MMP | Yes—Prostate samples/Serum | MT6-MMP expression upregulated in serum and tissue in PrCa. |
| Chu et al. [166] | 2006 | MT3-MMP | Yes—Prostate cell lines and mice | MT3-MMP expressed in PrCa tumors, especially in lymph node metastases. |
| Coulson-Thomas et al. [37] | 2010 | MT1-MMP * | Yes—Prostate cell lines | MT1-MMP expressed in the stromal cells during co-culture with metastatic PrCa cells, extending into the ECM |
| Daja et al. [38] | 2003 | MT1-, MT2- and MT3-MMP * | Yes—Prostate cell lines | MT1&3-MMP (but not MT2-MMP) expressed highly, particularly processed versions, in aggressive PrCa cell lines. |
| Jennbacken et al. [65] | 2006 | MT1-MMP * | Yes—Prostate cell lines | MT1-MMP expression increased in more invasive PrCa subline. |
| Jiang et al. [167] | 2017 | MT3-MMP | Yes—Prostate samples and prostate cell lines | High levels of MT3-MMP associated with advanced tumor stage and metastasis. Ablation of MT3-MMP decreased migration. |
| Jung et al. [168] | 2003 | MT1- and MT5-MMP | Yes—Prostate samples and prostate cell lines | MT1 and 5-MMP expressed in most PrCa cell lines and prostate tissue, with variable expression in metastatic lines and malignant tumors, with no correlation to tumor classification. |
| Khamis et al. [169] | 2016 | MT6-MMP | Yes—Prostate samples and prostate cell lines | MT6-MMP expression up-regulated in high grade prostate intraepithelial neoplasia but decreased with PrCa progression, with MT6-MMP expressing cells prone to apoptosis. |
| Latil et al. [78] | 2003 | MT1-MMP * | Yes—Patient samples | MT1-MMP expression decreased in PrCa tissue. |
| Lee et al. [170] | 2006 | MT6-MMP | Yes—Prostate samples | MT6-MMP expression increased in high-grade prostatic intraepithelial neoplasia but reduced in invasive cancer. |
| Lichtinghagen et al. [83] | 2003 | MT1-MMP * | Yes—Prostate samples | MT1-MMP expression observed in PrCa, but no correlation with grade, stage, or serum PSA. |
| Lin et al. [171] | 2013 | MT3-MMP | Yes—Prostate samples | MT3-MMP single nucleotide polymorphisms associated with PrCa aggressiveness. |
| Lin et al. [172] | 2016 | MT3-MMP | Yes—Prostate samples | MT3-MMP expression associated with PrCa aggressiveness. |
| Littlepage et al. [84] | 2010 | MT1-MMP * | Yes—Mice | Broad MMP inhibitor reduced tumor burden. |
| Liu et al. [173] | 2010 | MT1-MMP | Yes—Prostate cell lines | MT1-MMP ablation reduced susceptibility to immune-mediated killing. |
| Nagakawa et al. [174] | 2000 | MT1-MMP | Yes—Prostate cell lines | MT1-MMP expression increased in more metastatic PrCa lines. |
| Neuhaus et al. [101] | 2017 | MT1-MMP * | Yes—Prostate samples | MT1-MMP expression decreased in PrCa but increased in benign prostatic hyperplasia. |
| Nguyen et al. [161] | 2011 | MT1-MMP | Yes—Prostate cell lines | Enforced MT1-MMP expression increased invasion. |
| Reis et al. [112] | 2012 | MT1-MMP * | Yes—Prostate samples | MT1-MMP under-expressed in PrCa. |
| Riddick et al. [115] | 2005 | MT2-, MT5- and MT6-MMP * | Yes—Prostate samples | MT2- and MT6-MMP (but not MT5-MMP) expression correlated positively with Gleason score. |
| Sabbota et al. [160] | 2010 | MT1-MMP | Yes—Prostate cell lines | Enforced MT1-MMP expression enhanced tumor migration. |
| Sroka et al. [175] | 2008 | MT1-MMP | Yes—Prostate samples and prostate cell lines | MT1-MMP expression in apical regions in PIN and PrCa. |
| Udayakumar et al. [176] | 2003 | MT1-MMP | Yes—cells and patients | Ablation of MT1-MMP enhanced cell migration. |
| Upadhyay et al. [140] | 1999 | MT1-MMP * | Yes—Prostate samples | MT1-MMP localized in benign glands changing to cytoplasmic staining and then heterogenous as PrCa progressed. Increased vasculature when MT1-MMP co-localized with MMP-2. |
| Wang et al. [164] | 2009 | MT1-MMP | Yes—Prostate cell lines and mice | MT1-MMP expression increased in tumor cells.Enforced expression increased tumor growth in mice. |
| Zarrabi et al. [162] | 2011 | MT1-MMP | Yes—Prostate cell lines | MT1-MMP inhibition decreased cell migration, but not growth. |
| Zhang et al. [151] | 2002 | MT1- and MT3-MMP * | Yes—Prostate samples and prostate cell lines | MT1 and 3-MMP expressed in stromal and epithelial cells in PrCa. |
| Zhao et al. [155] | 2003 | MT6-MMP * | Yes—Prostate samples and prostate cell lines | MT6-MMP highly expressed in PrCa samples. Inhibition of MT6-MMP decreased invasion. |
| Authors | Year | TIMP | PrCa Platform | Role |
|---|---|---|---|---|
| Aalinkeel et al. [20] | 2004 | TIMP-1, 3 and 4 * | Yes—Prostate cell lines | TIMP-1 and 4 (but not TIMP-3) expressed higher in more metastatic PrCa cells. |
| Adissu et al. [181] | 2015 | TIMP-3 | Yes—Mice | TIMP-3 mouse knockout exhibited enhanced PrCa tumor growth and invasion. |
| Ashida et al. [178] | 2004 | TIMP-1 | Yes—Prostate samples | TIMP-1 expression down-regulated in the transition to PrCa. |
| Babichenko et al. [25] | 2014 | TIMP-1 * | Yes—Prostate samples | TIMP-1 expression lower in PrCa adenocarcinoma compared to benign prostatic hyperplasia. |
| Baker et al. [182] | 1994 | TIMP-1 and 2 * | Yes—Prostate samples | TIMP-1 expression higher and TIMP-2 lower in PrCa patients. |
| Brehmer et al. [32] | 2003 | TIMP-1 and 2 * | Yes—Prostate samples | TIMP-1 expression decreased in PrCa compared to normal tissue, whereas TIMP-2 expression not significantly different. |
| Daja et al. [38] | 2003 | TIMP-1 * | Yes—Prostate cell lines | TIMP-1 expression higher in more aggressive PrCa cell lines. |
| De Cicco et al. [39] | 2008 | TIMP-1 and 2 * | Yes—Prostate samples/Serum | TIMP-1 and 2 serum levels do not correlate with PrCa progression. |
| Deng et al. [183] | 2006 | TIMP-3 | Yes—Prostate cell lines | Enforced TIMP-3 expression caused apoptosis and increased sensitivity to chemotherapeutic agents. |
| Escaff et al. [48] | 2010 | TIMP-1, 2 and 3 * | Yes—Prostate samples | TIMP-1 expression significantly increased in PrCa and associated with Gleason score. |
| Escaff et al. [49] | 2011 | TIMP-1, 2 and 3 * | Yes—Prostate samples | Expression of TIMP-3 (but not TIMP-1 and 2) increased in mononuclear inflammatory cells in PrCa carcinoma. |
| Fernandez-Gomez et al. [52] | 2011 | TIMP-1, 2 and 3 * | Yes—Prostate samples | TIMP-2 expression in mononuclear inflammatory cells significantly associated with decreased tumor grade. TIMP-3 expression in stromal fibroblasts correlated with histological grade. |
| Gong et al. [184] | 2015 | TIMP-1 | Yes—Prostate samples and prostate cell lines | TIMP-1 highly expressed in tumors from castration-resistant PrCa patients. |
| Gravina et al. [55] | 2013 | TIMP-1, 2 and 3 * | Yes—Prostate cell lines | TIMP-1, 2 and 3 expression reduced in PrCa compared to benign prostatic hyperplasia. |
| Gustavsson et al. [185] | 2008 | TIMP-2 and 3 * | Yes—Prostate cell lines and mice | Expression of TIMP-2 (but not TIMP-3) higher in PrCa xenografts. |
| Hashimoto et al. [60] | 1998 | TIMP-1 * | Yes—Prostate samples | MMP-7/TIMP-1 ratio higher in advanced PrCa and correlated with increased invasion and elevated PSA. |
| Hoque et al. [186] | 2005 | TIMP-3 | Yes—Prostate samples/Urine | TIMP-3 gene promoter methylated in urine samples of PrCa patients. |
| Jerónimo et al. [187] | 2004 | TIMP-3 | Yes—Prostate samples | TIMP-3 gene promoter methylation significant in high-grade prostatic intraepithelial neoplasia and benign prostatic hyperplasia. |
| Jung et al. [66] | 1998 | TIMP-1 * | Yes—Prostate samples | TIMP-1 expression lower in PrCa versus normal tissue. |
| Jung et al. [68] | 1997 | TIMP-1 * | Yes—Prostate samples | TIMP-1 highly expressed in PrCa patients with metastasis compared to benign prostatic hyperplasia. TIMP-1 correlated with PrCa staging not grade. |
| Kamińska et al. [188] | 2019 | TIMP-2 | Yes—Prostate cell lines | TIMP-2 promoter hypermethylation resulting in decreased expression in PrCa. |
| Karan et al. [189] | 2003 | TIMP-3 * | Yes—Prostate samples and prostate cell lines | TIMP-3 not expressed in PrCa cell lines, only in benign prostatic hyperplasia. |
| Kim et al. [179] | 2012 | TIMP-1 * | Yes—Prostate samples | TIMP-1 expression downregulated in metastatic PrCa. |
| Kuefer et al. [190] | 2006 | TIMP-2 * | Yes—Prostate samples and prostate cell lines | TIMP-2 over-expressed in PrCa tissue. |
| Kwabi-Addo et al. [191] | 2010 | TIMP-3 | Yes—Patient samples | TIMP-3 promoter more highly methylated in PrCa versus controls. |
| Lee et al. [192] | 2012 | TIMP-2 | Yes—Prostate cell lines and mice | TIMP-2 administration decreased tumor growth. |
| Lein et al. [79] | 1999 | TIMP-1 * | Yes—Prostate samples | TIMP-1 plasma concentration significantly higher in PrCa and correlated with tumor stage. |
| Leshner et al. [80] | 2016 | TIMP-2 and 3 * | Yes—Prostate samples | TIMP-2 and 3 genes do not reposition during PrCa progression. |
| Lichtinghagen et al. [82] | 2002 | TIMP-1 * | Yes—Prostate samples | TIMP-1 protein, but not mRNA, decreased in PrCa tissue. |
| Lichtinghagen et al. [83] | 2003 | TIMP-1, 2 and 3 * | Yes—Prostate samples | Expression of TIMP-2 and 3 (but not TIMP-1) decreased in PrCa tissue, with TIMP-2 correlating with stage. |
| Liu et al. [177] | 2005 | TIMP-1 | Yes—Prostate samples | TIMP-1 protein levels decreased in PrCa samples, being located in secretory cells. |
| Lokeshwar et al. [86] | 1993 | TIMP * | Yes—Prostate samples | TIMP expression high in normal, but not neoplastic prostate. |
| Morgia et al. [95] | 2005 | TIMP-1 * | Yes—Prostate samples | TIMP-1 expression reduced in patients with metastatic PrCa. |
| Oh et al. [193] | 2011 | TIMP-1 | Yes—Prostate samples | Elevated plasma TIMP-1 correlated with decreased survival in metastatic PrCa. |
| Ozden et al. [105] | 2013 | TIMP-1 * | Yes—Prostate samples | TIMP-2 expression in normal glands associated with lower Gleason grade. |
| Pulukuri et al. [194] | 2007 | TIMP-2 | Yes—Prostate samples and prostate cell lines | Re-expression of TIMP-2 reduced tumor invasion. |
| Reis et al. [112] | 2012 | TIMP-2 * | Yes—Prostate samples | TIMP-2 under expressed in PrCa samples, but expression increased in higher grades. |
| Reis et al. [113] | 2015 | TIMP-1 and 2 * | Yes—Prostate samples | Reduced TIMP-1 expression associated with recurrence, whereas TIMP-2 expression negative in all cases. |
| Reis et al. [114] | 2011 | TIMP-1 * | Yes—Prostate samples | TIMP-1 under-expressed in PrCa samples but over-expressed in benign samples. |
| Riddick et al. [115] | 2005 | TIMP-3 and 4 * | Yes—Prostate samples | TIMP-3 and 4 expression negatively correlated with Gleason score. |
| Ross et al. [116] | 2003 | TIMP-2 * | Yes—Prostate samples | TIMP-2 expression correlated with advanced PrCa. |
| Ross et al. [195] | 2012 | TIMP-1 | Yes—Prostate samples | TIMP-1 expression in blood cells upregulated in PrCa. |
| Sehgal et al. [123] | 2003 | TIMP-1 * | Yes—Prostate cell lines | TIMP-1 expression reduced in metastatic PrCa subline. |
| Shinojima et al. [196] | 2012 | TIMP-3 | Yes—Prostate samples | TIMP-3 expression down regulated in PrCa versus normal due to promoter hypermethylation. |
| Srivastava et al. [131] | 2012 | TIMP-2 * | Yes—Prostate specimens | TIMP-2 GC polymorphism associated with PrCa progression not initiation, as well as cancer risk. |
| Stearns et al. [180] | 1995 | TIMP-1 * | Yes—Prostate cell lines | TIMP-1 expressed in PrCa cells. |
| Still et al. [134] | 2000 | TIMP-2 * | Yes—prostate specimens | MMP-2/TIMP-2 ratio increased in tumors of higher grade and stage. |
| Trudel et al. [136] | 2008 | TIMP-2 * | Yes—Prostate specimens | Higher TIMP-2 expression associated with longer disease-free survival. |
| Wood et al. [146] | 1997 | TIMP-1 and 2 * | Yes—Prostate samples | TIMP-1 and 2 expressed in stromal inversely correlated with Gleason score, with reduced expression in metastatic PrCa samples. |
| Yamanaka et al. [197] | 2003 | TIMP-3 | Yes—Prostate samples | TIMP-3 promoter methylation low, and unchanged between PrCa and benign samples. |
| Yaykaşli et al. [149] | 2014 | TIMP-2 * | Yes—Prostate samples | TIMP-2 polymorphism under-represented in PrCa patients. |
| Zhang et al. [151] | 2002 | TIMP-1 and 2 * | Yes—Prostate samples and prostate cell lines | TIMP-1 and 2 expressed in both stromal and epithelial cells in PrCa, with no difference between fibroblasts and smooth muscle cells. Tendency for higher TIMP-2 expression in cells derived from malignant PrCa tissue. |
| Zhang et al. [198] | 2010 | TIMP-3 | Yes—Prostate samples, Prostate cell lines and mice | Enforced TIMP-3 expression inhibited proliferation, survival, migration, invasion, and adhesion of cells, with reduced incidence and size of tumors in mice. |
| Authors | Year | ADAM | PrCa Platform | Role |
|---|---|---|---|---|
| Arima et al. [204] | 2007 | ADAM-10 | Yes—Prostate cell lines and prostate samples | ADAM-10 nuclear localization significantly increased in PrCa compared to benign and correlated with Gleason score. Ablation of ADAM-10 decreased cell proliferation. |
| Bilgin Doğru et al. [205] | 2014 | ADAM-12 | Yes—Prostate samples/urine | Serum and urine ADAM-12 levels significantly higher in PrCa patients compared to healthy controls, but no correlation with stage. |
| Burdelski et al. [199] | 2017 | ADAM-15 | Yes—Prostate samples | ADAM-15 expression correlated to stage, Gleason grade, lymph node metastasis and PSA recurrence. |
| Fritzsche et al. [206] | 2006 | ADAM-8 | Yes—Prostate samples | ADAM-8 expression correlated with higher Gleason score, but not PSA relapse-free survival. |
| Fritzsche et al. [207] | 2008 | ADAM-9 | Yes—Prostate samples | ADAM-9 expression significantly higher in PrCa compared to normal tissue, and associated with shortened PSA relapse-free survival, especially in androgen-ablated patients. |
| Hoyne et al. [208] | 2016 | ADAM-19 | Yes—Prostate samples and prostate cell lines | ADAM-19 expression decreased in PrCa compared to normal tissue, and positively correlated with lower grade and reduced relapse. Over-expression of ADAM-19 reduced proliferation and migration, but increased cell death. |
| Josson et al. [209] | 2011 | ADAM-9 | Yes—cells | Ablation of ADAM-9 increased apoptosis, increased sensitivity to radiation and chemotherapy, and induced epithelial phenotype. |
| Karan et al. [189] | 2003 | ADAM-9, 10 and 17 * | Yes—cells and patients | Expression of ADAM-17 (but not ADAM-9 or ADAM-10) increased in PrCa compared to benign samples. |
| Kuefer et al. [190] | 2006 | ADAM-15 * | Yes—Prostate samples and prostate cell lines | Expression of ADAM-15 significantly higher in PrCa and associated with increased Gleason score and angioinvasion. |
| Lin et al. [201] | 2012 | ADAM-17 | Yes—Prostate cell lines | Enforced ADAM-17 expression increased cell proliferation. |
| Lin et al. [210] | 2012 | ADAM-9 | Yes—Prostate samples | ADAM-9 expression reduced in castrate-resistant PrCa compared to hormone-sensitive PrCa, with low expression in castrate-resistant PrCa associated with shorter overall survival. |
| Liu et al. [211] | 2013 | ADAM-9 | Yes—Prostate cell lines and mice | Ablation of ADAM-9 decreased proliferation in vivo and tumor growth in mice. |
| McCulloch et al. [212] | 2000 | ADAM-9, 10, 11, 15 and 17 | Yes—Prostate cell lines | ADAM-9, 10, 11, 15 and 17 expressed in PrCa cell lines, with androgens increasing expression of ADAM-9 and 10 while ADAM-17 was downregulated. |
| McCulloch et al. [213] | 2004 | ADAM-10 | Yes—Prostate samples and prostate cell lines | ADAM-10 localized to secretory cells in PrCa with additional basal cell in benign glands. |
| Najy et al. [200] | 2008 | ADAM-15 | Yes—Cells | Ablation of ADAM-15 reduced migration and adhesion in vitro and decreased bone metastasis in mice. |
| Peduto et al. [214] | 2006 | ADAM-12 | Yes—Mice | ADAM-12 expressed in stromal cells adjacent to epithelial cells in PrCa. ADAM12 knock-out mice showed delayed tumor progression. |
| Peduto et al. [215] | 2005 | ADAM-9 | Yes—Mice | ADAM-9 expression elevated in mouse PrCa model. ADAM-9 knock-out resulted in well differentiated tumors. Overexpression of ADAM-9 led to epithelial hyperplasia and intraepithelial neoplasia. |
| Pen et al. [216] | 2012 | ADAM-9 | Yes—Prostate samples, prostate cell lines and mice | ADAM-9 nuclear expression observed in hormone refractory PrCa and in relapse patients, with levels correlated with the risk of relapse. |
| Rudnicka et al. [203] | 2016 | ADAM-28 | Yes—Prostate samples and prostate cell lines | ADAM-28 expression increased in PrCa samples compared to normal tissue and in PrCa cell lines. Over-expression of ADAM-28 stimulated proliferation and migration, whereas ablation of expression or activity reduced these phenotypes. |
| Shigemura et al. [217] | 2007 | ADAM-9 | Yes—Prostate cell lines | ADAM-9 expressed in AR-positive PrCa cells. |
| Sung et al. [218] | 2006 | ADAM-9 | Yes—Prostate samples and prostate cell lines | ADAM-9 expression elevated in in malignant compared to benign prostate tissue. ADAM-9 expression correlated to transition to androgen-independence and cellular stress. |
| Xiao et al. [202] | 2012 | ADAM-17 | Yes—Prostate cell lines | ADAM-17 expression correlated with invasiveness. Enforced expression of ADAM-17 increased invasiveness, whereas ablation decreased invasiveness. |
| Authors | Year | ADAMTS | PrCa Platform | Role |
|---|---|---|---|---|
| Binder et al. [221] | 2020 | ADAMTS-15 | Yes—Prostate samples, prostate cell lines and mice | ADAMTS-15 expressed and active in PrCa samples. Enforced ADAMTS-15 expression decreased migration and proliferation but increased survival in vitro and suppressed tumor growth in mice. |
| Cross et al. [222] | 2005 | ADAMTS-1, 4, 5, 9 and 15 | Yes—Prostate cell lines | ADAMTS-1, ADAMTS-4, ADAMTS-5, ADAMTS-9 and ADAMTS-15 expressed in PrCa. |
| Gustavsson et al. [185] | 2008 | ADAMTS-1 * | Yes—Prostate cell lines and mice | ADAMTS-1 expression decreased in PrCa cell line with enhanced angiogenic and tumorigenic properties, compared to parent. |
| Gustavsson et al. [220] | 2009 | ADAMTS-1 | Yes—Prostate samples | ADAMTS-1 expression decreased in prostate cancer cells compared to benign prostate glands. No correlation with Gleason score, but expression lower in patients with metastatic disease. |
| Gustavsson et al. [219] | 2010 | ADAMTS-1 | Yes—Prostate cell lines and mice | ADAMTS-1 ablation decreased tumor growth, but in other PrCa cells enforced expression inhibited tumor growth. |
| Jennbacken et al. [65] | 2009 | ADAMTS-1 | Yes—Prostate cell lines and mice | ADAMTS-1 expression increased in slow growing tumors in mice. |
| Kim et al. [179] | 2012 | ADAMTS-1 * | Yes—Prostate samples | ADAMTS-1 mRNA overexpressed in PrCa samples. |
| Molokwu et al. [223] | 2010 | ADAMTS-1 and 15 | Yes—Prostate cell lines | ADAMTS-15 (but not ADAMTS-1) expressed in PrCa. |
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Men, mice/rats or cells. | Involved other animal species or cells not related to PrCa progression. |
| Concept | All human metzincin superfamily members, including members of the MMP, MT-MMP, ADAM, ADAMTS, BMP1/TLL and Meprin subgroups, as well as the TIMP family of regulators. | Examining proteins other than metzincin superfamily members or TIMPs, or in different cancer types, other diseases, or normal biology. |
| Context | Studies that investigated the role of the metzincin superfamily in PrCa progression. | Did not specially look at the role of metzincin superfamily members in PrCa cancer progression. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binder, M.J.; Ward, A.C. The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review. Int. J. Mol. Sci. 2021, 22, 3608. https://doi.org/10.3390/ijms22073608
Binder MJ, Ward AC. The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review. International Journal of Molecular Sciences. 2021; 22(7):3608. https://doi.org/10.3390/ijms22073608
Chicago/Turabian StyleBinder, Marley J., and Alister C. Ward. 2021. "The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review" International Journal of Molecular Sciences 22, no. 7: 3608. https://doi.org/10.3390/ijms22073608
APA StyleBinder, M. J., & Ward, A. C. (2021). The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review. International Journal of Molecular Sciences, 22(7), 3608. https://doi.org/10.3390/ijms22073608







