Analysis of Gene Expression Changes in Plants Grown in Salty Soil in Response to Inoculation with Halophilic Bacteria
Abstract
:1. Introduction
1.1. Soil and Crop Loss Due to Rapidly Increasing Concentrations of Salinity Buildup in Soil
1.2. Limitations of Bioengineering Salt Tolerance
1.3. Survival Skills: How Halophytes Survive in Salty Soil
2. The Role of Halophilic Plant-Associated Bacteria in Plant Growth under Saline Conditions
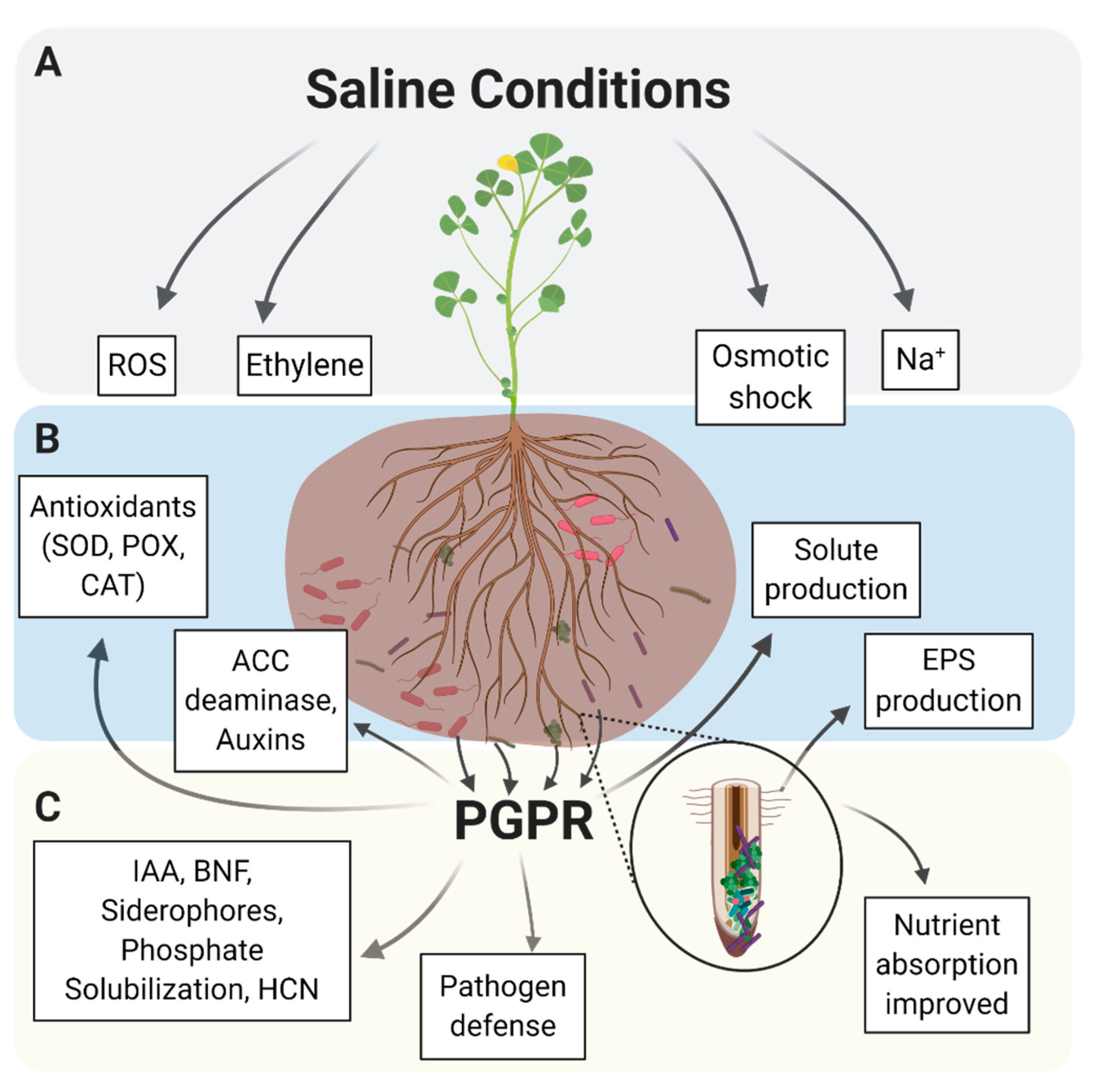
2.1. Potential Mechanisms for Plant Growth Stimulation by ST-PGPR
2.2. Changes in Plant Gene Expression in Salt-Grown Plants Inoculated with ST-PGPR
3. Overview of RNA Sequencing and Data Analysis
3.1. Approaches for Analyzing Changes in Plant Gene Expression in Response to Bacterial Inoculation
3.2. Mining Sequence Data for Candidate Genes
3.3. Limitations of RNA Sequencing and Analysis
4. Conclusions and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kearl, J.; McNary, C.; Lowman, S.J.; Mei, C.; Aanderug, Z.; Smith, S.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-tolerant halophyte rhizosphere bacteria stimulate growth of alfalfa in salty soil. Front. Microbiol. 2019, 10, 1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rima, F.S.; Biswas, S.; Sarker, P.; Islam, M.D.; Seraj, Z. Bacteria endemic to saline coastal belt and their ability to mitigate the effects of salt stress on rice growth and yields. Ann. Microbiol. 2018, 68, 525–535. [Google Scholar] [CrossRef]
- Nakayama, H.; Yoshida, K.; Ono, H.; Murooka, Y.; Shinmyo, A. Ectoine, the compatible solute of Halomonas elongata, confers hyperosmotic tolerance in cultured tobacco cells. Plant Physiol. 2020, 122, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, T. Enhanced salt tolerance of alfalfa (Medicago sativa) by rstB gene transformation. Plant Sci. 2015, 234, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zsigmond, L.; Szepi, A.; Tari, I.; Rigo, G.; Kiraly, A.; Szabados, L. Overexpression of the mitochondrial PPR40 gene improves salt tolerance in Arabidopsis. Plant Sci. 2012, 182, 87–93. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–34. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gao, Z.; Zhou, L.; Li, L.; Zhang, J.; Liu, Y.; Chen, H. Comparative transcriptome analysis reveals K+ transporter gene contributing to salt tolerance in eggplant. BMC Plant Biol. 2019, 19, 67. [Google Scholar] [CrossRef]
- Cen, H.; Ye, W.; Liu, Y.; Li, D.; Wang, K.; Zhang, W. Overexpression of a Chimeric Gene, OsDST-SRDX, Improved Salt Tolerance of Perennial Ryegrass. Sci. Rep. 2016, 6, 27320. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.; Golldack, D.; Ishitani, M.; Kamasani, U.; Rammesmayer, G.; Shen, B.; Sheveleva, E.; Jensen, R. Salt Tolerance Engineering Requires Multiple Gene Transfers. Ann. N. Y. Acad. Sci. 2006, 792, 115–125. [Google Scholar] [CrossRef]
- How GMOs Are Regulated for Food and Plant Safety in the United States. Available online: https://www.fda.gov/food/agricultural-biotechnology/how-gmos-are-regulated-food-and-plant-safety-united-states (accessed on 8 March 2021).
- Public Opinion about Genetically Modified Foods and Trust in Scientists. Available online: https://www.pewresearch.org/science/2016/12/01/public-opinion-about-genetically-modified-foods-and-trust-in-scientists-connected-with-these-foods/ (accessed on 10 March 2021).
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Tang, X.; Shao, H.; Wang, H. Salinity tolerance mechanism of economic halophytes from physiological to molecular hierarchy for improving food quality. Curr. Genom. 2016, 17, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- Sáenz-Mata, J.; Palacio-Rodríguez, R.; Sánchez-Galván, H.; Balagurusamy, N. Plant Growth Promoting Rhizobacteria Associated to Halophytes: Potential Applications in Agriculture. In Sabkha Ecosystems; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; Volume 48, pp. 411–425. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2010, 10, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etesami, H.; Beattie, G.A. Mining halophytes for Plant Growth-Promoting Halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Kundan, R.; Pant, G.; Jadon, N.; Agrawal, P.K. Plant growth promoting rhizobacteria: Mechanism and current prospective. J. Fertil. Pestic. 2015, 6, 155. [Google Scholar] [CrossRef]
- Malik, K.A.; Rasul, G.; Hassan, U.; Mehnaz, S.; Ashraf, M. Role of nitrogen fixing and growth hormone producing bacteria in improving growth of wheat and rice. Nitrogen Fixation with Non-Legumes. In Proceedings of the Sixth International Symposium on Nitrogen Fixation with Non-Legumes, Ismalia, Egypt, 6–10 September 1993; Hegazi, N.A., Fayez, M., Monib, M., Eds.; American University Cairo Press: Cairo, Egypt, 1993; pp. 409–422. [Google Scholar]
- Cattelan, A.; Hartel, P.G.; Fuhrmann, J.J. Screening for plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci. Soc. Am. J. 1999, 6, 1670–1680. [Google Scholar] [CrossRef]
- Glick, B.; Penrose, D.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef]
- Kim, S.; Lowman, S.; Hou, G.; Nowak, J.; Flinn, B.; Mei, C. Growth promotion and colonization of switchgrass (Panicum virgatum) cv. Alamo by bacterial endophyte Burkholderia phytofirmans strain PsJN. Biotechnol. Biofuels 2012, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Lara-Chavez, A.; Lowman, S.; Kim, S.; Tang, Y.; Zhang, J.; Udvardi, M.; Nowak, J.; Flinn, B.; Mei, C. Global gene expression profiling of two switchgrass cultivars following inoculation with Burkholderia phytofirmans strain PsJN. J. Exp. Botany 2015, 66, 4337–4350. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Geng, X.; Xie, R.; Fu, L.; Jiang, J.; Gao, L.; Sun, J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of hybrid Pennisetum. Biotech. Biofuels 2016, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Smith, M.; Glick, B.; Liang, Y. Effects of ACC deaminase containing rhizobacteria on plant growth and expression of TocGTPases in tomato (Solanum lycopersicum) under salt stress. Botany 2014, 92, 775–781. [Google Scholar] [CrossRef]
- Kumari, B.; Mallick, M.; Hora, A. Plant growth-promoting rhizobacteria (PGPR): Their potential for development of sustainable agriculture. In Bio-Exploitation for Sustainable Agriculture; Trivedi, P.C., Ed.; Avinskar Publishing: Jaipur, Rajasthan, 2016; pp. 1–19. [Google Scholar]
- Ilangumaran, G.; Smith, D. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.T.; Torky, Y.; Mourad, A.H.; Ayyash, M.M.; El-Keblawy, A.; Hilal-Alnaqbi, A.; AbuQamar, S.F.; El-Tarabily, K. Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef]
- Taj, Z.; Challabathula, D. Protection of photosynthesis by halotolerant Staphylococcus Sciuri ET101 in tomato (Lycoperiscon esculentum) and rice (Oryza sativa) plants during salinity stress: Possible interplay between carboxylation and oxygenation in stress mitigation. Front. Microbiol. 2021, 11, 547750. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chen, L.J.; Pan, S.Y.; Li, X.W.; Xu, M.J.; Zhang, C.M.; Xing, K.; Sheng, Q. Antifungal potential evaluation and alleviation of salt stress in tomato seedlings by a halotolerant plant growth-promoting Actinomycete Streptomyces sp. KLBMP5084. Rhizosphere 2020, 16, 100262. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Li, X.W.; Wang, T.T.; Gong, Y.; Zhang, C.M.; Xing, K.; Qin, S. Root exudates-driven rhizosphere recruitment of the plant growth-promoting rhizobacterium Bacillus flexus KLBMP 4941 and its growth-promoting effect on the coastal halophyte Limonium sinense under salt stress. Ecotoxicol. Environ. Saf. 2020, 194, 110374. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.; Barnawal, D.; Patel, V.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [Green Version]
- Safdarian, M.; Askan, H.; Shariati, V.; Nematzadeh, G. Transcriptional responses of wheat roots inoculated with Arthrobacter nitroguajacolicus to salt stress. Sci. Rep. 2019, 9, 1792. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Baek, D.; Rokibuzzaman, M.; Khan, A.; Kim, M.C.; Park, H.J.; Yun, D.-J.; Chung, Y.R. Plant-growth promoting Bacillus oryzicola YC7007 modulates stress-response gene expression and provides protection from salt stress. Front. Plant Sci. 2020, 10, 1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.; Zhu, J. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2020, 97, 3730–3734. [Google Scholar] [CrossRef]
- Gene Expression. Available online: https://www.nature.com/scitable/topicpage/gene-expression-14121669/ (accessed on 12 March 2021).
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, B.; Zhang, Y.; Gordon, W.; Du, S.; Paradis, T.; Vincent, M.; Von Schack, D. Bioinformatics for RNA-seq data analysis. InTechOpen 2016. [Google Scholar] [CrossRef] [Green Version]
- RNA Sample Collection, Protection, and Isolation Support-Troubleshooting. Available online: https://www.thermofisher.com/us/en/home/technical-resources/technical-reference-library/nucleic-acid-purification-analysis-support-center/rna-sample-collection-protection-isolation-support/rna-sample-collection-protection-isolation-support-troubleshooting.html (accessed on 14 March 2021).
- Methods of RNA Quality Assessment. Available online: https://www.promega.com/resources/pubhub/methods-of-rna-quality-assessment/#bioanalyzer (accessed on 14 March 2021).
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szczesniak, M.; Gaffney, D.; Elo, L.; Zang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Zadlock, F.; Zhang, Z.; Murphy, W.R.; Bentivegna, C.S. Comparison of de novo transcriptome assemblers and K-mer strategies using the Killifish, fundulus heteroclitus. PLoS ONE 2016, 11, e0153104. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genome wide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [Green Version]
- Adamski, M.; Gumann, P.; Baird, A. A method for quantitative analysis of standard and high-throughput qPCR expression data based on input sample quantity. PLoS ONE 2014, 9, e103917. [Google Scholar] [CrossRef] [Green Version]
- The Luna qPCR Mixes? Available online: https://www.neb.com/faqs/2016/11/14/what-is-the-difference-between-the-probe-and-dye-versions-of-the-luna-qpcr-mixes#:~:text=qPCR%20is%20typically%20measured%20in,sequence%20in%20the%20PCR%20amplicon (accessed on 19 March 2021).
- TaqMan vs. SYBR Chemistry for Real-Time PCR. Available online: https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-learning-center/real-time-pcr-basics/taqman-vs-sybr-chemistry-real-time-pcr.html (accessed on 18 March 2021).
- Explaining Multiple Peaks in qPCR Melt Curve Analysis. Available online: https://www.idtdna.com/pages/education/decoded/article/interpreting-melt-curves-an-indicator-not-a-diagnosis (accessed on 19 March 2021).
- Paszkiewicz, K.; Studholme, D.J. De novo assembly of short sequence reads. Brief. Bioinform. 2010, 11, 457–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, S.; Komori, H.; LaMere, S.; Whisenant, T.; Van Nieuwerburgh, F.; Salomon, D.; Ordoukhanian, P. Library construction for next-generation sequencing: Overviews and challenges. BioTechniques 2014, 56, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Considerations for RNA-seq Read Length and Coverage. Available online: https://support.illumina.com/bulletins/2017/04/considerations-for-rna-seq-read-length-and-coverage-.html (accessed on 13 March 2021).
- Baker, M. De novo genome assembly: What every biologist should know. Nat. Methods. 2012, 9, 333–337. [Google Scholar] [CrossRef]
- LRS vs. SRS Genomics. Available online: https://genome.vinbigdata.org/documentation/blog/2020/12/17/long-read-sequencing.html (accessed on 13 March 2021).
- Tuxedo Suite. Available online: https://support.illumina.com/help/BS_App_RNASeq_Alignment_OLH_1000000006112/Content/Source/Informatics/Apps/TuxedoSuite_RNASeqTools.htm (accessed on 13 March 2021).
- Rsubread. Available online: https://bioconductor.org/packages/release/bioc/html/Rsubread.html (accessed on 14 March 2021).
- RNA-seq Work Flows. Available online: http://bioconductor.org/help/course-materials/2014/SeattleOct2014/B02.1_RNASeq.html (accessed on 12 March 2021).
- Trapnell, C.; Pachter, L.; Salzberg, S. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.; Shi, W. The R Package Rsubread is easier, faster, cheaper, and better for alignment and quantification of RNA sequencing reads. Nucl. Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, A.K.; Nielsen, B.L. Analysis of Gene Expression Changes in Plants Grown in Salty Soil in Response to Inoculation with Halophilic Bacteria. Int. J. Mol. Sci. 2021, 22, 3611. https://doi.org/10.3390/ijms22073611
Miller AK, Nielsen BL. Analysis of Gene Expression Changes in Plants Grown in Salty Soil in Response to Inoculation with Halophilic Bacteria. International Journal of Molecular Sciences. 2021; 22(7):3611. https://doi.org/10.3390/ijms22073611
Chicago/Turabian StyleMiller, Ashley K., and Brent L. Nielsen. 2021. "Analysis of Gene Expression Changes in Plants Grown in Salty Soil in Response to Inoculation with Halophilic Bacteria" International Journal of Molecular Sciences 22, no. 7: 3611. https://doi.org/10.3390/ijms22073611
APA StyleMiller, A. K., & Nielsen, B. L. (2021). Analysis of Gene Expression Changes in Plants Grown in Salty Soil in Response to Inoculation with Halophilic Bacteria. International Journal of Molecular Sciences, 22(7), 3611. https://doi.org/10.3390/ijms22073611






