Silk Fiber-Reinforced Hyaluronic Acid-Based Hydrogel for Cartilage Tissue Engineering
Abstract
1. Introduction
2. Results
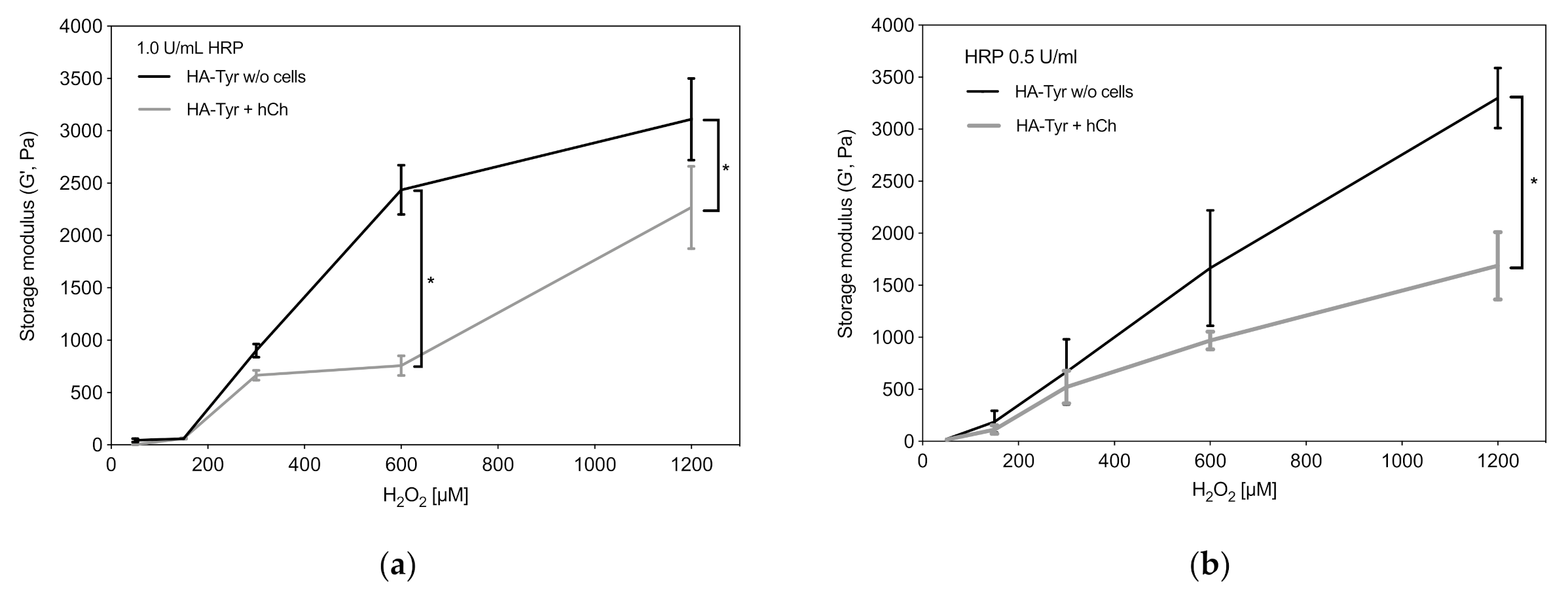
2.1. Viscoelastic Properties of HA-Tyr Vary Depending on Cell Content and Crosslinking Agents
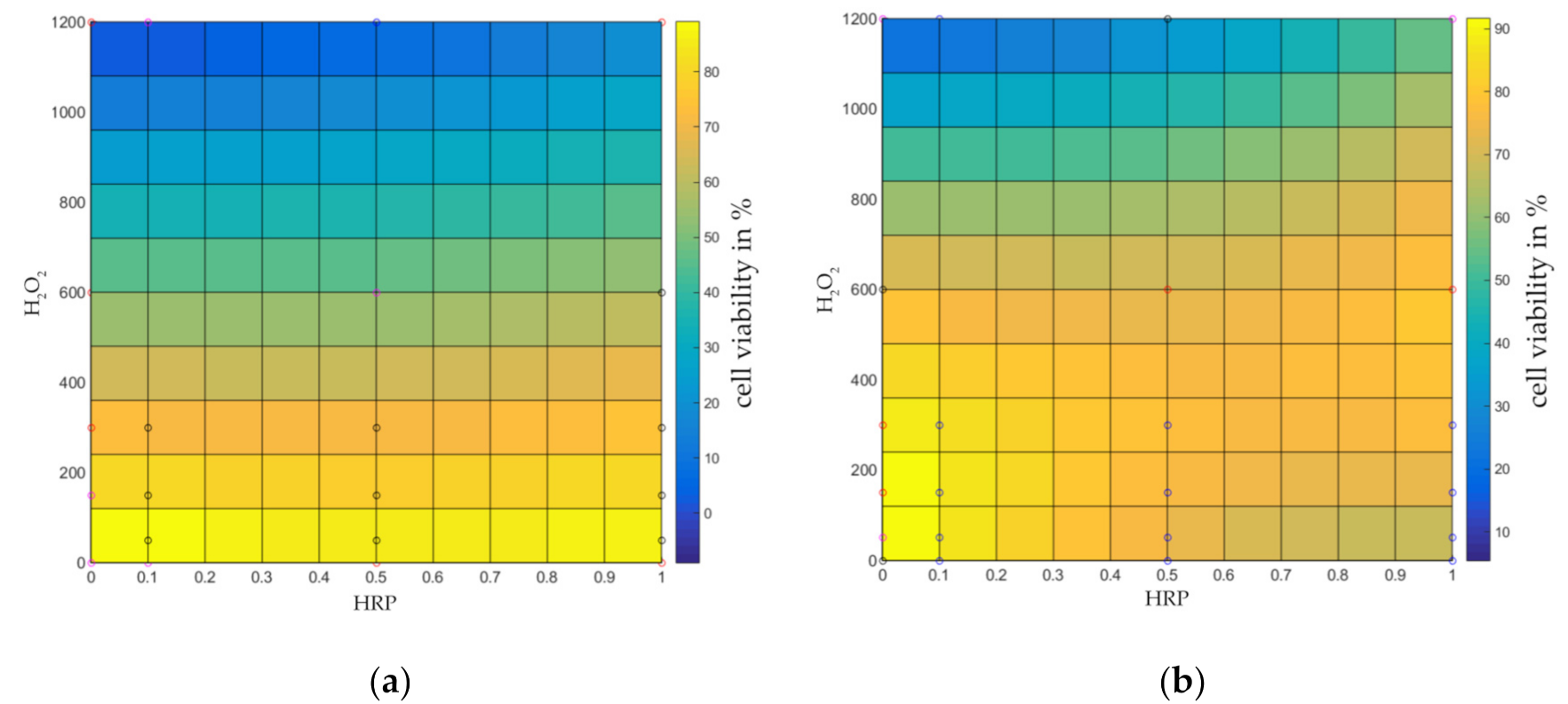
2.2. Identification of a Cytocompatibility Range of HA-Tyr Crosslinking Agents
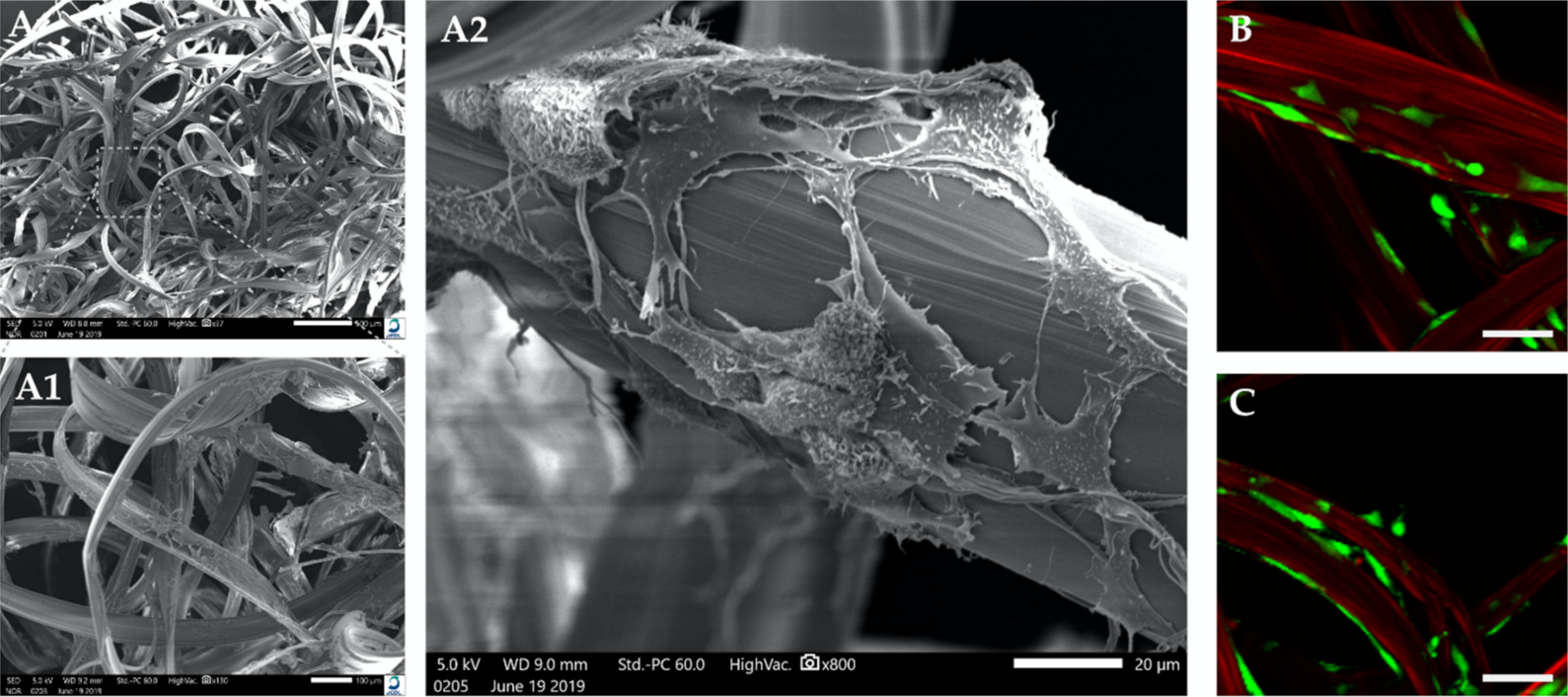
2.3. Cell Viability and Morphological Changes in 3D Cultures
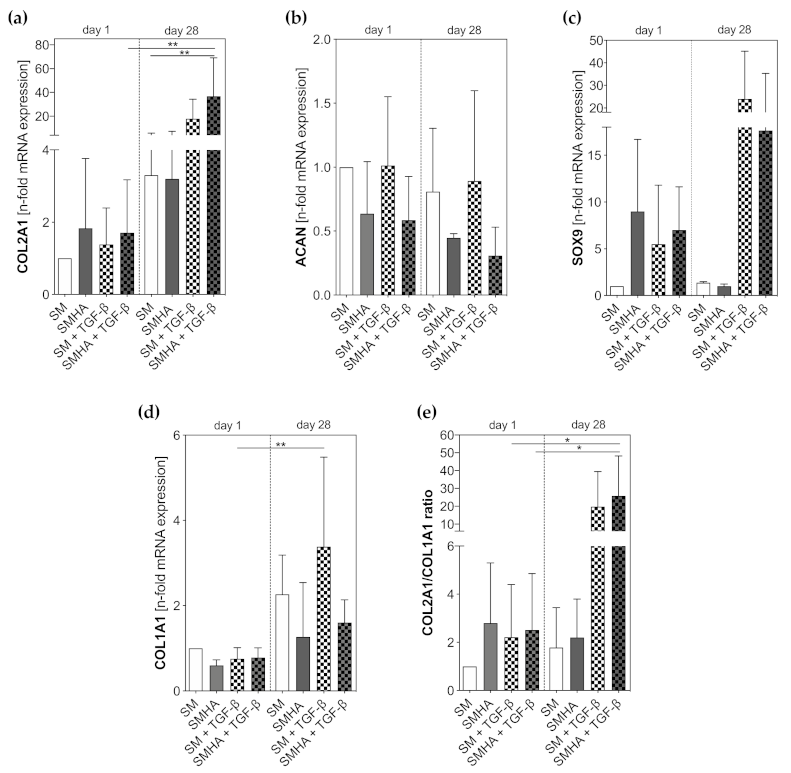
2.4. Chondrogenic Gene Expression Profile
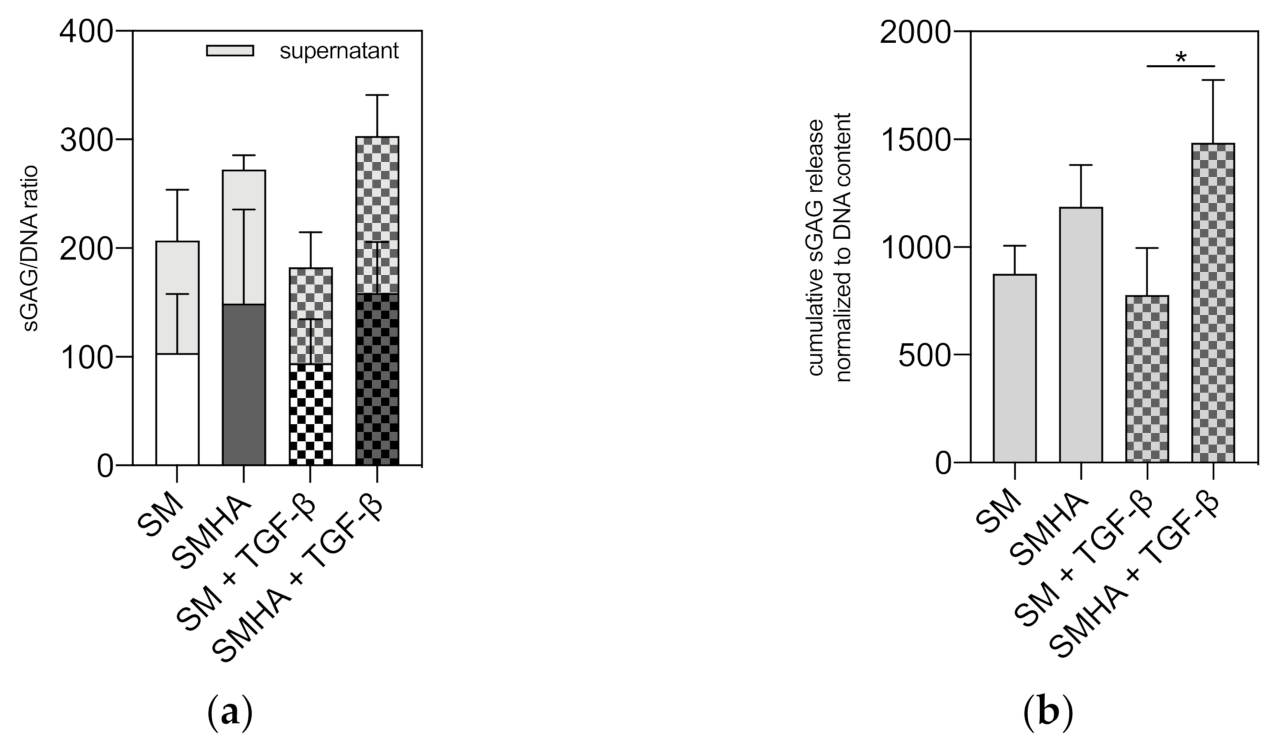
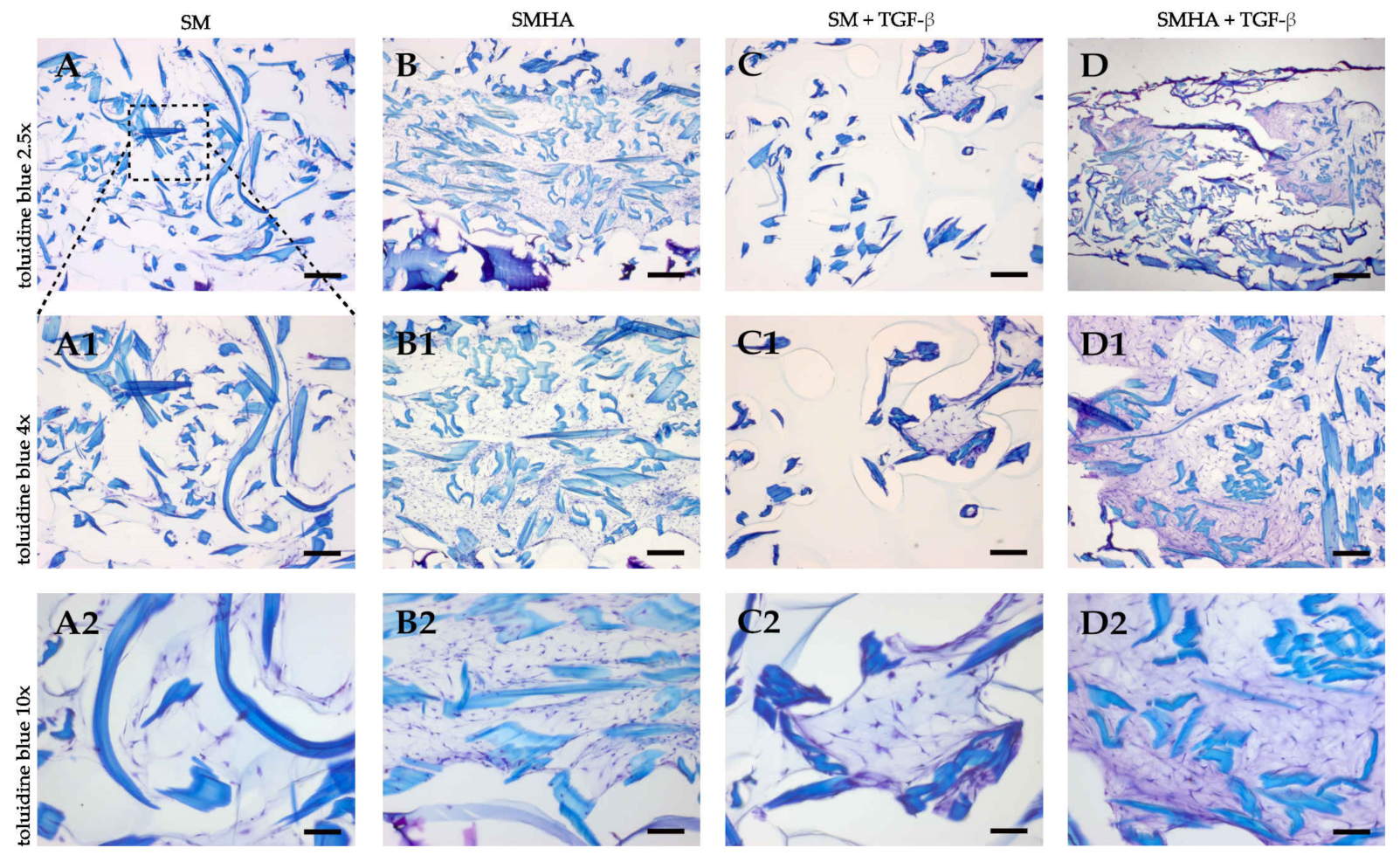
2.5. Chondrogenic ECM Neosynthesis
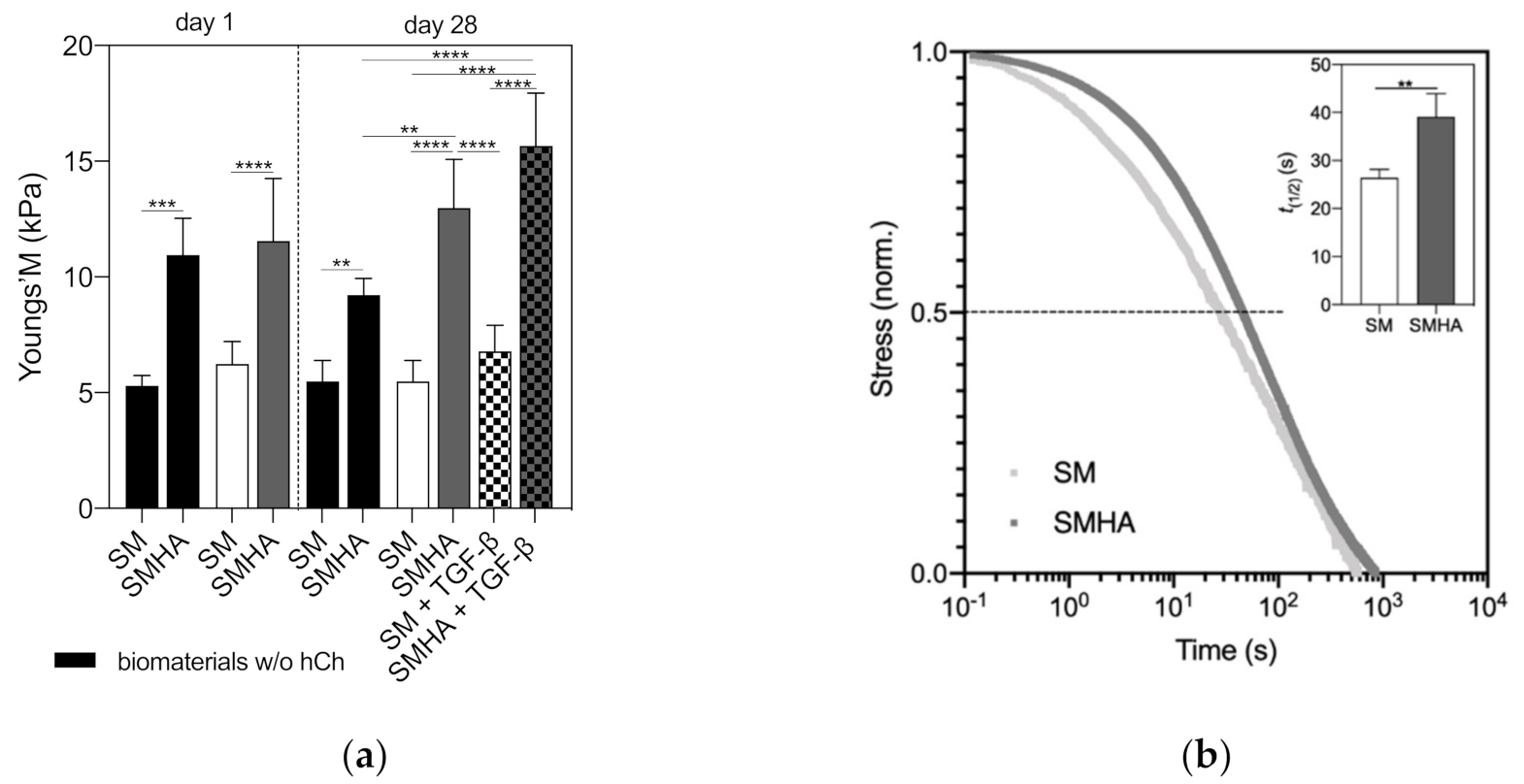
2.6. Biomechanical Analysis
3. Discussion
4. Materials and Methods
4.1. Articular Chondrocyte Isolation and Culture
4.2. Embedding of Cells in 3-Dimensional Silk Matrices
4.3. Viscoelastic Properties in Dependency on Cell Encapsulation and Varying H2O2 and HRP Concentrations
4.4. Cytocompatibility Range of HA-Tyr and Cell Viability
4.5. Scaffold Architecture and Cell Morphology
4.6. Gene Expression Analyses by Quantitative Real-Time qPCR
4.7. DNA Quantification and Glycosaminoglycan Synthesis
4.8. Histological and Immunohistochemical Analysis
4.9. Biomaterial Stiffness and Stress Relaxation
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cicuttini, F.; Ding, C.; Wluka, A.; Davis, S.; Ebeling, P.R.; Jones, G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: A prospective study. Arthritis Rheum. 2005, 52, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Cicuttini, F.; Jones, G. Tibial subchondral bone size and knee cartilage defects: Relevance to knee osteoarthritis. Osteoarthr. Cartil. 2007, 15, 479–486. [Google Scholar] [CrossRef]
- Niemeyer, P.; Albrecht, D.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee 2016, 23, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuźma, T.; Widuchowski, W.; Fickert, S. A Prospective, Randomized, Open-Label, Multicenter, Phase III Noninferiority Trial to Compare the Clinical Efficacy of Matrix-Associated Autologous Chondrocyte Implantation With Spheroid Technology Versus Arthroscopic Microfracture for Cartilage Defects of the Knee. Orthop. J. Sports Med. 2019, 7, 2325967119854442. [Google Scholar] [CrossRef]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Strauss, E.; Bosco, J., 3rd. Advances in the Surgical Management of Articular Cartilage Defects: Autologous Chondrocyte Implantation Techniques in the Pipeline. Cartilage 2013, 4, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Drescher, W.; Rath, B.; Tingart, M.; Fischer, H. Supporting Biomaterials for Articular Cartilage Repair. Cartilage 2012, 3, 205–221. [Google Scholar] [CrossRef]
- Stoop, R. Smart biomaterials for tissue engineering of cartilage. Injury 2008, 39, 77–87. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef]
- Font Tellado, S.; Bonani, W.; Balmayor, E.R.; Foehr, P.; Motta, A.; Migliaresi, C.; van Griensven, M. Fabrication and Characterization of Biphasic Silk Fibroin Scaffolds for Tendon/Ligament-to-Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, D.K.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk Fibroin-Based Scaffold for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 371–387. [Google Scholar] [CrossRef]
- Frauchiger, D.A.; May, R.D.; Bakirci, E.; Tekari, A.; Chan, S.C.W.; Wöltje, M.; Benneker, L.M.; Gantenbein, B. Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. J. Funct. Biomater. 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Blasioli, D.J.; Kim, H.J.; Kim, H.S.; Kaplan, D.L. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials 2006, 27, 4434–4442. [Google Scholar] [CrossRef]
- Brünler, R.; Aibibu, D.; Wöltje, M.; Anthofer, A.M.; Cherif, C. In silico modeling of structural and porosity properties of additive manufactured implants for regenerative medicine. Mater. Sci. Eng. C 2017, 76, 810–817. [Google Scholar] [CrossRef]
- Wu, S.C.; Chang, J.K.; Wang, C.K.; Wang, G.J.; Ho, M.L. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials 2010, 31, 631–640. [Google Scholar] [CrossRef]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Späth, S.S.; Nehrer, S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J. Inflamm. (Lond.) 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Loebel, C.; D’Este, M.; Alini, M.; Zenobi-Wong, M.; Eglin, D. Precise tailoring of tyramine-based hyaluronan hydrogel properties using DMTMM conjugation. Carbohydr. Polym. 2015, 115, 325–333. [Google Scholar] [CrossRef]
- Behrendt, P.; Ladner, Y.; Stoddart, M.J.; Lippross, S.; Alini, M.; Eglin, D.; Armiento, A.R. Articular Joint-Simulating Mechanical Load Activates Endogenous TGF-beta in a Highly Cellularized Bioadhesive Hydrogel for Cartilage Repair. Am. J. Sports Med. 2020, 48, 210–221. [Google Scholar] [CrossRef]
- Fujita, Y.; Kitagawa, M.; Nakamura, S.; Azuma, K.; Ishii, G.; Higashi, M.; Kishi, H.; Hiwasa, T.; Koda, K.; Nakajima, N.; et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002, 528, 101–108. [Google Scholar] [CrossRef]
- Nuernberger, S.; Cyran, N.; Albrecht, C.; Redl, H.; Vécsei, V.; Marlovits, S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011, 32, 1032–1040. [Google Scholar] [CrossRef]
- Vainieri, M.L.; Lolli, A.; Kops, N.; D’Atri, D.; Eglin, D.; Yayon, A.; Alini, M.; Grad, S.; Sivasubramaniyan, K.; van Osch, G. Evaluation of biomimetic hyaluronic-based hydrogels with enhanced endogenous cell recruitment and cartilage matrix formation. Acta Biomater. 2020, 101, 293–303. [Google Scholar] [CrossRef]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar] [CrossRef]
- Jooybar, E.; Abdekhodaie, M.J.; Alvi, M.; Mousavi, A.; Karperien, M.; Dijkstra, P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019, 83, 233–244. [Google Scholar] [CrossRef]
- Mohan, R.; Mohan, N.; Vaikkath, D. Hyaluronic Acid Dictates Chondrocyte Morphology and Migration in Composite Gels. Tissue Eng. Part A 2018, 24, 1481–1491. [Google Scholar] [CrossRef]
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Alini, M.; Eglin, D.; Grad, S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. C 2021, 120, 111701. [Google Scholar] [CrossRef]
- Benya, P.D.; Padilla, S.R.; Nimni, M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 1978, 15, 1313–1321. [Google Scholar] [CrossRef]
- Benya, P. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, P.; Cui, L.; Yu, Y.; Deng, C.; Wang, Q.; Fan, X. Self-Crosslinking of Silk Fibroin Using H(2)O(2)-Horseradish Peroxidase System and the Characteristics of the Resulting Fibroin Membranes. Appl. Biochem. Biotechnol. 2017, 182, 1548–1563. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef]
- Rheinnecker, M.; Kohlhaas, S.; Zimmat, R. Method and Apparatus for Extraction of Arthropod Gland. U.S. Patent US8105633B2, 31 January 2012. [Google Scholar]
- Loening, A.M.; James, I.E.; Levenston, M.E.; Badger, A.M.; Frank, E.H.; Kurz, B.; Nuttall, M.E.; Hung, H.H.; Blake, S.M.; Grodzinsky, A.J.; et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch. Biochem. Biophys. 2000, 381, 205–212. [Google Scholar] [CrossRef]
- Gille, J.; Kunow, J.; Boisch, L.; Behrens, P.; Bos, I.; Hoffmann, C.; Köller, W.; Russlies, M.; Kurz, B. Cell-Laden and Cell-Free Matrix-Induced Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage 2010, 1, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Kurz, B.; Domm, C.; Jin, M.; Sellckau, R.; Schünke, M. Tissue engineering of articular cartilage under the influence of collagen I/III membranes and low oxygen tension. Tissue Eng. 2004, 10, 1277–1286. [Google Scholar] [CrossRef] [PubMed]


| Human Target | Sequence (5′–3′) Sense | Sequence (5′–3′) Antisense |
|---|---|---|
| ACAN | GAGGCCAGCAGAGAAGATTCTG | GACGCCTCGCCTTCTTGAA |
| COL2A1 | CAACACTGCCAACGTCCAGAT | CTGCTTCGTCCAGATAGGCAAT |
| SOX9 | CTCGGAGACTTCTGAACGAGAG | CGTTCTTCACCGACTTCCTCC |
| COL1A1 | AATTCCAAGGCCAAGAAGCATG | GGTAGCCATTTCCTTGGTGGTT |
| GAPDH | GCCTCAAGATCATCAGCAATGC | TGGTCATGAGTCCTTCCACGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weitkamp, J.-T.; Wöltje, M.; Nußpickel, B.; Schmidt, F.N.; Aibibu, D.; Bayer, A.; Eglin, D.; Armiento, A.R.; Arnold, P.; Cherif, C.; et al. Silk Fiber-Reinforced Hyaluronic Acid-Based Hydrogel for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 3635. https://doi.org/10.3390/ijms22073635
Weitkamp J-T, Wöltje M, Nußpickel B, Schmidt FN, Aibibu D, Bayer A, Eglin D, Armiento AR, Arnold P, Cherif C, et al. Silk Fiber-Reinforced Hyaluronic Acid-Based Hydrogel for Cartilage Tissue Engineering. International Journal of Molecular Sciences. 2021; 22(7):3635. https://doi.org/10.3390/ijms22073635
Chicago/Turabian StyleWeitkamp, Jan-Tobias, Michael Wöltje, Bastian Nußpickel, Felix N. Schmidt, Dilbar Aibibu, Andreas Bayer, David Eglin, Angela R. Armiento, Philipp Arnold, Chokri Cherif, and et al. 2021. "Silk Fiber-Reinforced Hyaluronic Acid-Based Hydrogel for Cartilage Tissue Engineering" International Journal of Molecular Sciences 22, no. 7: 3635. https://doi.org/10.3390/ijms22073635
APA StyleWeitkamp, J.-T., Wöltje, M., Nußpickel, B., Schmidt, F. N., Aibibu, D., Bayer, A., Eglin, D., Armiento, A. R., Arnold, P., Cherif, C., Lucius, R., Smeets, R., Kurz, B., & Behrendt, P. (2021). Silk Fiber-Reinforced Hyaluronic Acid-Based Hydrogel for Cartilage Tissue Engineering. International Journal of Molecular Sciences, 22(7), 3635. https://doi.org/10.3390/ijms22073635








