Discovery of Hyperactive Antifreeze Protein from Phylogenetically Distant Beetles Questions Its Evolutionary Origin
Abstract
:1. Introduction
2. Results and Discussion
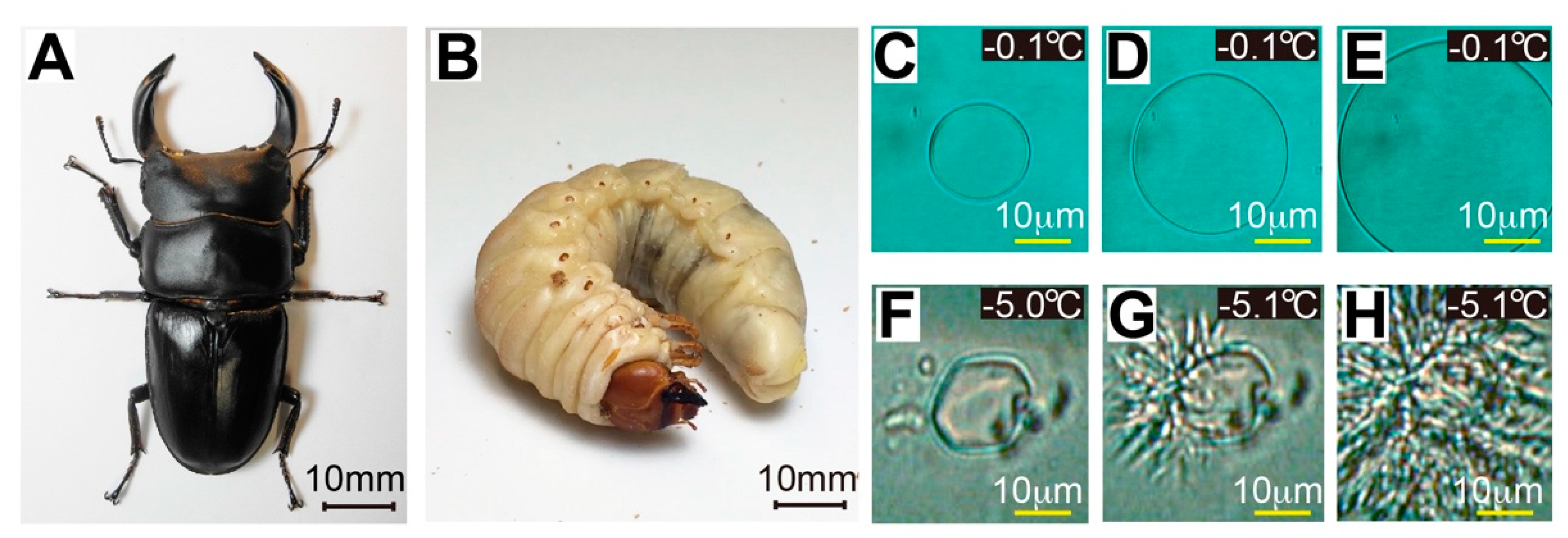
2.1. Hemolymph of Dorcus Hopei Binodulosus (Dhb) Exhibits Antifreeze Activity
2.2. Dhb Larvae Tolerate−5 °C-Preservation for 24 Hours
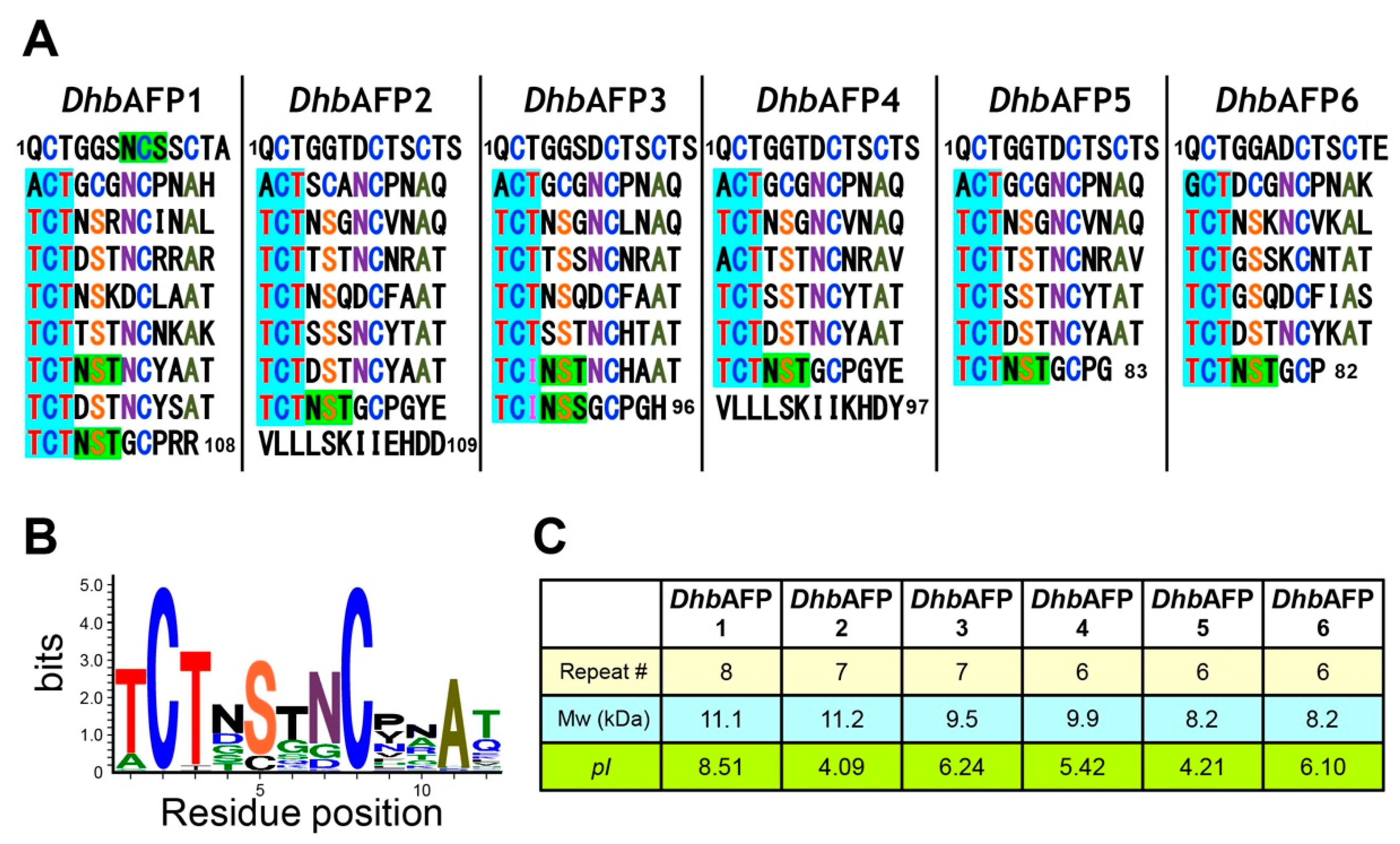
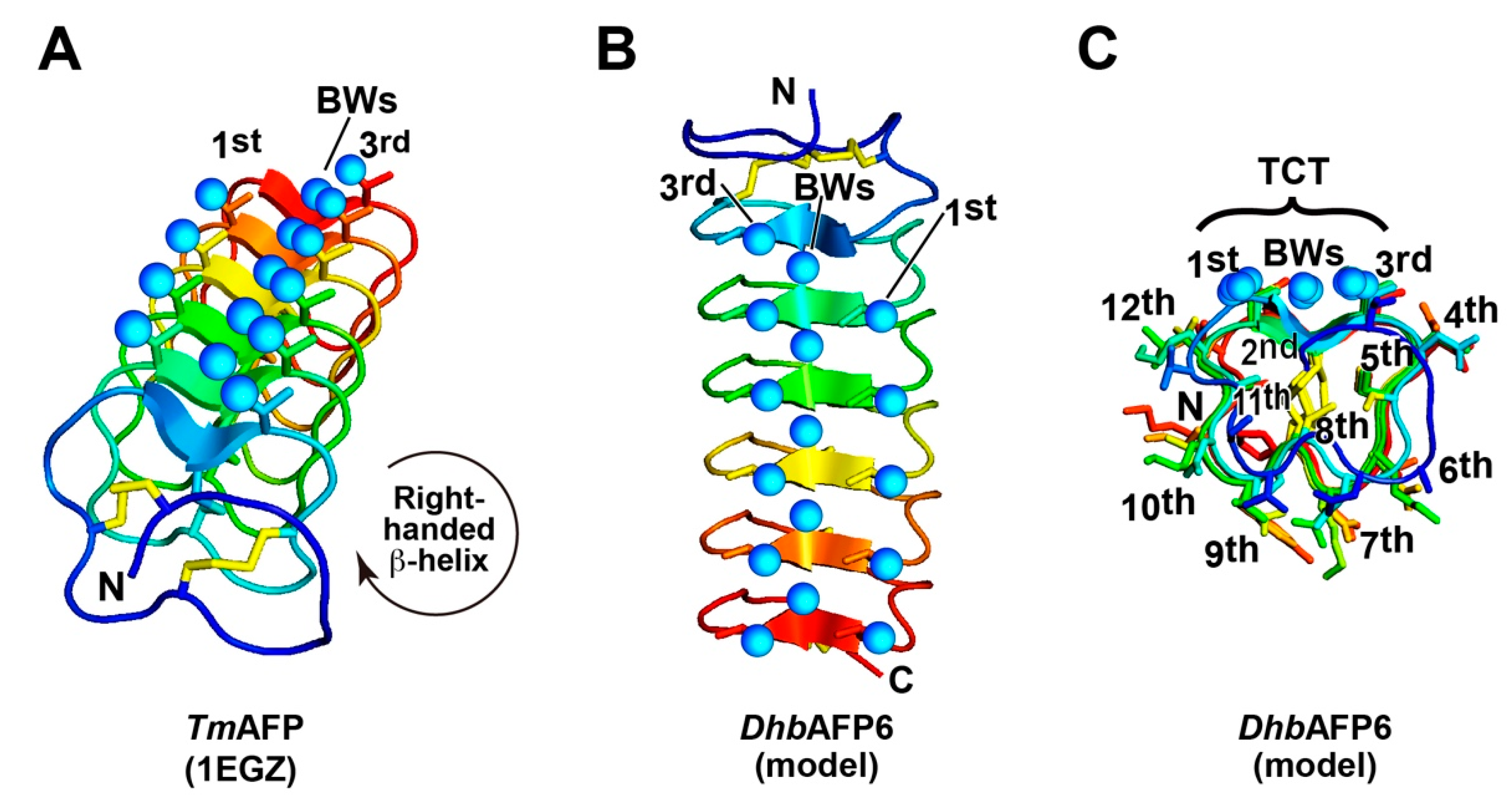
2.3. DhbAFP and Tenebrio Molitor AFP Show Significant Similarities
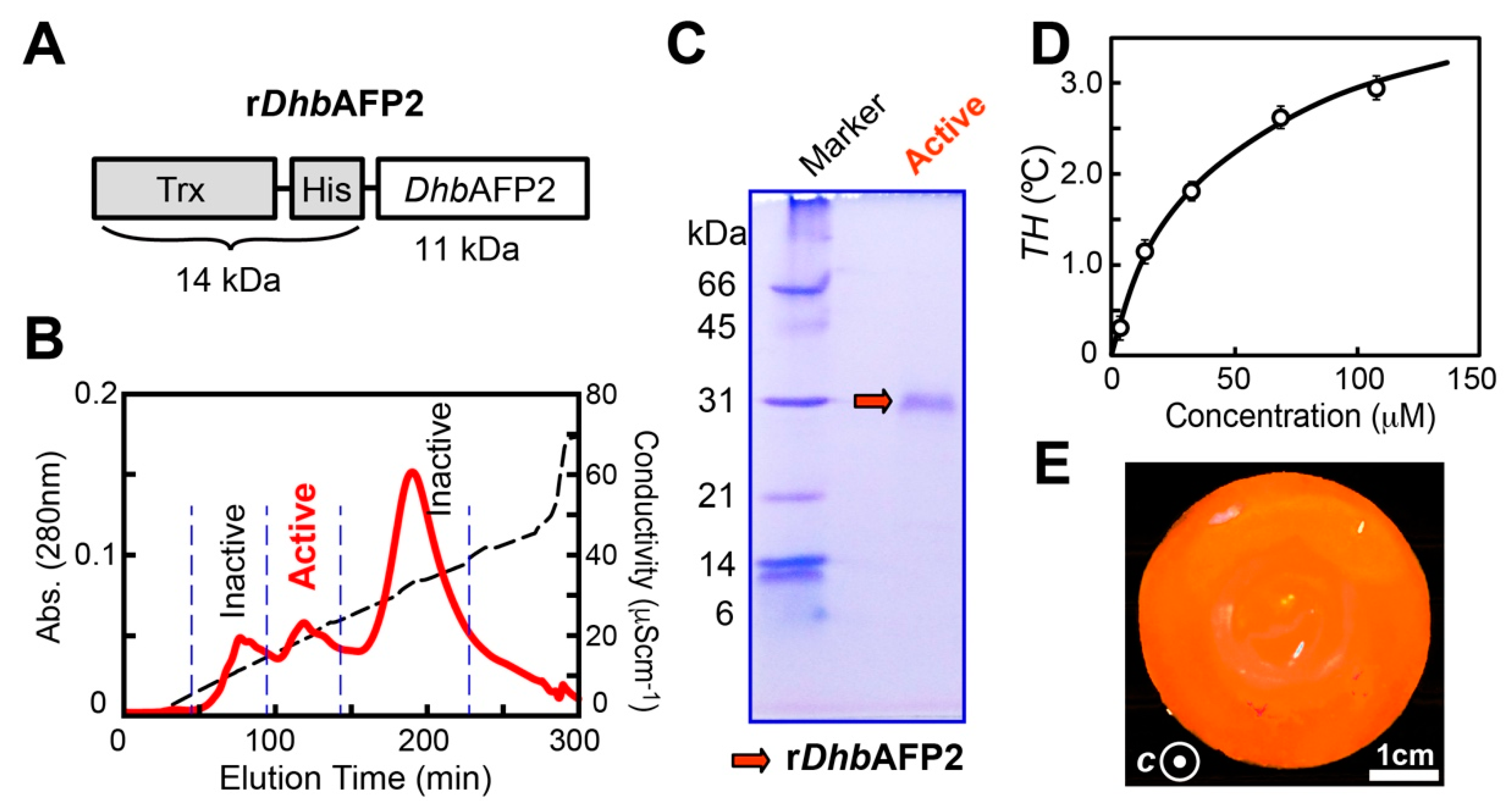
2.4. A DhbAFP Isoform Equips the Properties of Hyperactive AFP
2.5. Unrevealed Gene Transfer Mechanism May Exist between Dhb and Tm
3. Materials and Methods
3.1. Construction of cDNA Library from Dhb Larva
3.2. Sequence Determination of cDNA Encoding DhbAFP
3.3. Expression and Purification of rDhbAFP2 Isoform
3.4. Thermal Hysteresis (TH) Measurement for rDhbAFP2
3.5. Fluorescence-Based Ice Plane Affinity (FIPA) Measurement for rDhbAFP2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Antifreeze protein |
| Dhb | Dorcus hopei binodulosus |
| Tm | Tenebrio molitor |
| TH | Thermal hysteresis |
| FIPA | Fluorescence-based ice plane affinity |
| NCBI | National Center for Biotechnology Information |
References
- Kim, S.I.; Farrel, B.D. Phylogeny of world stag beetles (Coleoptera: Lucanidae) reveals a Gondwanan origin of Darwin’s stag beetle. Mol. Phylogenet. Evol. 2015, 86, 35–48. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, J.I. Review of family Lucanidae (Insecta: Coleoptera) in Korea with the description of one new species. Entomol. Res. 2010, 40, 55–81. [Google Scholar] [CrossRef]
- Tournant, P.; Joseph, L.; Goka, K.; Couchamp, F. The rarity and overexploitation paradox: Stag beetle collections in Japan. Biodivers. Conserv. 2012, 21, 1425–1440. [Google Scholar] [CrossRef]
- Goka, K.; Kojima, H.; Okabe, K. Biological invasion caused by commercialization of stag beetles in Japan. Glob. Environ. Res. 2004, 8, 67–74. [Google Scholar]
- Brown’s Beetles. Available online: https://abrowntks.weebly.com/dorcus-hopei.html (accessed on 14 March 2021).
- Scotter, A.J.; Marshall, C.B.; Graham, L.A.; Gilbert, J.A.; Garnham, C.P.; Davies, P.L. The basis for hyperactivity of antifreeze proteins. Cryobiology 2006, 53, 229–239. [Google Scholar] [CrossRef]
- Graham, L.A.; Liou, Y.-C.; Walker, V.K.; Davies, P.L. Hyperactive antifreeze protein from beetles. Nature 1997, 388, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Duman, J.G.; Verleye, N.L.-D.; Goetz, F.W.; Wu, D.W.; Andorfer, C.A.; Benjamin, T.; Parmelee, D.D. Molecular characterization and sequencing of antifreeze proteins from larvae of the beetle Dendroides canadensis. J. Comp. Physiol. B 1998, 168, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, F.; Ma, J. Seasonal changes in cold tolerance of desert beetle Anatolica polita borealis (Coleoptera: Tenebrionidae) and their physiological mechanisms. Acta Entomol. Sin. 2009, 52, 372–379. [Google Scholar]
- Qiu, L.; Wang, Y.; Wang, J.; Zhang, F.; Ma, J. Expression of biologically active recombinant antifreeze protein His-MpAFP149 from the desert beetle (Microdera punctipennis dzungarica) in Escherichia coli. Mol. Biol. Rep. 2010, 37, 1725–1732. [Google Scholar] [CrossRef]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef]
- Kristiansen, E.; Zachariassen, K.E. The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology 2005, 51, 262–280. [Google Scholar] [CrossRef]
- Duman, J.G.; Newton, S.S. Insect Antifreeze Proteins. In Antifreeze Proteins; Ramløv, H., Eriis, D.S., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 1, pp. 131–188. [Google Scholar] [CrossRef]
- Mahatabuddin, S.; Tsuda, S. Applications of Antifreeze proteins: Practical use of the quality products from Japanese fishes. Adv. Exp. Med. Biol. 2018, 1081, 321–327. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. TiBS 2017, 39, 548–555. [Google Scholar] [CrossRef]
- Liou, Y.-C.; Thibault, P.; Walker, V.K.; Davies, P.L.; Graham, L.A. A complex family of highly heterogeneous and internally repetitive hyperactive antifreeze proteins from the beetle Tenebrio molitor. Biochemistry 1999, 38, 11415–11424. [Google Scholar] [CrossRef]
- Graether, S.P.; Kuiper, M.J.; Gagné, S.M.; Walker, V.K.; Jia, Z.; Sykes, B.D.; Davies, P.L. Helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature 2000, 406, 325–328. [Google Scholar] [CrossRef]
- Kristiansen, E.; Ramløv, H.; Højrup, P.; Pedersen, S.A.; Hagen, L.; Zachariassen, K.E. Structural characteristics of a novel antifreeze protein from the longhorn beetle Rhagium inquisitor. Insect Biochem. Mol. Biol. 2011, 41, 109–117. [Google Scholar] [CrossRef]
- Lin, F.-H.; Davies, P.L.; Graham, L.A. The Thr- and Ala-rich hyperactive antifreeze protein from Inchworm folds as a flat silk-like β-helix. Biochemistry 2011, 50, 4467–4478. [Google Scholar] [CrossRef]
- Graham, L.A.; Davies, P.L. Glycine-rich antifreeze proteins from snow fleas. Science 2005, 310, 461. [Google Scholar] [CrossRef]
- Hosoya, T.; Honda, M.; Araya, K. Genetic variation of 16S rRNA gene observed in Ceruchus lignarius and Dorcus rectus rectus (Coleoptera; Lucanidae). Entomol. Sci. 2001, 4, 335–344. [Google Scholar]
- Chen, Y.; Liu, J.; Cao, Y.; Zhou, S.; Wan, X. Two new complete mitochondrial genomes of Dorcus stag beetles (Coleoptera, Lucanidae). Gens Genom. 2018, 40, 873–880. [Google Scholar] [CrossRef]
- Bar-Dolev, M.; Celik, Y.; Wettlaufer, J.S.; Davies, P.L.; Braslavsky, I. New insights into ice growth and melting modifications by antifreeze proteins. J. R. Soc. Interface 2012, 9, 3249–3259. [Google Scholar] [CrossRef]
- Graham, L.A.; Walker, V.K.; Davies, P.L. Developmental and environmental regulation of antifreeze proteins in the mealworm beetle Tenebrio molitor. Eur. J. Biochem. 2000, 267, 6452–6458. [Google Scholar] [CrossRef] [Green Version]
- Asahina, E.; Ohyama, Y. Cold Resistance in insects wintering in decayed wood. Low Temp. Sci. 1969, 27, 143–152. Available online: https://eprints.lib.hokudai.ac.jp/dspace/bitstream/2115/17760/1/27_p143-152.pdf (accessed on 3 March 2021).
- Takamichi, M.; Nishimiya, Y.; Miura, A.; Tsuda, S. Effect of annealing time of an ice crystal on the activity of type III antifreeze protein. FEBS J. 2007, 274, 6469–6476. [Google Scholar] [CrossRef]
- Wang, L.; Duman, J.G. Antifreeze proteins of the beetle Dendroides canadensis enhance one another’s activities. Biochemistry 2005, 44, 10305–10312. [Google Scholar] [CrossRef]
- Graham, L.A.; Qin, W.; Lougheed, S.C.; Davies, P.L.; Walker, V.K. Evolution of hyperactive, repetitive antifreeze proteins in beetles. J. Mol. Evol. 2007, 64, 387–398. [Google Scholar] [CrossRef]
- Qin, W.; Walker, V.K. Tenebrio molitor antifreeze protein gene identification and regulation. Gene 2006, 376, 142–149. [Google Scholar] [CrossRef]
- Wang, S.; Amornwittawat, N.; Juwita, V.; Kao, Y.; Duman, J.G.; Pascal, T.A.; Goddard, W.A., III; Wen, X. Arginine, a key residue for the enhancing ability of an antifreeze protein of. the beetle Dendroides canadensis. Biochemistry 2009, 48, 9696–9703. [Google Scholar] [CrossRef] [Green Version]
- LaVallie, E.R.; Lu, Z.E.; Diblasio-Smith, A.; Collins-Racie, L.A.; McCoy, J.M. Thioredoxin as a fusion partner for production of soluble recombinant proteins in Escherichia coli. Methods Enzymol. 2000, 326, 322–340. [Google Scholar] [CrossRef]
- Liou, Y.-C.; Daley, M.E.; Graham, L.A.; Kay, C.M.; Walker, V.K.; Sykes, B.D.; Davies, P.L. Folding and structural characterization of highly disulfide-bonded beetle antifreeze protein produced in bacteria. Protein Expr. Purif. 2000, 19, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Basu, K.; Garnham, C.P.; Nishimiya, Y.; Tsuda, S.; Braslavsky, I.; Davies, P.L. Determining the ice-binding planes of antifreeze proteins by fluorescence-based ice plane affinity. J. Vis. Exp. 2014, 83, e51185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, Y.-C.; Tociij, A.; Davies, P.L.; Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a β-helix antifreeze protein. Nature 2000, 406, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Graether, S.P.; Sykes, B.D. Cold survival in freeze-intolerant insects: The structure and function of β-helical antifreeze proteins. Eur. J. Biochem. 2004, 271, 3285–3296. [Google Scholar] [CrossRef]
- Marshall, C.B.; Daley, M.E.; Sykes, B.D.; Davies, P.L. Enhancing the activity of a β-helical antifreeze protein by the engineered addition of Coils. Biochemistry 2004, 43, 11637–11646. [Google Scholar] [CrossRef]
- Emmanuel, F.A.; Toussaint, A.; Seidel, M.; Arriaga-Varela, E.; Hájek, J.; Král, D.; Sekerka, L.; Short, A.E.Z.; Fikáček, M. The peril of dating beetles. Syst. Entomol. 2017, 42, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Henderson, C.M.; Shen, S.Z.; Gradstein, F.M.; Agterberg, F.P. The Permian Period. In The Geologic Time Scale 2020; Gradstein, F.M., Ogg, J.G., Schmitz, M.D., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 653–679. [Google Scholar] [CrossRef]
- Graham, L.A.; Boddington, M.E.; Holmstrup, M.; Davies, P.L. Antifreeze protein complements cryoprotective dehydration in the freeze-avoiding springtail Megaphorura arctica. Sci. Rep. 2010, 10, 3047. [Google Scholar] [CrossRef] [Green Version]
- Stayton, C.T. What does convergent evolution mean? The interpretation of convergence and its implications in the search for limits to evolution. Interface Focus 2015, 5, 20150039. [Google Scholar] [CrossRef]
- Chen, L.; DeVries, A.L.; Cheng, C.-H.C. Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc. Natl. Acad. Sci. USA 1997, 94, 3811–3816. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; DeVries, A.L.; Cheng, C.-H.C. Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod. Proc. Natl. Acad. Sci. USA 1997, 94, 3817–3822. [Google Scholar] [CrossRef] [Green Version]
- Keeling, P.J.; Palmer, J.D. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 2008, 9, 605–618. [Google Scholar] [CrossRef]
- Drezen, J.-M.; Josse, T.; Bézier, A.; Gauthier, J.; Huguet, E.; Herniou, E.A. Impact of lateral transfers on the genomes of Lepidoptera. Genes 2017, 8, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharoc, I.A. Horizontal Gene Transfer into the Genomes of Insects. Russ. J. Genet. 2016, 52, 702–707. [Google Scholar] [CrossRef]
- Mao, X.; Liu, Z.; Ma, J.; Pang, H.; Zhang, F. Characterization of a novel β-helix antifreeze protein from the desert beetle Anatolica polita. Cryobiology 2011, 62, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, P.V. Appendix A Miller-Bravais indices. In Ice Physics; Oxford University Press: London, UK, 1974; pp. 725–726. ISBN 9780199587711. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, T.; Yamauchi, A.; Miura, A.; Kondo, H.; Nishimiya, Y.; Sasaki, Y.C.; Tsuda, S. Discovery of Hyperactive Antifreeze Protein from Phylogenetically Distant Beetles Questions Its Evolutionary Origin. Int. J. Mol. Sci. 2021, 22, 3637. https://doi.org/10.3390/ijms22073637
Arai T, Yamauchi A, Miura A, Kondo H, Nishimiya Y, Sasaki YC, Tsuda S. Discovery of Hyperactive Antifreeze Protein from Phylogenetically Distant Beetles Questions Its Evolutionary Origin. International Journal of Molecular Sciences. 2021; 22(7):3637. https://doi.org/10.3390/ijms22073637
Chicago/Turabian StyleArai, Tatsuya, Akari Yamauchi, Ai Miura, Hidemasa Kondo, Yoshiyuki Nishimiya, Yuji C. Sasaki, and Sakae Tsuda. 2021. "Discovery of Hyperactive Antifreeze Protein from Phylogenetically Distant Beetles Questions Its Evolutionary Origin" International Journal of Molecular Sciences 22, no. 7: 3637. https://doi.org/10.3390/ijms22073637
APA StyleArai, T., Yamauchi, A., Miura, A., Kondo, H., Nishimiya, Y., Sasaki, Y. C., & Tsuda, S. (2021). Discovery of Hyperactive Antifreeze Protein from Phylogenetically Distant Beetles Questions Its Evolutionary Origin. International Journal of Molecular Sciences, 22(7), 3637. https://doi.org/10.3390/ijms22073637







