Alpha-1 Antitrypsin-Induced Endoplasmic Reticulum Stress Promotes Invasion by Extravillous Trophoblasts
Abstract
1. Introduction
2. Results
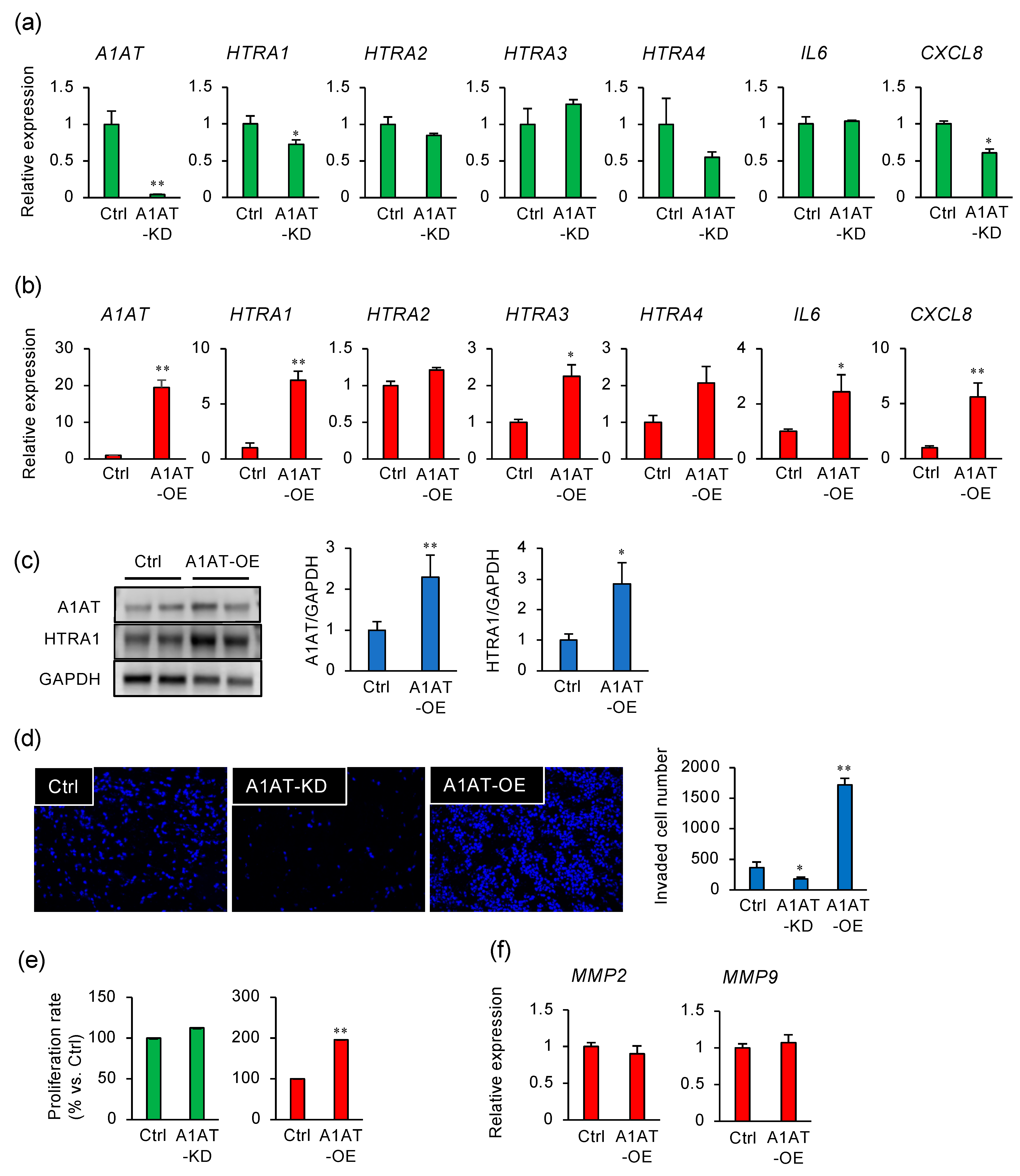
2.1. Alpha-1 Antitrypsin (A1AT) Regulates the Expression of HTRA1 in an Extravillous Trophoblast Cell Line
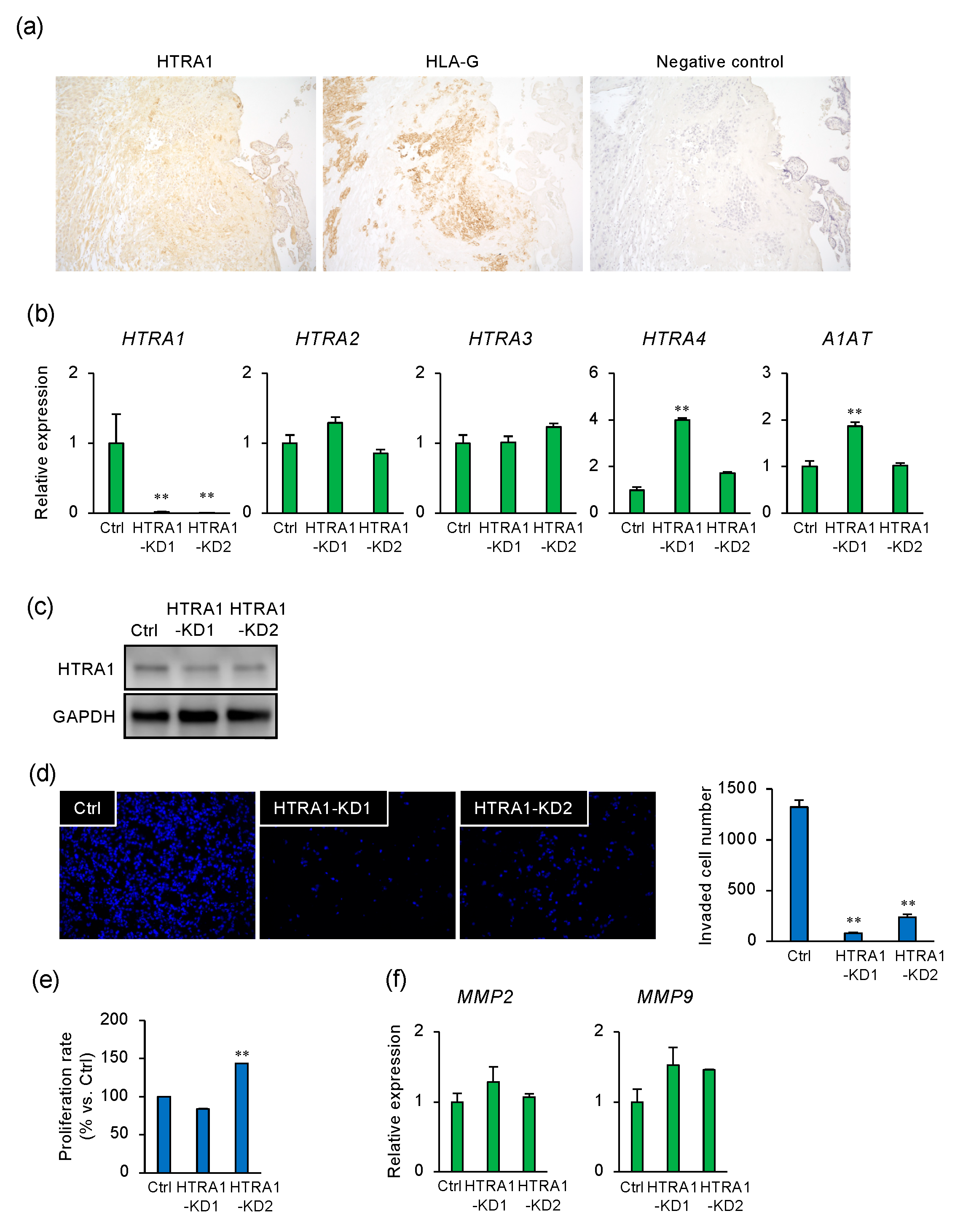
2.2. HTRA1 Regulates Invasion by Extravillous Trophoblast Cells
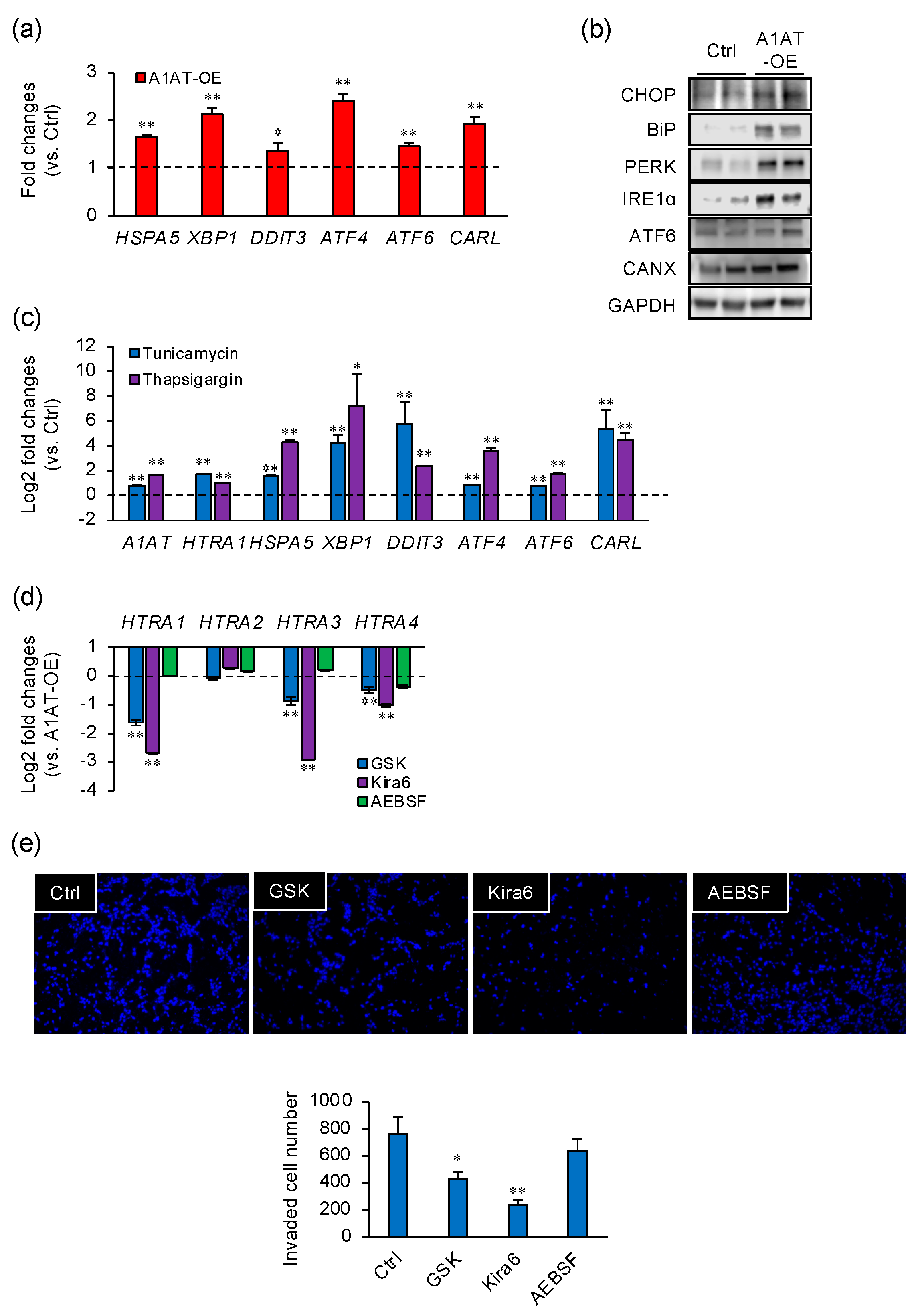
2.3. A1AT-Induced HTRA1 Expression and Cell Invasion Are Regulated by UPR Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transfection of Small Interfering (si)RNA
4.3. Transfection of the SERPINA1 Plasmid Construct
4.4. RNA Extraction and Quantitative RT-PCR
4.5. Western Blotting
4.6. Invasion Assay
4.7. Cell Viability and Proliferation Assays
4.8. Immunohistochemistry
4.9. Reagents
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lim, K.H.; Zhou, Y.; Janatpour, M.; McMaster, M.; Bass, K.; Chun, S.H.; Fisher, S.J. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 1997, 151, 1809–1818. [Google Scholar]
- Brown, C.M.; Garovic, V.D. Mechanisms and management of hypertension in pregnant women. Curr. Hypertens. Rep. 2011, 13, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.M.; Tuder, R. Alpha 1 anti-trypsin: One protein, many functions. Curr. Mol. Med. 2012, 12, 827–835. [Google Scholar] [CrossRef]
- Tamura, K.; Takashima, H.; Fumoto, K.; Kajihara, T.; Uchino, S.; Ishihara, O.; Yoshie, M.; Kusama, K.; Tachikawa, E. Possible Role of alpha1-Antitrypsin in Endometriosis-Like Grafts From a Mouse Model of Endometriosis. Reprod. Sci. 2015, 22, 1088–1097. [Google Scholar] [CrossRef]
- Starodubtseva, N.; Nizyaeva, N.; Baev, O.; Bugrova, A.; Gapaeva, M.; Muminova, K.; Kononikhin, A.; Frankevich, V.; Nikolaev, E.; Sukhikh, G. SERPINA1 Peptides in Urine as A Potential Marker of Preeclampsia Severity. Int. J. Mol. Sci. 2020, 21, 914. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Al-Omari, M.; Maegel, L.; Müller, M.; Izykowski, N.; Hong, J.; Hong, K.; Kim, S.H.; Dorsch, M.; Mahadeva, R.; et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. USA 2013, 110, 15007–15012. [Google Scholar] [CrossRef]
- Frochaux, V.; Hildebrand, D.; Talke, A.; Linscheid, M.W.; Schlüter, H. Alpha-1-antitrypsin: A novel human high temperature requirement protease A1 (HTRA1) substrate in human placental tissue. PLoS ONE 2014, 9, e109483. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Endo, Y.; Nie, G. Decidual HtrA3 negatively regulates trophoblast invasion during human placentation. Hum. Reprod. 2011, 26, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lim, R.; Nie, G. HtrA4 may play a major role in inhibiting endothelial repair in pregnancy complication preeclampsia. Sci. Rep. 2019, 9, 2728. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.E.; Dickens, J.A.; Marciniak, S.J. Measuring the effects of alpha1 -antitrypsin polymerisation on the structure and biophysical properties of the endoplasmic reticulum. Biol. Cell 2018, 110, 249–255. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef]
- Fung, T.S.; Huang, M.; Liu, D.X. Coronavirus-induced ER stress response and its involvement in regulation of coronavirus-host interactions. Virus Res. 2014, 194, 110–123. [Google Scholar] [CrossRef]
- Ando, Y.; Kuroda, A.; Kusama, K.; Matsutani, T.; Matsuda, A.; Tamura, K. Impact of serine protease inhibitor alpha1-antitrypsin on expression of endoplasmic reticulum stress-induced proinflammatory factors in adipocytes. Biochem. Biophys. Rep. 2021, 26. [Google Scholar] [CrossRef]
- Lee, C.L.; Veerbeek, J.H.W.; Rana, T.K.; van Rijn, B.B.; Burton, G.J.; Yung, H.W. Role of Endoplasmic Reticulum Stress in Proinflammatory Cytokine-Mediated Inhibition of Trophoblast Invasion in Placenta-Related Complications of Pregnancy. Am. J. Pathol. 2019, 189, 467–478. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chuang, P.Y.; Chen, C.P.; Chiu, Y.H.; Lo, H.F.; Cheong, M.L.; Huang, J.Y.; Kuo, P.L.; Chen, H. Functional antagonism between high temperature requirement protein A (HtrA) family members regulates trophoblast invasion. J. Biol. Chem. 2014, 289, 22958–22968. [Google Scholar] [CrossRef]
- Skorko-Glonek, J.; Zurawa-Janicka, D.; Koper, T.; Jarzab, M.; Figaj, D.; Glaza, P.; Lipinska, B. HtrA protease family as therapeutic targets. Curr. Pharm. Des. 2013, 19, 977–1009. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kaner, Z.; Ochayon, D.E.; Shahaf, G.; Baranovski, B.M.; Bahar, N.; Mizrahi, M.; Lewis, E.C. Acute phase protein α1-antitrypsin reduces the bacterial burden in mice by selective modulation of innate cell responses. J. Infect. Dis. 2015, 211, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, N.; Xu, J.; Zou, J.; Liang, X.; Liu, H.; Chen, Y. Alpha-1-antitrypsin functions as a protective factor in preeclampsia through activating Smad2 and inhibitor of DNA binding 4. Oncotarget 2017, 8, 113002–113012. [Google Scholar] [CrossRef][Green Version]
- Feng, Y.; Xu, J.; Zhou, Q.; Wang, R.; Liu, N.; Wu, Y.; Yuan, H.; Che, H. Alpha-1 Antitrypsin Prevents the Development of Preeclampsia Through Suppression of Oxidative Stress. Front. Physiol. 2016, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Yin, Y.X.; Ding, J.; Yuan, H.; Yang, L.; Xu, J.J.; Hu, L.Q. Alpha-1-antitrypsin suppresses oxidative stress in preeclampsia by inhibiting the p38MAPK signaling pathway: An in vivo and in vitro study. PLoS ONE 2017, 12, e0173711. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Zhou, C.J.; Li, X.M.; Liang, X.Q. Alpha-1-antitrypsin acts as a preeclampsia-related protein: A proteomic study. Gynecol. Obstet. Investig. 2012, 73, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Twina, G.; Sheiner, E.; Shahaf, G.; Yaniv Salem, S.; Madar, T.; Baron, J.; Wiznitzer, A.; Mazor, M.; Holcberg, G.; Lewis, E.C. Lower circulation levels and activity of α-1 antitrypsin in pregnant women with severe preeclampsia. J. Matern. Fetal Neonatal Med. 2012, 25, 2667–2670. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, I.A.; Zhao, G.; Funai, E.F.; Harris, N.; Sasson, I.E.; Bernstein, I.M.; Saade, G.R.; Buhimschi, C.S. Proteomic profiling of urine identifies specific fragments of SERPINA1 and albumin as biomarkers of preeclampsia. Am. J. Obstet. Gynecol. 2008, 199, 551.e1–551.e16. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Nayeri, U.A.; Zhao, G.; Shook, L.L.; Pensalfini, A.; Funai, E.F.; Bernstein, I.M.; Glabe, C.G.; Buhimschi, C.S. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci. Transl. Med. 2014, 6, 245ra292. [Google Scholar] [CrossRef]
- Gerasimova, E.M.; Fedotov, S.A.; Kachkin, D.V.; Vashukova, E.S.; Glotov, A.S.; Chernoff, Y.O.; Rubel, A.A. Protein Misfolding during Pregnancy: New Approaches to Preeclampsia Diagnostics. Int. J. Mol. Sci. 2019, 20, 6183. [Google Scholar] [CrossRef]
- Lechowicz, U.; Rudzinski, S.; Jezela-Stanek, A.; Janciauskiene, S.; Chorostowska-Wynimko, J. Post-Translational Modifications of Circulating Alpha-1-Antitrypsin Protein. Int. J. Mol. Sci. 2020, 21, 9187. [Google Scholar] [CrossRef]
- Wongwananuruk, T.; Sato, T.; Kajihara, T.; Matsumoto, S.; Akita, M.; Tamura, K.; Brosens, J.J.; Ishihara, O. Endometrial androgen signaling and decidualization regulate trophoblast expansion and invasion in co-culture: A time-lapse study. Placenta 2016, 47, 56–62. [Google Scholar] [CrossRef]
- Kusama, K.; Tamura, K.; Bai, H.; Sakurai, T.; Nishi, H.; Isaka, K.; Imakawa, K.; Yoshie, M. Exchange protein directly activated by cAMP (EPAC) promotes transcriptional activation of the decidual prolactin gene via CCAAT/enhancer-binding protein in human endometrial stromal cells. Reprod. Fertil. Dev. 2018, 30, 1454–1461. [Google Scholar] [CrossRef]
- Kusama, K.; Miyagawa, M.; Ota, K.; Kuwabara, N.; Saeki, K.; Ohnishi, Y.; Kumaki, Y.; Aizawa, T.; Nakasone, T.; Okamatsu, S.; et al. Cordyceps militaris Fruit Body Extract Decreases Testosterone Catabolism and Testosterone-Stimulated Prostate Hypertrophy. Nutrients 2020, 13, 50. [Google Scholar] [CrossRef]
- Kusama, K.; Bai, R.; Imakawa, K. Regulation of human trophoblast cell syncytialization by transcription factors STAT5B and NR4A3. J. Cell. Biochem. 2018, 119, 4918–4927. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, M.; Kashima, H.; Bessho, T.; Takeichi, M.; Isaka, K.; Tamura, K. Expression of stathmin, a microtubule regulatory protein, is associated with the migration and differentiation of cultured early trophoblasts. Hum. Reprod. 2008, 23, 2766–2774. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Yoshie, M.; Tamura, K.; Daikoku, T.; Takarada, T.; Tachikawa, E. Possible roles of the cAMP-mediators EPAC and RAP1 in decidualization of rat uterus. Reproduction 2014, 147, 897–906. [Google Scholar] [CrossRef] [PubMed]

| Name (Accession No.) | Sequence (5′---3′) | Product Length (bp) |
|---|---|---|
| GAPDH NM_002046.7 | AGCCACATCGCTCAGACA | 66 |
| GCCCAATACGACCAAATCC | ||
| SERPINA1 NM_001127704.2 | TCAAGGAGCTTGACAGAGACAC | 94 |
| TCGGTGTCCTTGACTTCAAAGG | ||
| HTRA1 NM_002775.5 | AACACCTACGCCAACCTGTG | 127 |
| GCAAACTGTTGGGATCTTCCTG | ||
| HTRA2 NM_013247.5 | TGAAGGTCACAGCTGGAATCTC | 147 |
| TGGGACTCAGGGTCAGCATC | ||
| HTRA3 NM_053044.5 | ACAAGAAGTCGGACATTGCC | 139 |
| ACTGTGTTCTGTAGGGCGAAG | ||
| HTRA4 | GACTACGTCCAGATTGATGCC | 145 |
| NM_153692.4 | ACTGCCTAACTCGATCTGAAGG | |
| IL6 | CAGGAGCCCAGCTATGAACT | 85 |
| NM_000600.5 | AGCAGGCAACACCAGGAG | |
| IL8 | AAGCATACTCCAAACCTTTCCA | 123 |
| NM_000584.4 | CCAGACAGAGCTCTCTTCCA | |
| HSPA5 | CTGTCCAGGCTGGTGTGCTCT | 143 |
| NM_005347.5 | CTTGGTAGGCACCACTGTGTTC | |
| DDIT3 | AGAACCAGGAAACGGAAACAGA | 67 |
| NM_001195053.1 | TCTCCTTCATGCGCTGCTTT | |
| Spliced XBP1 | CTGAGTCCGAATCAGGTGCAG | 59 |
| NM_001079539.2 | ATCCATGGGGAGATGTTCTGG | |
| ATF4 | GTTCTCCAGCGACAAGGCTA | 88 |
| NM_001675.4 | ATCCTGCTTGCTGTTGTTGG | |
| ATF6 | CAGACAGTACCAACGCTTATGCC | 133 |
| NM_007348.4 | GCAGAACTCCAGGTGCTTGAAG | |
| MMP2 | AGCGAGTGGATGCCGCCTTTAA | 138 |
| NM_004530.6 | CATTCCAGGCATCTGCGATGAG | |
| MMP9 | GCCACTACTGTGCCTTTGAGTC | 125 |
| NM_004994.3 | CCCTCAGAGAATCGCCAGTACT | |
| CARL | GACCTCTGGCAGGTCAAGTC | 71 |
| NM_004343.4 | TCAGCGTATGCCTCATCGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Kusama, K.; Fukushima, Y.; Ohmaru-Nakanishi, T.; Kato, K.; Tamura, K. Alpha-1 Antitrypsin-Induced Endoplasmic Reticulum Stress Promotes Invasion by Extravillous Trophoblasts. Int. J. Mol. Sci. 2021, 22, 3683. https://doi.org/10.3390/ijms22073683
Yoshida K, Kusama K, Fukushima Y, Ohmaru-Nakanishi T, Kato K, Tamura K. Alpha-1 Antitrypsin-Induced Endoplasmic Reticulum Stress Promotes Invasion by Extravillous Trophoblasts. International Journal of Molecular Sciences. 2021; 22(7):3683. https://doi.org/10.3390/ijms22073683
Chicago/Turabian StyleYoshida, Kanoko, Kazuya Kusama, Yuta Fukushima, Takako Ohmaru-Nakanishi, Kiyoko Kato, and Kazuhiro Tamura. 2021. "Alpha-1 Antitrypsin-Induced Endoplasmic Reticulum Stress Promotes Invasion by Extravillous Trophoblasts" International Journal of Molecular Sciences 22, no. 7: 3683. https://doi.org/10.3390/ijms22073683
APA StyleYoshida, K., Kusama, K., Fukushima, Y., Ohmaru-Nakanishi, T., Kato, K., & Tamura, K. (2021). Alpha-1 Antitrypsin-Induced Endoplasmic Reticulum Stress Promotes Invasion by Extravillous Trophoblasts. International Journal of Molecular Sciences, 22(7), 3683. https://doi.org/10.3390/ijms22073683






