Effects of Lifestyle Intervention in Tissue-Specific Lipidomic Profile of Formerly Obese Mice
Abstract
1. Introduction
2. Results
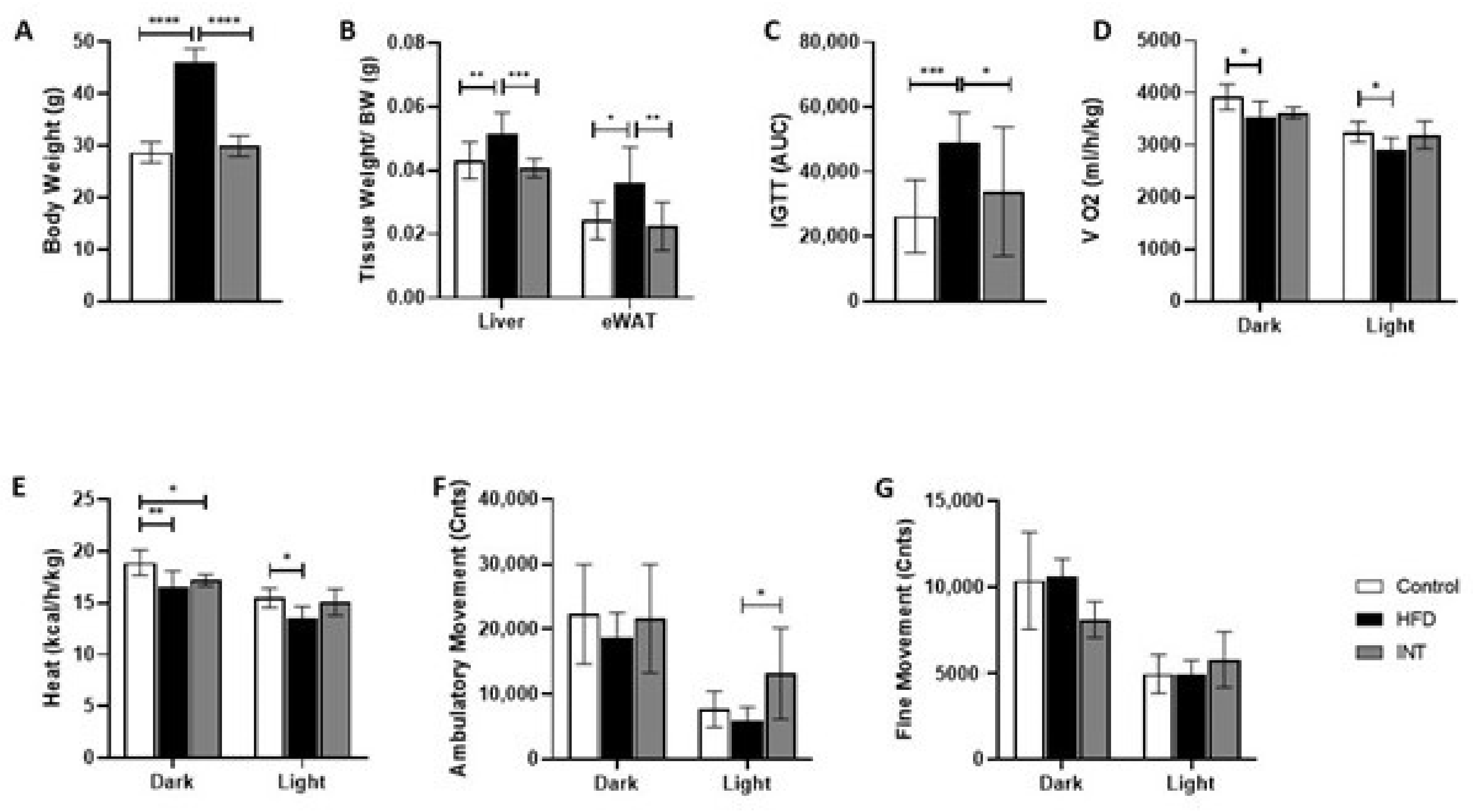
2.1. High-Fat Diet Induced Phenotype Is Reversed by Nutritional and Exercise Intervention
2.2. Lipid Profile Is Tissue-Specific
2.3. HFD and Nutritional and Exercise Intervention Did Not Alter the Lipid Profile of Hypothalamus
2.4. Lipid Profile of Gastrocnemius Was Sensitive to Alterations in Energy Balance
2.5. High-Fat Diet Induced Increase in Most Lipid Classes Content, and Its Reversibility after Intervention Was a Marker of the Liver Lipid Profile
2.6. eWAT Lipid Content Mirrored the Composition of the Diet
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Nutritional and Exercise Intervention
4.3. Glucose Homeostasis In Vivo Functional Assay
4.4. Indirect Calorimetry
4.5. Sample Preparation for NMR Metabolomics
4.6. Nuclear Magnetic Resonance (NMR) Metabolomics Analysis
4.7. NMR Data Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huynh, K.; Martins, R.N.; Meikle, P.J. Lipidomic Profiles in Diabetes and Dementia. J. Alzheimers Dis. 2017, 59, 433–444. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—An allostatic perspective. BBA Mol. Cell Biol. Lipids 2010, 1801, 338–349. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin resistance, obesity and lipotoxicity. In Obesity and Lipotoxicity: Advances in Experimental Medicine and Biology; Engin, A.B., Engin, A., Eds.; Springer Nature: Cham, Switzerland, 2017; Volume 960, pp. 277–304. [Google Scholar]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K.C. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 266–271. [Google Scholar] [CrossRef]
- Engin, A.B. What is lipotoxicity. In Obesity and Lipotoxicity: Advances in Experimental Medicine and Biology; Engin, A.B., Engin, A., Eds.; Springer Nature: Cham, Switzerland, 2017; Volume 960, pp. 197–220. [Google Scholar]
- Jain, M.; Ngoy, S.; Sheth, S.A.; Swanson, R.A.; Rhee, E.P.; Liao, R.; Clish, C.B.; Mootha, V.K.; Nilsson, R. A systematic survey of lipids across mouse tissues. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Lydic, T.A.; Goo, Y.-H. Lipidomics unveils the complexity of the lipidome in metabolic diseases. Lydic. Goo. Clin. Trans. Med. 2018, 7, 4. [Google Scholar] [CrossRef]
- Pradas, I.; Huynh, K.; Cabré, R.; Ayala, V.; Meikle, P.J.; Jové, M.; Pamplona, R. Lipidomics reveals a tissue-specific fingerprint. Front. Physiol. 2018, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J. Circadian regulation of lipid metabolism. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2016; Volume 75, pp. 440–450. [Google Scholar]
- Mizunoya, W.; Iwamoto, Y.; Shirouchi, B.; Sato, M.; Komiya, Y.; Razin, F.R.; Tatsumi, R.; Sato, Y.; Nakamura, M.; Ikeuchi, Y. Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle. PLoS ONE 2013, 8, e80152. [Google Scholar] [CrossRef]
- Lynes, M.D.; Shamsi, F.; Sustarsic, E.G.; Leiria, L.O.; Su, S.; Huang, T.L.; Gao, F.; Narain, N.R.; Chen, E.Y.; Cypess, A.M.; et al. Cold-Activated Lipid Dynamics in Adipose Tissue Highlights a Role for Cardiolipin in Thermogenic Metabolism. Cell Rep. 2018, 24, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; You, W.; Zhou, Y.; Chen, W.; Wang, Y.; Shan, T. Cold-induced lipid dynamics and transcriptional programs in white adipose tissue. BMC Biol. 2019, 17, 74. [Google Scholar] [CrossRef]
- Lee, S.T.; Lee, J.C.; Kim, J.W.; Cho, S.Y.; Seong, J.K.; Moon, M.H. Global Changes in Lipid Profiles of Mouse Cortex, Hippocampus, and Hypothalamus Upon p53 Knockout. Sci. Rep. 2016, 6, 36510. [Google Scholar] [CrossRef] [PubMed]
- Id, F.I.; Fretts, A.; Id, M.M.; Korat, A.V.A.; Yang, W.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.; Id, K.W.; et al. Fatty acid biomarkers of dairy fat consumption and incidence of T2DM, a pooled analysis of prospective Cohort Studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef]
- Id, M.G.; Id, M.H.S.; Lo, C. V Lipidomic and transcriptomic analysis of western diet-induced nonalcoholic steatohepatitis (NASH) in female Ldlr−/− mice. PLoS ONE 2019, 14, e0214387. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Hernandez-Carretero, A.; Weber, N.; La Frano, M.R.; Ying, W.; Lantero Rodriguez, J.; Sears, D.D.; Wallenius, V.; Börgeson, E.; Newman, J.W.; Osborn, O.; et al. Obesity-induced changes in lipid mediators persist after weight loss. Int. J. Obes. 2018, 42, 728–736. [Google Scholar] [CrossRef]
- Hou, B.; Zhao, Y.; He, P.; Xu, C.; Ma, P.; Lam, S.M.; Li, B.; Gil, V.; Shui, G.; Qiang, G.; et al. Targeted lipidomics and transcriptomics profiling reveal the heterogeneity of visceral and subcutaneous white adipose tissue. Life Sci. 2020, 245, 117352. [Google Scholar] [CrossRef]
- Cortie, C.H.; Hulbert, A.J.; Hancock, S.E.; Mitchell, T.W.; McAndrew, D.; Else, P.L. Of mice, pigs and humans: An analysis of mitochondrial phospholipids from mammals with very different maximal lifespans. Exp. Gerontol. 2015, 70, 135–143. [Google Scholar] [CrossRef]
- Turner, N.; Kowalski, G.M.; Leslie, S.J.; Risis, S.; Yang, C.; Lee-Young, R.S.; Babb, J.R.; Meikle, P.J.; Lancaster, G.I.; Henstridge, D.C.; et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 2013, 56, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.L.; Omran, S.F.; Weir, J.; Meikle, P.J.; Watt, M.J. Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J. Physiol. 2012, 590, 4377–4389. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Park, S.M.; Kim, I.Y.; Sung, H.; Seong, J.K.; Moon, M.H. High-fat diet-induced lipidome perturbations in the cortex, hippocampus, hypothalamus, and olfactory bulb of mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 980–990. [Google Scholar] [CrossRef]
- Mannock, D.A.; Lewis, R.N.A.H.; Mcmullen, T.P.W.; Mcelhaney, R.N. The effect of variations in phospholipid and sterol structure on the nature of lipid—Sterol interactions in lipid bilayer model membranes. Chem. Phys. Lipids 2010, 163, 403–448. [Google Scholar] [CrossRef]
- Morgan, A.E.; Auley, M.T.M. Cholesterol homeostasis: An in silico investigation into how aging disrupts its key hepatic regulatory mechanisms. Biology 2020, 9, 314. [Google Scholar] [CrossRef]
- Van Der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. BBA Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.E.; Marcucci, M.J.; Cline, G.W.; Bell, K.; Barucci, N.; Lee, D.; Goodyear, L.J.; Kraegen, E.W.; White, M.F.; Shulman, G.I. Free Fatty Acid–Induced Insulin Resistance Is Associated with Activation of Protein Kinase C theta and Alterations in the Insulin Signaling Cascade. Diabetes 1999, 48, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Dresner, A.; Laurent, D.; Marcucci, M.; Griffin, M.E.; Dufour, S.; Cline, G.W.; Slezak, L.A.; Andersen, D.K.; Hundal, R.S.; Rothman, D.L.; et al. Effects of free fatty acids on glucose transport and IRS-1—Associated phosphatidylinositol 3-kinase activity. J. Clin. Investig. 1999, 103, 253–259. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Ruiz, J. Mechanisms of fatty acid-induced inhibition of glucose uptake. J. Clin. Investig. 1994, 93, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef]
- Morales, P.E.; Bucarey, J.L.; Espinosa, A. Muscle lipid metabolism: Role of lipid droplets and perilipins. J. Diabetes Res. 2017, 2017, 1789395. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2013, 16, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Sarah, E.; Goodpaster, B.H. Exercise-Induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2013, 294, E882–E888. [Google Scholar] [CrossRef]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, 61–79. [Google Scholar] [CrossRef]
- Kim, J.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Lipid, J.A.H. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Hulver, M.W.; Berggren, J.R.; Cortright, R.N.; Dudek, R.W.; Thompson, R.P.; Pories, W.J.; MacDonald, K.G.; Cline, G.W.; Shulman, G.I.; Dohm, G.L.; et al. Skeletal muscle lipid metabolism with obesity. Am. J. Physiol. Endocrinol. Metab. 2003, 284, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.P.; Bonen, A.; Spriet, L.L. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am. J. Clin. Nutr. 2009, 89, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Roves, P.; Huss, J.M.; Han, D.; Hancock, C.R.; Iglesias-Gutierrez, E.; Chen, M.; Holloszy, J.O. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc. Natl. Acad. Sci. USA 2007, 104, 10709–10713. [Google Scholar] [CrossRef] [PubMed]
- Holmström, M.H.; Iglesias-Gutierrez, E.; Zierath, J.R.; Garcia-Roves, P.M. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Bruce, C.R.; Beale, S.M.; Hoehn, K.L.; So, T.; Rolph, M.S.; Cooney, G.J. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle. Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance. Diabetes 2007, 56, 2085–2092. [Google Scholar] [CrossRef]
- Bosma, M. Lipid homeostasis in exercise. Drug. Discov. Today 2014, 19, 1019–1023. [Google Scholar] [CrossRef]
- Kiens, B. Skeletal Muscle Lipid Metabolism in Exercise and Insulin Resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Exercise metabolism: Fuels for the fire. Cold Spring Harb. Perspect. Med. 2018, 8, a029744. [Google Scholar] [CrossRef]
- Madsbad, S. Dietary intervention increases n-3 long-chain polyunsaturated fatty acids in skeletal muscle membrane phospholipids of obese subjects. Implications for insulin sensitivity. Clin. Endocrinol. 2006, 64, 169–178. [Google Scholar] [CrossRef]
- Turner, N.; Lee, J.S.; Bruce, C.R.; Mitchell, T.W.; Else, P.L.; Hulbert, A.J.; Hawley, J.A.; Lee, J.S.; Bruce, C.R.; Todd, W.; et al. Greater effect of diet than exercise training on the fatty acid profile of rat skeletal muscle. J. Appl. Physiol. 2021, 974–980. [Google Scholar] [CrossRef]
- Ibrahim, A.; Natarajan, S. Substituting dietary linoleic acid with a -linolenic acid improves insulin sensitivity in sucrose fed rats. BBA 2005, 1733, 67–75. [Google Scholar] [CrossRef]
- Van Der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H.; Nash, B. The effects of physical exercise on fatty liver disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Fuente, F.P.; Quezada, L.; Sepúlveda, C.; Monsalves-Alvarez, M.; Rodríguez, J.M.; Sacristán, C.; Chiong, M.; Llanos, M.; Espinosa, A.; Troncoso, R. Exercise regulates lipid droplet dynamics in normal and fatty liver. BBA Mol. Cell Biol. Lipids 2019, 1864, 158519. [Google Scholar] [CrossRef]
- Trentzsch, M.; Nyamugenda, E.; Miles, T.K.; Grif, H.; Russell, S.; Koss, B.; Cooney, K.A.; Phelan, K.D.; Tackett, A.J.; Iyer, S.; et al. Delivery of phosphatidylethanolamine blunts stress in hepatoma cells exposed to elevated palmitate by targeting the endoplasmic reticulum. Cell Death Discov. 2020, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Signaling, I.; Zorzano, A.; Sebastia, D.; Mun, J.P. Mitofusin 2 as a Driver That Controls Energy. Antioxid. Redox Signal. 2015, 22, 1020–1031. [Google Scholar] [CrossRef]
- Golabi, P.; Locklear, C.T.; Austin, P.; Afdhal, S.; Byrns, M.; Gerber, L.; Younossi, Z.M.; Golabi, P.; Austin, P.; Afdhal, S.; et al. Effectiveness of exercise in hepatic fat mobilization in non- alcoholic fatty liver disease: Systematic review. World J. Gastroenterol. 2016, 22, 6318–6327. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Grzybek, M.; Palladini, A.; Alexaki, V.I.; Surma, M.A.; Simons, K. Comprehensive and quantitative analysis of white and brown adipose tissue by shotgun lipidomics. Mol. Metab. 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell. Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, N.; Molavi, B.; Elbein, S.C.; Kern, P.A. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes. Metab. 2007, 9, 1–10. [Google Scholar] [CrossRef]
- Caesar, R.; Manieri, M.; Kelder, T.; Boekschoten, M.; Evelo, C.; Kooistra, T.; Cinti, S.; Kleemann, R.; Drevon, C.A. A combined transcriptomics and lipidomics analysis of subcutaneous, epididymal and mesenteric adipose tissue reveals marked functional differences. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.; Poirier, P. Ectopic fat and cardiac metabolism. Expert Rev. Endocrinol. Metab. 2018, 13, 213–221. [Google Scholar] [CrossRef]
- Zabielski, P.; Ksi, M. The impact of oMEGA-3 fatty acids supplementation on insulin resistance and content of adipocytokines and biologically active lipids in adipose tissue of high-fat diet fed rats. Nutrients 2019, 11, 835. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S.; Rodríguez, A.; Gonçalves, I.O.; Moreira, A.; Maciel, E.; Santos, S.; Domingues, M.R.; Frühbeck, G.; Ascensão, A. Impact of physical exercise on visceral adipose tissue fatty acid profile and inflammation in response to a high-fat diet regimen. Int. J. Biochem. Cell Biol. 2017, 87, 114–124. [Google Scholar] [CrossRef]
- Biorxiv, the Preprint Server for Biology. Available online: https://www.biorxiv.org/content/10.1101/2020.07.08.194167v1 (accessed on 11 July 2020).
- Vinaixa, M.; Miguel, A.; Rull, A.; Joven, J.; Correig, X. Metabolomic assessment of the effect of dietary cholesterol in the progressive development of fatty liver disease. J. Proteome Res. 2010, 9, 2527–2538. [Google Scholar] [CrossRef]





| Chow Diet | High-Fat Diet | Intervention Diet | ||
|---|---|---|---|---|
| Caloric Density (kcal/g) | 2.9 | 4.7 | 4.7 | |
| Protein (%) | 20 | 20 | 20 | |
| Carbohydrates (%) | 67 | 35 | 35 | |
| Fat (%) | 13 | 45 | 45 | |
| Total carbohydrates | ||||
| Source (%) | Corn starch | 21.1 | 63.8 | |
| Maltodextrin | 28.9 | 36.2 | ||
| Sucrose | 50 | - | ||
| Total fat | ||||
| Source (%) | Soybean oil | 12.3 | - | |
| Lard | 87.7 | - | ||
| Flaxseed oil | - | 55.6 | ||
| Olive oil | - | 44.4 | ||
| Composition (%) | Saturated fat | 17.6 | 31.7 | 11.8 |
| Monounsaturated fat | 20.6 | 35.6 | 43.8 | |
| Polyunsaturated fat | 61.8 | 32.7 | 44.4 |
| High-Fat Diet | Intervention Diet | |||
|---|---|---|---|---|
| Sources (g) | Daily Intake (mg) | Sources (g) | Daily Intake (mg) | |
| Total fat | 202.5 | 600 | 202.5 | 450 |
| C16, Palmitic | 36.8 | 109.0 | 14.9 | 33.1 |
| C18, Stearic | 19.8 | 58.7 | 5.6 | 12.4 |
| C18:1, Oleic | 64.1 | 189.9 | 83.7 | 186.0 |
| C18:2, Linoleic | 56.2 | 166.5 | 29.7 | 66.0 |
| C18:3, Linolenic (ALA) | 4.2 | 12.4 | 62.4 | 138.7 |
| C20:4, Arachidonic (ARA) | 0.5 | 1.5 | 0 | 0 |
| C20:5, Eicosapentaenoic (EPA) | 0 | 0 | 0 | 0 |
| C22:6, Docosahexaenoic (DHA) | 0 | 0 | 0 | 0 |
| Ctrl | HFD | INT | |
|---|---|---|---|
| Daily Intake (g) | 3.08 | 2.55 | 1.9 |
| Caloric Density (kcal/g) | 2.90 | 4.73 | 4.73 |
| Daily Caloric Intake (kcal) | 8.93 | 12.06 | 8.99 |
| Daily Intake of Macronutrients (g) | |||
| Protein | 0.45 | 0.60 | 0.45 |
| Carbohydrate | 1.50 | 1.06 | 0.79 |
| Fat | 0.13 | 0.60 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahdah, N.; Gonzalez-Franquesa, A.; Samino, S.; Gama-Perez, P.; Herrero, L.; Perales, J.C.; Yanes, O.; Malagón, M.D.M.; Garcia-Roves, P.M. Effects of Lifestyle Intervention in Tissue-Specific Lipidomic Profile of Formerly Obese Mice. Int. J. Mol. Sci. 2021, 22, 3694. https://doi.org/10.3390/ijms22073694
Dahdah N, Gonzalez-Franquesa A, Samino S, Gama-Perez P, Herrero L, Perales JC, Yanes O, Malagón MDM, Garcia-Roves PM. Effects of Lifestyle Intervention in Tissue-Specific Lipidomic Profile of Formerly Obese Mice. International Journal of Molecular Sciences. 2021; 22(7):3694. https://doi.org/10.3390/ijms22073694
Chicago/Turabian StyleDahdah, Norma, Alba Gonzalez-Franquesa, Sara Samino, Pau Gama-Perez, Laura Herrero, José Carlos Perales, Oscar Yanes, Maria Del Mar Malagón, and Pablo Miguel Garcia-Roves. 2021. "Effects of Lifestyle Intervention in Tissue-Specific Lipidomic Profile of Formerly Obese Mice" International Journal of Molecular Sciences 22, no. 7: 3694. https://doi.org/10.3390/ijms22073694
APA StyleDahdah, N., Gonzalez-Franquesa, A., Samino, S., Gama-Perez, P., Herrero, L., Perales, J. C., Yanes, O., Malagón, M. D. M., & Garcia-Roves, P. M. (2021). Effects of Lifestyle Intervention in Tissue-Specific Lipidomic Profile of Formerly Obese Mice. International Journal of Molecular Sciences, 22(7), 3694. https://doi.org/10.3390/ijms22073694







