The Role of Formyl Peptide Receptors in Permanent and Low-Grade Inflammation: Helicobacter pylori Infection as a Model
Abstract
:1. Introduction
2. Formyl Peptide Receptors
2.1. Formyl Peptide Receptors: Cell Distribution and Classification
2.2. FPRs: G-Protein Coupled Receptors
2.3. N-Formylated Peptides: Signal Peptides Detected by FPRs
2.4. More Than N-Formylated Peptides: Other Ligands Detected by FPRL1
3. Formyl Peptide Receptors in Helicobacter pylori Chronic Infection
3.1. Helicobacter pylori and Chronic Inflammatory Response
3.2. Helicobacter pylori Hp(2-20) Modulated the Host Immune Response by Interacting with FPRL1
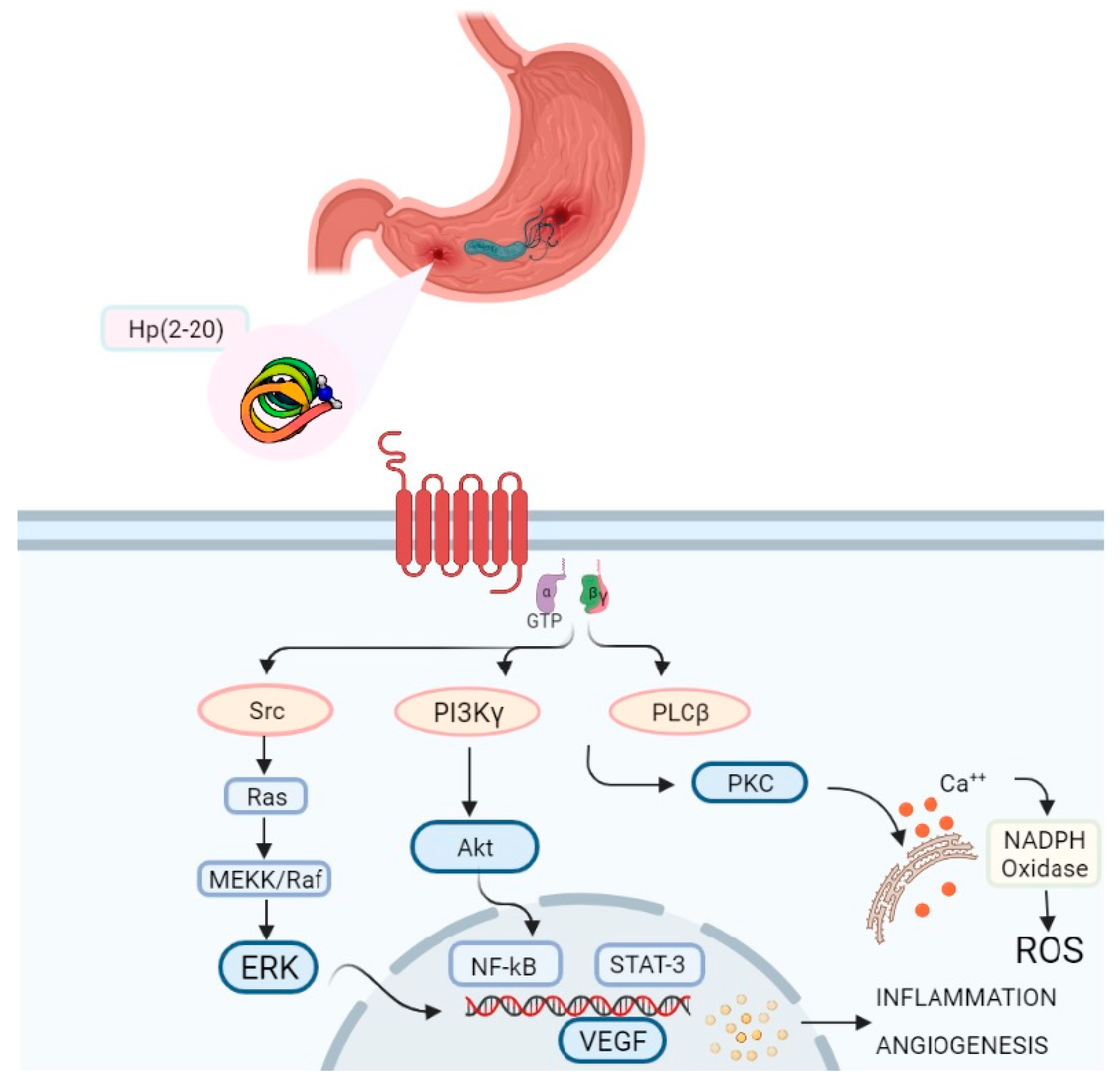
3.3. Hp(2-20) and FPRL1: Intracellular Signalling Cascade
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yatim, K.M.; Lakkis, F.G. A brief journey through the immune system. Clin. J. Am. Soc. Nephrol. 2015, 10, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basset, C.; Holton, J.; O’Mahony, R.; Roitt, I. Innate immunity and pathogen-host interaction. Vaccine 2003, 21, S12–S23. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoni, G.; Neumann, P.A.; Sumagin, R.; Denning, T.L.; Nusrat, A. Wound repair: Role of immune-epithelial interactions. Mucosal Immunol. 2015, 8, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Bae, Y.S. Formyl peptide receptors in the mucosal immune system. Exp. Mol. Med. 2020, 52, 1694–1704. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Filep, J.G.; Sekheri, M.; El Kebir, D. Targeting formyl peptide receptors to facilitate the resolution of inflammation. Eur. J. Pharmacol. 2018, 833, 339–348. [Google Scholar] [CrossRef]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413. [Google Scholar] [CrossRef]
- Xiao, T.S. Innate immunity and inflammation. Cell. Mol. Immunol. 2017, 14, 1–3. [Google Scholar] [CrossRef]
- Zhong, J.; Shi, G. Editorial: Regulation of Inflammation in Chronic Disease. Front. Immunol. 2019, 10, 737. [Google Scholar] [CrossRef] [Green Version]
- Weiß, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef]
- Ye, R.D.; Boulay, F.; Ji, M.W.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Lee, H.-Y.; Kim, M.-K.; Park, K.S.; Park, Y.M.; Kwak, J.-Y.; Bae, Y.-S. The Synthetic Peptide Trp-Lys-Tyr-Met-Val-D-Met Inhibits Human Monocyte-Derived Dendritic Cell Maturation via Formyl Peptide Receptor and Formyl Peptide Receptor-Like 2. J. Immunol. 2005, 175, 685–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.Y.; Lee, M.; Bae, Y.-S. Formyl Peptide Receptors in Cellular Differentiation and Inflammatory Diseases. J. Cell. Biochem. 2017, 118, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017, 85, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Migeotte, I.; Communi, D.; Parmentier, M. Formyl peptide receptors: A promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006, 17, 501–519. [Google Scholar] [CrossRef]
- He, H.-Q.; Ye, R. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef]
- Krepel, S.A.; Wang, J.M. Chemotactic Ligands that Activate G-Protein-Coupled Formylpeptide Receptors. Int. J. Mol. Sci. 2019, 20, 3426. [Google Scholar] [CrossRef] [Green Version]
- Le, Y.; Murphy, P.M.; Wang, J.M. Formyl-peptide receptors revisited. Trends Immunol. 2002, 23, 541–548. [Google Scholar] [CrossRef]
- Le, Y.; Oppenheim, J.J.; Wang, J.M. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 2001, 12, 91–105. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, J.M.; Jo, S.H.; Lee, H.Y.; Lee, S.Y.; Shim, J.W.; Seo, S.-K.; Yun, J.; Bae, Y.-S. Functional Expression of Formyl Peptide Receptor Family in Human NK Cells. J. Immunol. 2009, 183, 5511–5517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.K.; Min, D.S.; Park, Y.J.; Kim, J.H.; Ryu, S.H.; Bae, Y.S. Expression and functional role of formyl peptide receptor in human bone marrow-derived mesenchymal stem cells. FEBS Lett. 2007, 581, 1917–1922. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.C.; Kwon, Y.W.; Jang, I.H.; Jeong, G.O.; Yoon, J.W.; Kim, C.D.; Kwon, S.M.; Bae, Y.S.; Kim, J.H. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells 2014, 32, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Schröder, N.; Schaffrath, A.; Welter, J.A.; Putzka, T.; Griep, A.; Ziegler, P.; Brandt, E.; Samer, S.; Heneka, M.T.; Kaddatz, H.; et al. Inhibition of formyl peptide receptors improves the outcome in a mouse model of Alzheimer disease. J. Neuroinflamm. 2020, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Zhang, S.; Lu, J.; Li, Y.; Yi, X.; Tang, L.; Su, L.; Ding, Y. Serum amyloid A, an acute phase protein, stimulates proliferative and proinflammatory responses of keratinocytes. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [Green Version]
- VanCompernolle, S.E.; Clark, K.L.; Rummel, K.A.; Todd, S.C. Expression and Function of Formyl Peptide Receptors on Human Fibroblast Cells. J. Immunol. 2003, 171, 2050–2056. [Google Scholar] [CrossRef] [Green Version]
- McCoy, R.; Haviland, D.L.; Molmenti, E.P.; Ziambaras, T.; Wetsel, R.A.; Perlmutter, D.H. N-formylpeptide and complement C5a receptors are expressed in liver cells and mediate hepatic acute phase gene regulation. J. Exp. Med. 1995, 182, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Liu, M.; Liu, Y.; Yoshimura, T.; Shen, W.; Le, Y.; Durum, S.; Gong, W.; Wang, C.; Gao, J.L.; et al. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J. Clin. Investig. 2013, 123, 1694–1704. [Google Scholar] [CrossRef] [Green Version]
- Bufe, B.; Schumann, T.; Kappl, R.; Bogeski, I.; Kummerow, C.; Podgórska, M.; Smola, S.; Hoth, M.; Zufall, F. Recognition of bacterial signal peptides by mammalian formyl peptide receptors: A new mechanism for sensing pathogens. J. Biol. Chem. 2015, 290, 7369–7387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenceslau, C.F.; Szasz, T.; McCarthy, C.G.; Baban, B.; NeSmith, E.; Webb, R.C. Mitochondrial N-formyl peptides cause airway contraction and lung neutrophil infiltration via formyl peptide receptor activation. Pulm. Pharmacol. Ther. 2016, 37, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lind, S.; Gabl, M.; Holdfeldt, A.; Mårtensson, J.; Sundqvist, M.; Nishino, K.; Dahlgren, C.; Mukai, H.; Forsman, H. Identification of Residues Critical for FPR2 Activation by the Cryptic Peptide Mitocryptide-2 Originating from the Mitochondrial DNA–Encoded Cytochrome b. J. Immunol. 2019, 202, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- de Paulis, A.; Prevete, N.; Rossi, F.W.; Rivellese, F.; Salerno, F.; Delfino, G.; Liccardo, B.; Avilla, E.; Montuori, N.; Mascolo, M.; et al. Helicobacter pylori Hp(2–20) Promotes Migration and Proliferation of Gastric Epithelial Cells by Interacting with Formyl Peptide Receptors In Vitro and Accelerates Gastric Mucosal Healing In Vivo. J. Immunol. 2009, 183, 3761–3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, J.S. Peptides derived from HIV-1, HIV-2, Ebola virus, SARS coronavirus and coronavirus 229E exhibit high affinity binding to the formyl peptide receptor. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Proost, P.; Li, B.; Gong, W.; Le, Y.; Sargeant, R.; Murphy, P.M.; Van Damme, J.; Wang, J.M. Activation of the chemotactic peptide receptor FPRL1 in monocytes phosphorylates the chemokine receptor CCR5 and attenuates cell responses to selected chemokines. Biochem. Biophys. Res. Commun. 2000, 272, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Le, Y.; Jiang, S.; Hu, J.; Gong, W.; Su, S.; Dunlop, N.M.; Shen, W.; Li, B.; Wang, J.M. N36, a synthetic N-terminal heptad repeat domain of the HIV-1 envelope protein gp41, is an activator of human phagocytes. Clin. Immunol. 2000, 96, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Bellner, L.; Thorén, F.; Nygren, E.; Liljeqvist, J.-Å.; Karlsson, A.; Eriksson, K. A Proinflammatory Peptide from Herpes Simplex Virus Type 2 Glycoprotein G Affects Neutrophil, Monocyte, and NK Cell Functions. J. Immunol. 2005, 174, 2235–2241. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Fang, D.; Hou, X.; Le, Y.; Fang, J.; Wen, F.; Gong, W.; Chen, K.; Wang, J.M.; Su, S.B. HCV Peptide (C5A), an Amphipathic α-Helical Peptide of Hepatitis Virus C, Is an Activator of N-Formyl Peptide Receptor in Human Phagocytes. J. Immunol. 2011, 186, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Hebeda, C.B.; Sandri, S.; Benis, C.M.; de Paula-Silva, M.; Loiola, R.A.; Reutelingsperger, C.; Perretti, M.; Farsky, S.H.P. Annexin A1/Formyl Peptide Receptor Pathway Controls Uterine Receptivity to the Blastocyst. Cells 2020, 9, 1188. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, Q.; Xu, L.; Guo, Z.N.; Ou, Y.; He, Y.; Yin, C.; Sun, X.; Tang, J.; Zhang, J.H. Lipoxin A4 Reduces Inflammation Through Formyl Peptide Receptor 2/p38 MAPK Signaling Pathway in Subarachnoid Hemorrhage Rats. Stroke 2016, 47, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Hinrichs, B.H.; Matthews, J.D.; Siuda, D.; O’Leary, M.N.; Wolfarth, A.A.; Saeedi, B.J.; Nusrat, A.; Neish, A.S. Serum Amyloid A1 Is an Epithelial Prorestitutive Factor. Am. J. Pathol. 2018, 188, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Kim, Y.N.; Jang, Y.-S. Cutting Edge: LL-37–Mediated Formyl Peptide Receptor-2 Signaling in Follicular Dendritic Cells Contributes to B Cell Activation in Peyer’s Patch Germinal Centers. J. Immunol. 2016, 198, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Christophe, T.; Karlsson, A.; Dugave, C.; Rabiet, M.J.; Boulay, F.; Dahlgren, C. The Synthetic Peptide Trp-Lys-Tyr-Met-Val-Met-NH2 Specifically Activates Neutrophils through FPRL1/Lipoxin A4 Receptors and is an Agonist for the Orphan Monocyte-expressed Chemoattractant Receptor FPRL2. J. Biol. Chem. 2001, 276, 21585–21593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepetkin, I.A.; Khlebnikov, A.I.; Giovannoni, M.P.; Kirpotina, L.N.; Cilibrizzi, A.; Quinn, M.T. Development of Small Molecule Non-peptide Formyl Peptide Receptor (FPR) Ligands and Molecular Modeling of Their Recognition. Curr. Med. Chem. 2014, 21, 1478–1504. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, F.; Parisi, M.; Fioretti, T.; Sarnataro, D.; Esposito, G.; Ammendola, R. Nuclear localization of Formyl-Peptide Receptor 2 in human cancer cells. Arch. Biochem. Biophys. 2016, 603, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef]
- Wang, X.; Iyer, A.; Lyons, A.B.; Körner, H.; Wei, W. Emerging roles for G-protein coupled receptors in development and activation of macrophages. Front. Immunol. 2019, 10, 2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, F.; Guerra, G.; Parisi, M.; De Marinis, M.; Tafuri, D.; Cinelli, M.; Ammendola, R. Cell-Surface Receptors Transactivation Mediated by G Protein-Coupled Receptors. Int. J. Mol. Sci. 2014, 15, 19700–19728. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Ye, R.D. Role of G protein-coupled receptors in inflammation. Acta Pharmacol. Sin. 2012, 33, 342–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilder, A.S.; Wang, L.; Natali, L.; Karimi-Mostowfi, N.; Brifault, C.; Gonias, S.L. Pertussis toxin is a robust and selective inhibitor of high grade glioma cell migration and invasion. PLoS ONE 2016, 11, e0168418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locht, C.; Coutte, L.; Mielcarek, N. The ins and outs of pertussis toxin. FEBS J. 2011, 278, 4668–4682. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Liu, H.; Zhou, X.E.; Verma, R.K.; de Waal, P.W.; Jang, W.; Xu, T.H.; Wang, L.; Meng, X.; Zhao, G.; et al. Structure of formylpeptide receptor 2-Gi complex reveals insights into ligand recognition and signaling. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ivankov, D.N.; Payne, S.H.; Galperin, M.Y.; Bonissone, S.; Pevzner, P.A.; Frishman, D. How many signal peptides are there in bacteria? Environ. Microbiol. 2013, 15, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Bufe, B.; Zufall, F. The sensing of bacteria: Emerging principles for the detection of signal sequences by formyl peptide receptors. Biomol. Concepts 2016, 7, 205–214. [Google Scholar] [CrossRef]
- Tjalsma, H.; Bolhuis, A.; Jongbloed, J.D.H.; Bron, S.; van Dijl, J.M. Signal Peptide-Dependent Protein Transport inBacillus subtilis: A Genome-Based Survey of the Secretome. Microbiol. Mol. Biol. Rev. 2000, 64, 515–547. [Google Scholar] [CrossRef] [Green Version]
- Von Heijne, G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: Implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984, 3, 2315–2318. [Google Scholar] [CrossRef]
- Piatkov, K.I.; Vu, T.T.M.; Hwang, C.-S.; Varshavsky, A. Formyl-methionine as a degradation signal at the N-termini of bacterial proteins. Microb. Cell 2015, 2, 376. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.; Laverny, G.; Bernardi, L.; Charles, A.L.; Alsaleh, G.; Pottecher, J.; Sibilia, J.; Geny, B. Mitochondria: An organelle of bacterial origin controlling inflammation. Front. Immunol. 2018, 9, 536. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.L.; Flower, R.J.; Perretti, M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raabe, C.A.; Gröper, J.; Rescher, U. Biased perspectives on formyl peptide receptors. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Gottesmann, M.; Paraskevopoulou, V.; Mohammed, A.; Falcone, F.H.; Hensel, A. BabA and LPS inhibitors against Helicobacter pylori: Pectins and pectin-like rhamnogalacturonans as adhesion blockers. Appl. Microbiol. Biotechnol. 2020, 104, 351–363. [Google Scholar] [CrossRef]
- Cuomo, P.; Papaianni, M.; Fulgione, A.; Guerra, F.; Capparelli, R.; Medaglia, C. An Innovative Approach to Control H. pylori-Induced Persistent Inflammation and Colonization. Microorganisms 2020, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Polk, D.B.; Peek, R.M. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, P.; Papaianni, M.; Sansone, C.; Iannelli, A.; Iannelli, D.; Medaglia, C.; Paris, D.; Motta, A.; Capparelli, R. An In Vitro Model to Investigate the Role of Helicobacter pylori in Type 2 Diabetes, Obesity, Alzheimer’s Disease and Cardiometabolic Disease. Int. J. Mol. Sci. 2020, 21, 8369. [Google Scholar] [CrossRef]
- Lamb, A.; Chen, L.F. Role of the Helicobacter pylori-Induced inflammatory response in the development of gastric cancer. J. Cell. Biochem. 2013, 114, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Soto, L.F.; Haas, R. The CagA toxin of Helicobacter pylori: Abundant production but relatively low amount translocated. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, M. Structure and function of helicobacter pylori caga, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef] [Green Version]
- Bylund, J.; Christophe, T.; Boulay, F.; Nyström, T.; Karlsson, A.; Dahlgren, C. Proinflammatory activity of a cecropin-like antibacterial peptide from Helicobacter pylori. Antimicrob. Agents Chemother. 2001, 45, 1700–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paulis, A.; Prevete, N.; Fiorentino, I.; Walls, A.F.; Curto, M.; Petraroli, A.; Castaldo, V.; Ceppa, P.; Fiocca, R.; Marone, G. Basophils Infiltrate Human Gastric Mucosa at Sites of Helicobacter pylori Infection, and Exhibit Chemotaxis in Response to H. pylori- derived Peptide Hp(2–20). J. Immunol. 2004, 172, 7734–7743. [Google Scholar] [CrossRef] [Green Version]
- Capparelli, R.; De Chiara, F.; Nocerino, N.; Montella, R.C.; Iannaccone, M.; Fulgione, A.; Romanelli, A.; Avitabile, C.; Blaiotta, G.; Capuano, F. New perspectives for natural antimicrobial peptides: Application as antinflammatory drugs in a murine model. BMC Immunol. 2012, 13, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peek, R.M.; Fiske, C.; Wilson, K.T. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol. Rev. 2010, 90, 831–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, F.; Parisi, M.; Ammendola, R. Distinct Signaling Cascades Elicited by Different Formyl Peptide Receptor 2 (FPR2) Agonists. Int. J. Mol. Sci. 2013, 14, 7193–7230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contaldi, F.; Capuano, F.; Fulgione, A.; Cigliano, R.A.; Sanseverino, W.; Iannelli, D.; Medaglia, C.; Capparelli, R. The hypothesis that Helicobacter pylori predisposes to Alzheimer’s disease is biologically plausible. Sci. Rep. 2017, 7, 7817. [Google Scholar] [CrossRef]
- Blaser, M.J.; Kirschner, D. The equilibria that allow bacterial persistence in human hosts. Nature 2007, 449, 843–849. [Google Scholar] [CrossRef]
- Dorward, D.A.; Lucas, C.D.; Chapman, G.B.; Haslett, C.; Dhaliwal, K.; Rossi, A.G. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 2015, 185, 1172–1184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, G.; Chen, X.; Xue, X.; Guo, Q.; Liu, M.; Zhao, J. Formyl peptide receptors promotes neural differentiation in mouse neural stem cells by ROS generation and regulation of PI3KAKT signaling. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Bae, Y.S.; Song, J.Y.; Kim, Y.; He, R.; Ye, R.D.; Kwak, J.Y.; Suh, P.G.; Ryu, S.H. Differential activation of formyl peptide receptor signaling by peptide ligands. Mol. Pharmacol. 2003, 64, 841–847. [Google Scholar] [CrossRef]
- Cattaneo, F.; Russo, R.; Castaldo, M.; Chambery, A.; Zollo, C.; Esposito, G.; Pedone, P.V.; Ammendola, R. Phosphoproteomic analysis sheds light on intracellular signaling cascades triggered by Formyl-Peptide Receptor 2. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Rossi, F.W.; Prevete, N.; Montuori, N.; Ragno, P.; Selleri, C.; Marone, G.; de Paulis, A. Hp(2-20) peptide of Helicobacter py-lori and the innate immune receptors: Specific role(s) of the formyl peptide receptors. Le Infezioni Medicina 2012, 20 (Suppl. S2), 19–25. [Google Scholar]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFκB and the essentialness of NFκB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.H.; Lim, J.W.; Kim, H. Differential Role of ERK and p38 on NF-κB Activation in Helicobacter pylori-Infected Gastric Epithelial Cells. J. Cancer Prev. 2013, 18, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kasembeli, M.; Bharadwaj, U.; Robinson, P.; Tweardy, D. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.B.; Nabha, S.M.; Atanaskova, N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003, 22, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.E. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr. Opin. Gastroenterol. 2005, 21, 32–38. [Google Scholar] [PubMed]
- Hou, X.L.; Ji, C.D.; Tang, J.; Wang, Y.; Wang, Y.X.; Xiang, D.F.; Li, H.Q.; Liu, W.; Liu, W.W.; Wang, J.X.; et al. FPR2 promotes invasion and metastasis of gastric cancer cells and predicts the prognosis of patients. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, R.; Iannelli, D. Genetics of Host Protection against Helicobacter pylori Infections. Int. J. Mol. Sci. 2021, 22, 3192. [Google Scholar] [CrossRef]

| Cells/Tissues | Formyl Peptide Receptors | Role | References | |
|---|---|---|---|---|
| Innate Immune Cell Expression | Neutrophils | FPR1, FPRL1 | Chemotaxis, phagocytosis, superoxide generation | [16,21,22] |
| Natural killer cells | FPR1, FPRL1 | Interferonγ production | [16,23] | |
| Immature dendritic cells | FPR1, FPRL2 | Chemotaxis | [15,21,22] | |
| Mature dendritic cells | FPRL2 | Chemotaxis | [15,21,22] | |
| Monocytes | FPR1, FPRL1, FPRL2 | Chemotaxis, pro-inflammatory activity | [16] | |
| Macrophages | FPR1, FPRL1, FPRL2 | Chemotaxis, pro-inflammatory activity | [16] | |
| Adaptative Immune Cell Expression | Naïve CD4 T cells (CD3+, CD4+, CD45RA+, CD45RO–, CCR7+) | FPRL1 | Interferon-γ production | [16,17] |
| Th1 cells | FPRL1 | – | [16,17] | |
| Th2 | FPRL1 | – | [16,17] | |
| Th17 | FPRL1 | – | [16,17] | |
| Non-Immune Cells, Organ/Tissue Expression | Epithelial cells | FPRL1 | Chemotaxis | [18,24] |
| Endothelial cells | FPRL1 | Chemotaxis, angiogenesis, and cell proliferation | [25] | |
| Microglial cells | FPRL1, FPRL2 | Inflammation and neurogenerative activity | [18,26] | |
| Keratinocytes | FPRL1 | Cell proliferation and pro-inflammatory activity | [27] | |
| Fibroblasts | FPRL1 | Chemotaxis and innate immune response stimulation | [28] | |
| Astrocytes | FPRL1 | Inflammation and neurogenerative activity | [18,26] | |
| Hepatocytes | FPRL1 | Chemotaxis, angiogenesis | [18,29] | |
| Intestinal epithelial cells | FPR1, FPRL1 | Cell proliferation, inflammation, and tumorigenesis | [30] | |
| Brain | FPRL1 | Inflammation and neurodegenerative activity | [16,17,18] | |
| Spleen | FPRL1, FPRL2 | Innate immune response | [16,17,18] | |
| Placenta | FPRL1, FPRL2 | Innate immune response | [16,17,18] | |
| Lung | FPRL1, FPRL2 | Innate immune response | [16,17,18] | |
| Testis | FPRL1 | Innate immune response | [16,17,18] | |
| Trachea | FPRL2 | Innate immune response | [16,17,18] | |
| Lymph nodes | FPRL2 | Innate immune response | [16,17,18] |
| Classification | Ligand | Origin | Signaling | Selectivity | References |
|---|---|---|---|---|---|
| Formylated Bacterial Peptides | f-MLF | E. coli | Ca++ mobilization, superoxide generation | FPR1 | [14] |
| f-MKNFKG | Bacillus | Ca++ mobilization, superoxide generation | FPRL1 | [31] | |
| f-MGFFIS | Streptococcus | Ca++ mobilization, superoxide generation | FPR1, FPRL1 | [31] | |
| f-MAMKKL | Salmonella | Ca++ mobilization, superoxide generation | FPR1 | [31] | |
| f-MVMKFK | Haemophilus | Ca++ mobilization, superoxide generation | FPR1, FPRL1 | [31] | |
| f-MFIYYCK | Staphylococcus | Ca++ mobilization, superoxide generation | FPR1 | [31] | |
| f-MKKIML | Listeria | Ca++ mobilization, superoxide generation | FPR1, FPRL1 | [31] | |
| f-MKKNLV | Clostridium | Ca++ mobilization, superoxide generation | FPRL1 | [31] | |
| Formylated Mitochondria Peptides | f-MMYALF | Mitochondrion | Superoxide generation | FPRL1 | [32] |
| f-MLKIV | Mitochondrion | Ca++ mobilization, ERK activation | FPRL1 | [32] | |
| f-MYFINILTL | Mitochondrion | Ca++ mobilization, ERK activation | FPRL1 | [32] | |
| f-MFADRW | Mitochondrion | Ca++ mobilization, ERKs activation | FPRL1 | [32] | |
| Mitocryptide-2 | Mitochondrion | Ca++ mobilization, ERK activation | FPRL1 | [33] | |
| Microbe-Derived Non-Formylated Peptides | Hp(2-20) | Helicobacter pylori | Superoxide generation, cell proliferation, Akt and STAT3 activation, VEGFA secretion | FPRL1 | [34] |
| OC43 Coronavirus protein | OC43 Coronavirus | Unknown | Not clear | [35] | |
| 229E Coronavirus protein | 229E Coronavirus | Unknown | Not clear | [35] | |
| NL36 Coronavirus protein | NL36 Coronavirus | Unknown | Not clear | [35] | |
| spike protein | Ebola virus | Unknown | Not clear | [35] | |
| T20/DP178 | HIV gp41 | Ca++ mobilization | FPR1 | [20] | |
| T21/DP107 | HIV gp41 | Ca++ mobilization | FPR1, FPRL1 | [20] | |
| V3 peptide | HIV gp120 | Ca++ mobilization, CCR5 desensitization | FPRL1 | [36] | |
| N36 peptide | HIV gp41 | Ca++ mobilization, chemokine receptorsn desensitization, NF-kB activation | FPRL1 | [37] | |
| gG-2p20 | Herpes simplex virus | Superoxide generation, NADPH oxidase activation, apoptosis | FPR1 | [38] | |
| C5a HCV peptide | Hepatitis C virus | Ca++ mobilization, degranulation, superoxide generation, MAPK activation | FPRL1 | [39] | |
| Host-Derived Molecules | Annexin 1 | Host | ERK phosphorylation, NF-kB pathway | FPRL1 | [40] |
| Lipoxin-A4 | Host | Ca++ mobilization, ERKs, PI3K, Akt phosphorylation | FPRL1 | [41] | |
| SAA | Host | Ca++ mobilization, ERKs, JNK and p38MAPK activation, cytokine release, NF-kB and COX2 induction | FPRL1 | [42] | |
| Aβ-42 | host | PI3K/Akt pathway activation | FPRL1 | [26] | |
| LL-37 | host | Ca++ mobilization, Bcl-xL expression, caspase-3 inhibition, MAPK and JAK/STAT signaling | FPRL1 | [43] | |
| Synthetic Peptides | WKYMVm | synthetic | Ca++ mobilization, NADPH oxidase activation, ERK phosphorylation, MAPK and JNK activation, PKC activation | FPRL1 | [44] |
| Synthetic Molecules | Quinazolinones | Synthetic | Ca++ mobilization, ERK activation | FPRL1 | [45] |
| Benzimidazoles | Synthetic | Ca++ mobilization | FPR1 | [45] | |
| Pyrazolones | Synthetic | Ca++ mobilization, desensitization of chemokine receptors | FPRL1 | [45] | |
| Pyridazin-3(2H)-ones | Synthetic | Ca++ mobilization | FPR1 | [45] | |
| Chiral pyridazines | Synthetic | Ca++ mobilization | FPR1, FPRL1 | [45] | |
| N-phenylureas | Synthetic | Ca++ mobilization | FPRL1 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuomo, P.; Papaianni, M.; Capparelli, R.; Medaglia, C. The Role of Formyl Peptide Receptors in Permanent and Low-Grade Inflammation: Helicobacter pylori Infection as a Model. Int. J. Mol. Sci. 2021, 22, 3706. https://doi.org/10.3390/ijms22073706
Cuomo P, Papaianni M, Capparelli R, Medaglia C. The Role of Formyl Peptide Receptors in Permanent and Low-Grade Inflammation: Helicobacter pylori Infection as a Model. International Journal of Molecular Sciences. 2021; 22(7):3706. https://doi.org/10.3390/ijms22073706
Chicago/Turabian StyleCuomo, Paola, Marina Papaianni, Rosanna Capparelli, and Chiara Medaglia. 2021. "The Role of Formyl Peptide Receptors in Permanent and Low-Grade Inflammation: Helicobacter pylori Infection as a Model" International Journal of Molecular Sciences 22, no. 7: 3706. https://doi.org/10.3390/ijms22073706






