Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment
Abstract
1. Introduction
1.1. Bipolar Disorder
1.2. Composition and Development of the Gut Microbiota
1.3. Function of Gut Microbiota
1.4. Dysfunctions of Gut Microbiota
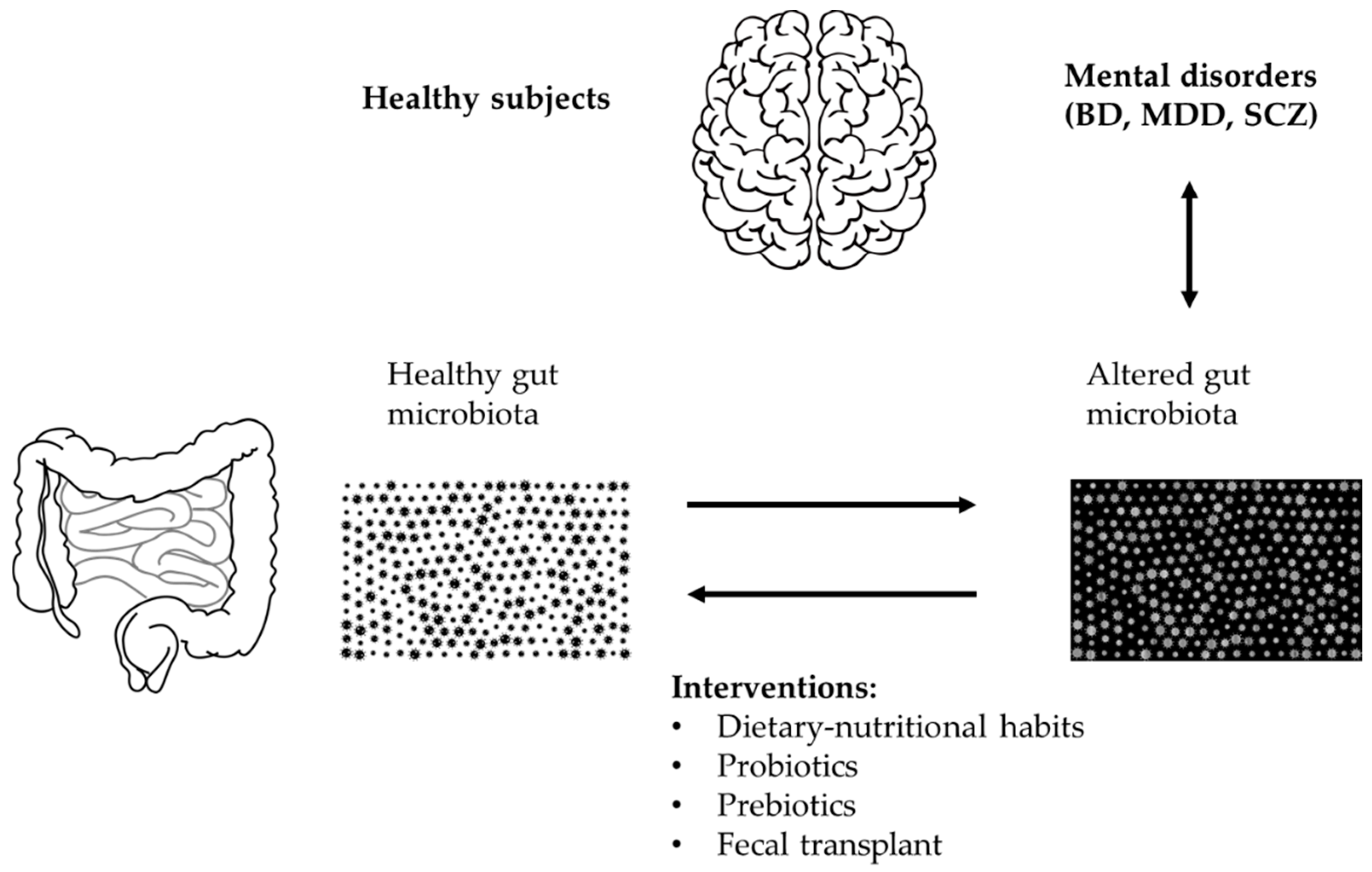
1.5. Aims
2. Microbiota and Bipolar Disorder
2.1. The Pathogenetic Role of the Gut Microbiota in Bipolar Disorder
2.2. Composition of Gut Microbiota and Bipolar Disorder
2.3. Composition of Gut Microbiota and Mania
2.4. Composition of Gut Microbiota and Bipolar Depression
3. Psychotropic Drugs and Alterations of Microbiota Composition
4. Therapeutic Potential of Microbiota Composition Modification in Bipolar Disorder
4.1. Dietary-Nutritional Habits
4.2. Probiotics
4.3. Prebiotics
4.4. Faecal Transplant
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar Disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Angst, J.; Azorin, J.M.; Bowden, C.L.; Perugi, G.; Vieta, E.; Gamma, A.; Young, A.H. Prevalence and Characteristics of Undiagnosed Bipolar Disorders in Patients with a Major Depressive Episode: The BRIDGE Study. Arch. Gen. Psychiatry 2011, 68, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Craddock, N.; Sklar, P. Bipolar Disorder 1—Genetics of Bipolar Disorder. Lancet 2013, 381, 1654–1662. [Google Scholar] [CrossRef]
- Jukic, M.M.; Carrillo-Roa, T.; Bar, M.; Becker, G.; Jovanovic, V.M.; Zega, K.; Binder, E.B.; Brodski, C. Abnormal Development of Monoaminergic Neurons Is Implicated in Mood Fluctuations and Bipolar Disorder. Neuropsychopharmacology 2015, 40, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Fries, G.R.; Kunz, M.; Kapczinski, F. The Role of BDNF as a Mediator of Neuroplasticity in Bipolar Disorder. Psychiatry Investig. 2010, 7, 243–250. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The Interplay between the Intestinal Microbiota and the Brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, J.; Deng, S.; Tan, Y.; Qiu, C.; Zhang, M.; Ni, X.; Lu, H.; Wang, Z.; Li, L.; et al. Bibliometric and Visual Analysis of Research on the Links Between the Gut Microbiota and Depression From 1999 to 2019. Front. Psychiatry 2020, 11, 587670. [Google Scholar] [CrossRef] [PubMed]
- Tralau, T.; Sowada, J.; Luch, A. Insights on the Human Microbiome and Its Xenobiotic Metabolism: What Is Known about Its Effects on Human Physiology? Expert Opin. Drug Metab. Toxicol. 2015, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genomics Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lankelma, J.M.; Nieuwdorp, M.; de Vos, W.M.; Wiersinga, W.J. The Gut Microbiota in Internal Medicine: Implications for Health and Disease. Neth. J. Med. 2015, 73, 61–68. [Google Scholar] [PubMed]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The Impact of Gut Microbiota on Brain and Behaviour: Implications for Psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the Infant Microbiome Community Structure and Function across Multiple Body Sites and in Relation to Mode of Delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Koleva, P.T.; Kim, J.S.; Scott, J.A.; Kozyrskyj, A.L. Microbial Programming of Health and Disease Starts during Fetal Life. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 265–277. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-Related Changes in Gut Microbiota Composition from Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.H.; Gadkari, M.; Zhou, Q.; Yu, S.; Gao, N.; Guan, Y.; Schady, D.; Roshan, T.N.; Chen, M.H.; Laritsky, E.; et al. Postnatal Epigenetic Regulation of Intestinal Stem Cells Requires DNA Methylation and Is Guided by the Microbiome. Genome Biol. 2015, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Dill-McFarland, K.A.; Tang, Z.Z.; Kemis, J.H.; Kerby, R.L.; Chen, G.; Palloni, A.; Sorenson, T.; Rey, F.E.; Herd, P. Close Social Relationships Correlate with Human Gut Microbiota Composition. Sci. Rep. 2019, 9, 703. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-Related Psychiatric Disorders. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and Mental Health: A Review. Brain. Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Ekström, L.D.; Ekström, H.; Dal, H.; Kosidou, K.; Gustafsson, U.O. Childhood Appendectomy and Adult Mental Disorders: A Population-Based Cohort Study. Depress. Anxiety 2020, 37, 1108–1117. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut Microbiota in Autism and Mood Disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey-Neufeld, K.A. Gut-Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From Gut Dysbiosis to Altered Brain Function and Mental Illness: Mechanisms and Pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, R.; Stone, T.W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016, 41, 2819–2835. [Google Scholar] [CrossRef]
- Vinberg, M.; Ottesen, N.M.; Meluken, I.; Sørensen, N.; Pedersen, O.; Kessing, L.V.; Miskowiak, K.W. Remitted Affective Disorders and High Familial Risk of Affective Disorders Associate with Aberrant Intestinal Microbiota. Acta Psychiatr. Scand. 2019, 139, 174–184. [Google Scholar] [CrossRef]
- Naaldijk, Y.M.; Bittencourt, M.C.; Sack, U.; Ulrich, H. Kinins and Microglial Responses in Bipolar Disorder: A Neuroinflammation Hypothesis. Biol. Chem. 2016, 397, 283–296. [Google Scholar] [CrossRef]
- Sayana, P.; Colpo, G.D.; Simões, L.R.; Giridharan, V.V.; Teixeira, A.L.; Quevedo, J.; Barichello, T. A Systemic Review of Evidence for the Role of Inflammatory Biomarkers in Bipolar Patients. J. Psychiatr. Res. 2017, 92, 160–182. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut Microbiota–Brain Axis in Depression: The Role of Neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Kelly, J.R.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbiota Axis: Challenges for Translation in Psychiatry. Ann. Epidemiol. 2016, 26, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanssen, T.F.S.; Cowan, C.S.M.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making Sense of … the Microbiome in Psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52. [Google Scholar] [CrossRef]
- Yolken, R.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; Dickerson, F. Individuals Hospitalized with Acute Mania Have Increased Exposure to Antimicrobial Medications. Bipolar Disord. 2016, 18, 404–409. [Google Scholar] [CrossRef]
- Dickerson, F.; Severance, E.; Yolken, R. The Microbiome, Immunity, and Schizophrenia and Bipolar Disorder. Brain. Behav. Immun. 2017, 62, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Gressitt, K.L.; Yang, S.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Alaedini, A.; Dickerson, F.B.; Yolken, R.H. Seroreactive Marker for Inflammatory Bowel Disease and Associations with Antibodies to Dietary Proteins in Bipolar Disorder. Bipolar Disord. 2014, 16, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Boukouaci, W.; Chevalier, G.; Regnault, A.; Eberl, G.; Hamdani, N.; Dickerson, F.; Macgregor, A.; Boyer, L.; Dargel, A.; et al. The “Psychomicrobiotic”: Targeting Microbiota in Major Psychiatric Disorders: A Systematic Review. Pathol. Biol. 2015, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Weinhard, L.; Di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia Remodel Synapses by Presynaptic Trogocytosis and Spine Head Filopodia Induction. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Strakowski, S.M.; Adler, C.M.; Almeida, J.; Altshuler, L.L.; Blumberg, H.P.; Chang, K.D.; Delbello, M.P.; Frangou, S.; McIntosh, A.; Phillips, M.L.; et al. The Functional Neuroanatomy of Bipolar Disorder: A Consensus Model. Bipolar Disord. 2012, 14, 313–325. [Google Scholar] [CrossRef]
- Erny, D.; De Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P. Gut Microbiota: A Potential Regulator of Neurodevelopment. Front. Cell. Neurosci. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Eltokhi, A.; Janmaat, I.E.; Genedi, M.; Haarman, B.C.M.; Sommer, I.E.C. Dysregulation of Synaptic Pruning as a Possible Link between Intestinal Microbiota Dysbiosis and Neuropsychiatric Disorders. J. Neurosci. Res. 2020, 98, 1335–1369. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The Gut Microbiome Composition Associates with Bipolar Disorder and Illness Severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A Step Ahead: Exploring the Gut Microbiota in Inpatients with Bipolar Disorder during a Depressive Episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L.; Salonen, A.; Kekkonen, R.A.; Salojärvi, J.; Jalanka-Tuovinen, J.; Palva, A.; Orešič, M.; de Vos, W.M. Associations between the Human Intestinal Microbiota, Lactobacillus Rhamnosus GG and Serum Lipids Indicated by Integrated Analysis of High-Throughput Profiling Data. PeerJ 2013, 2013, 1–25. [Google Scholar] [CrossRef]
- Thompson, W.K.; Kupfer, D.J.; Fagiolini, A.; Scott, J.A.; Frank, E. Prevalence and Clinical Correlates of Medical Comorbidities in Patients with Bipolar I Disorder: Analysis of Acute-Phase Data from a Randomized Controlled Trial. J. Clin. Psychiatry 2006, 67, 783–788. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.P.; Bedora-Faure, M.; K’ouas, G.; Alauzet, C.; Mory, F. Proposal to Unify Clostridium Orbiscindens Winter et al. 1991 and Eubacterium plautii (Séguin 1928) Hofstad and Aasjord 1982, with Description of Flavonifractor plautii Gen. Nov., Comb. Nov., and Reassignment of Bacteroides capillosus to Pseudoflavonifrac. Int. J. Syst. Evol. Microbiol. 2010, 60, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.C.; Andreazza, A.C.; Young, L.T. An Updated Meta-Analysis of Oxidative Stress Markers in Bipolar Disorder. Psychiatry Res. 2014, 218, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mané, A.; Fernández-Expósito, M.; Bergé, D.; Gómez-Pérez, L.; Sabaté, A.; Toll, A.; Diaz, L.; Diez-Aja, C.; Perez, V. Relationship between Cannabis and Psychosis: Reasons for Use and Associated Clinical Variables. Psychiatry Res. 2015, 229, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Coello, K.; Hansen, T.H.; Sørensen, N.; Munkholm, K.; Kessing, L.V.; Pedersen, O.; Vinberg, M. Gut Microbiota Composition in Patients with Newly Diagnosed Bipolar Disorder and Their Unaffected First-Degree Relatives. Brain. Behav. Immun. 2019, 75, 112–118. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Subramaniapillai, M.; Shekotikhina, M.; Carmona, N.E.; Lee, Y.; Mansur, R.B.; Brietzke, E.; Fus, D.; Coles, A.S.; Iacobucci, M.; et al. Characterizing the Gut Microbiota in Adults with Bipolar Disorder: A Pilot Study. Nutr. Neurosci. 2021, 24, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, F.; Işık, Ü.; Demirdaş, A.; Doğuç, D.K.; Bozkurt, M. Serum Zonulin and Claudin-5 Levels in Patients with Bipolar Disorder. J. Affect. Disord. 2020, 266, 37–42. [Google Scholar] [CrossRef]
- Rudzki, L.; Szulc, A. “Immune Gate” of Psychopathology-The Role of Gut Derived Immune Activation in Major Psychiatric Disorders. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Stallings, C.; Origoni, A.; Vaughan, C.; Khushalani, S.; Alaedini, A.; Yolken, R. Markers of Gluten Sensitivity and Celiac Disease in Bipolar Disorder. Bipolar Disord. 2011, 13, 52–58. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.; Origoni, A.; Vaughan, C.; Khushalani, S.; Yolken, R. Markers of Gluten Sensitivity in Acute Mania: A Longitudinal Study. Psychiatry Res. 2012, 196, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, A.; Huang, T.; Lai, J.; Li, J.; Sublette, M.E.; Lu, H.; Lu, Q.; Du, Y.; Hu, Z.; et al. Gut Microbiota Changes in Patients with Bipolar Depression. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Koga, N.; Hattori, K.; Ota, M.; Kunugi, H. Bifidobacterium and Lactobacillus Counts in the Gut Microbiota of Patients with Bipolar Disorder and Healthy Controls. Front. Psychiatry 2019, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Xie, X.H.; Zhao, J.; Lai, W.T.; Wang, M.B.; Xu, D.; Liu, Y.H.; Guo, Y.Y.; Xu, S.X.; Deng, W.F.; et al. Similarly in Depression, Nuances of Gut Microbiota: Evidences from a Shotgun Metagenomics Sequencing Study on Major Depressive Disorder versus Bipolar Disorder with Current Major Depressive Episode Patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, D.; Stoyanov, D.; Leunis, J.-C.; Carvalho, A.F.; Kubera, M.; Murdjeva, M.; Maes, M. Increased Serum Immunoglobulin Responses to Gut Commensal Gram-Negative Bacteria in Unipolar Major Depression and Bipolar Disorder Type 1, Especially When Melancholia Is Present. Neurotox. Res. 2020, 37, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Yang, J.; Li, Y.; Wu, J.; Liang, W.; Yin, B.; Tan, X.; Huang, Y.; Chai, T.; Zhang, H.; et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bengesser, S.A.; Mörkl, S.; Painold, A.; Dalkner, N.; Birner, A.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Hamm, C.; Maget, A.; et al. Epigenetics of the Molecular Clock and Bacterial Diversity in Bipolar Disorder. Psychoneuroendocrinology 2019, 101, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Grunze, H.; Vieta, E.; Young, A.; Yatham, L.; Blier, P.; Kasper, S.; Moeller, H.J. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: The Clinical Guidelines. Int. J. Neuropsychopharmacol. 2017, 20, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 Guidelines for the Management of Patients with Bipolar Disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Cussotto, S.; Strain, C.R.; Fouhy, F.; Strain, R.G.; Peterson, V.L.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Differential Effects of Psychotropic Drugs on Microbiome Composition and Gastrointestinal Function. Psychopharmacology 2019, 236, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Horton-Sparks, K.; Hull, V.; Li, R.W.; Martínez-Cerdeño, V. The Valproic Acid Rat Model of Autism Presents with Gut Bacterial Dysbiosis Similar to That in Human Autism. Mol. Autism 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; Davis, J.H.; Mangat, C.S.; Williamson, J.R.; Brown, E.D. Discovery of a Small Molecule That Inhibits Bacterial Ribosome Biogenesis. Elife 2014, 3, e03574. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Ward, K.M.; Clark, C.T. The Gut Microbiome in Bipolar Disorder and Pharmacotherapy Management. Neuropsychobiology 2020, 79, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T.; Dempsey, J.L.; Mimche, S.M.; Lamb, T.J.; Kulkarni, S.; Cui, J.Y.; Jeong, H.; Slitt, A.L. Physiological Regulation of Drug Metabolism and Transport: Pregnancy, Microbiome, Inflammation, Infection, and Fasting. Drug Metab. Dispos. 2018, 46, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Davey, K.J.; O’Mahony, S.M.; Schellekens, H.; O’Sullivan, O.; Bienenstock, J.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gender-Dependent Consequences of Chronic Olanzapine in the Rat: Effects on Body Weight, Inflammatory, Metabolic and Microbiota Parameters. Psychopharmacology 2012, 221, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Davey, K.J.; Cotter, P.D.; O’Sullivan, O.; Crispie, F.; Dinan, T.G.; Cryan, J.F.; O’Mahony, S.M. Antipsychotics and the Gut Microbiome: Olanzapine-Induced Metabolic Dysfunction Is Attenuated by Antibiotic Administration in the Rat. Transl. Psychiatry 2013, 3, e309. [Google Scholar] [CrossRef] [PubMed]
- Bahr, S.M.; Tyler, B.C.; Wooldridge, N.; Butcher, B.D.; Burns, T.L.; Teesch, L.M.; Oltman, C.L.; Azcarate-Peril, M.A.; Kirby, J.R.; Calarge, C.A. Use of the Second-Generation Antipsychotic, Risperidone, and Secondary Weight Gain Are Associated with an Altered Gut Microbiota in Children. Transl. Psychiatry 2015, 5, e652-6. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Evans, S.J.; Ward, K.M.; McInnis, M.G.; Ellingrod, V.L. Interaction Between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy 2017, 37, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Baxter, N.T.; Ward, K.M.; Kraal, A.Z.; McInnis, M.G.; Schmidt, T.M.; Ellingrod, V.L. Effects of Atypical Antipsychotic Treatment and Resistant Starch Supplementation on Gut Microbiome Composition in a Cohort of Patients with Bipolar Disorder or Schizophrenia. Pharmacotherapy 2019, 39, 161–170. [Google Scholar] [CrossRef]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the Development of the Human Intestinal Microbiome. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional Medicine as Mainstream in Psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The Gut Microbiome and Diet in Psychiatry: Focus on Depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, Neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Wetzlmair, L.C.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Pilz, R.; Hamm, C.; Maget, A.; Koidl, C.; et al. The Impact of Probiotic Supplements on Cognitive Parameters in Euthymic Individuals with Bipolar Disorder: A Pilot Study. Neuropsychobiology 2020, 79, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sheil, B.; Shanahan, F.; O’Mahony, L. Probiotic Effects on Inflammatory Bowel Disease. J. Nutr. 2007, 137, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, J.E.; Walker, W.A. Nutritional Impact of Pre- and Probiotics as Protective Gastrointestinal Organisms. Annu. Rev. Nutr. 2002, 22, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 Improves Behavioral, Cognitive, and Biochemical Aberrations Caused by Chronic Restraint Stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Lyte, M.; Meyer, E.; Cryan, J.F. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience. Int. J. Neuropsychopharmacol. 2016, 19, pyv114. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; Goga, J.; et al. Adjunctive Probiotic Microorganisms to Prevent Rehospitalization in Patients with Acute Mania: A Randomized Controlled Trial. Bipolar Disord. 2018, 20, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of Consuming a Milk Drink Containing a Probiotic on Mood and Cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Healthy Human Volunteers. Gut Microbes 2011, 2, 37–41. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.E.; Tzortzis, G.; Burnet, P.W.J. Prebiotic Feeding Elevates Central Brain Derived Neurotrophic Factor, N-Methyl-d-Aspartate Receptor Subunits and d-Serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Mörkl, S.; Sandhu, K.V.; Cryan, J.F.; Dinan, T.G. The Gut Microbiome and Mental Health: What Should We Tell Our Patients?: Le Microbiote Intestinal et La Santé Mentale: Que Devrions-Nous Dire à Nos Patients? Can. J. Psychiatry 2019, 64, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Kahn, S.; Kashyap, P.; Laine, L.; Rubin, D.; Atreja, A.; Moore, T.; Wu, G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015, 149, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.C.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The Effect of Fecal Microbiota Transplantation on Psychiatric Symptoms among Patients with Irritable Bowel Syndrome, Functional Diarrhea and Functional Constipation: An Open-Label Observational Study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef]
- Hinton, R. A Case Report Looking at the Effects of Faecal Microbiota Transplantation in a Patient with Bipolar Disorder. Aust. N. Z. J. Psychiatry 2020, 54, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lai, J.; Lu, H.; Ng, C.; Huang, T.; Zhang, H.; Ding, K.; Wang, Z.; Jiang, J.; Hu, J.; et al. Gut Microbiota in Bipolar Depression and Its Relationship to Brain Function: An Advanced Exploration. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, E.Z.; Wetzlmair, L.C.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Pilz, R.; Hamm, C.; Maget, A.; Rieger, A.; et al. Probiotic Treatment in Individuals with Euthymic Bipolar Disorder: A Pilot-Study on Clinical Changes and Compliance. Neuropsychobiology 2020, 79, 71–79. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucidi, L.; Pettorruso, M.; Vellante, F.; Di Carlo, F.; Ceci, F.; Santovito, M.C.; Di Muzio, I.; Fornaro, M.; Ventriglio, A.; Tomasetti, C.; et al. Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment. Int. J. Mol. Sci. 2021, 22, 3723. https://doi.org/10.3390/ijms22073723
Lucidi L, Pettorruso M, Vellante F, Di Carlo F, Ceci F, Santovito MC, Di Muzio I, Fornaro M, Ventriglio A, Tomasetti C, et al. Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment. International Journal of Molecular Sciences. 2021; 22(7):3723. https://doi.org/10.3390/ijms22073723
Chicago/Turabian StyleLucidi, Lorenza, Mauro Pettorruso, Federica Vellante, Francesco Di Carlo, Franca Ceci, Maria Chiara Santovito, Ilenia Di Muzio, Michele Fornaro, Antonio Ventriglio, Carmine Tomasetti, and et al. 2021. "Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment" International Journal of Molecular Sciences 22, no. 7: 3723. https://doi.org/10.3390/ijms22073723
APA StyleLucidi, L., Pettorruso, M., Vellante, F., Di Carlo, F., Ceci, F., Santovito, M. C., Di Muzio, I., Fornaro, M., Ventriglio, A., Tomasetti, C., Valchera, A., Gentile, A., Kim, Y.-K., Martinotti, G., Fraticelli, S., Di Giannantonio, M., & De Berardis, D. (2021). Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment. International Journal of Molecular Sciences, 22(7), 3723. https://doi.org/10.3390/ijms22073723












