Synaptic Reshaping and Neuronal Outcomes in the Temporal Lobe Epilepsy
Abstract
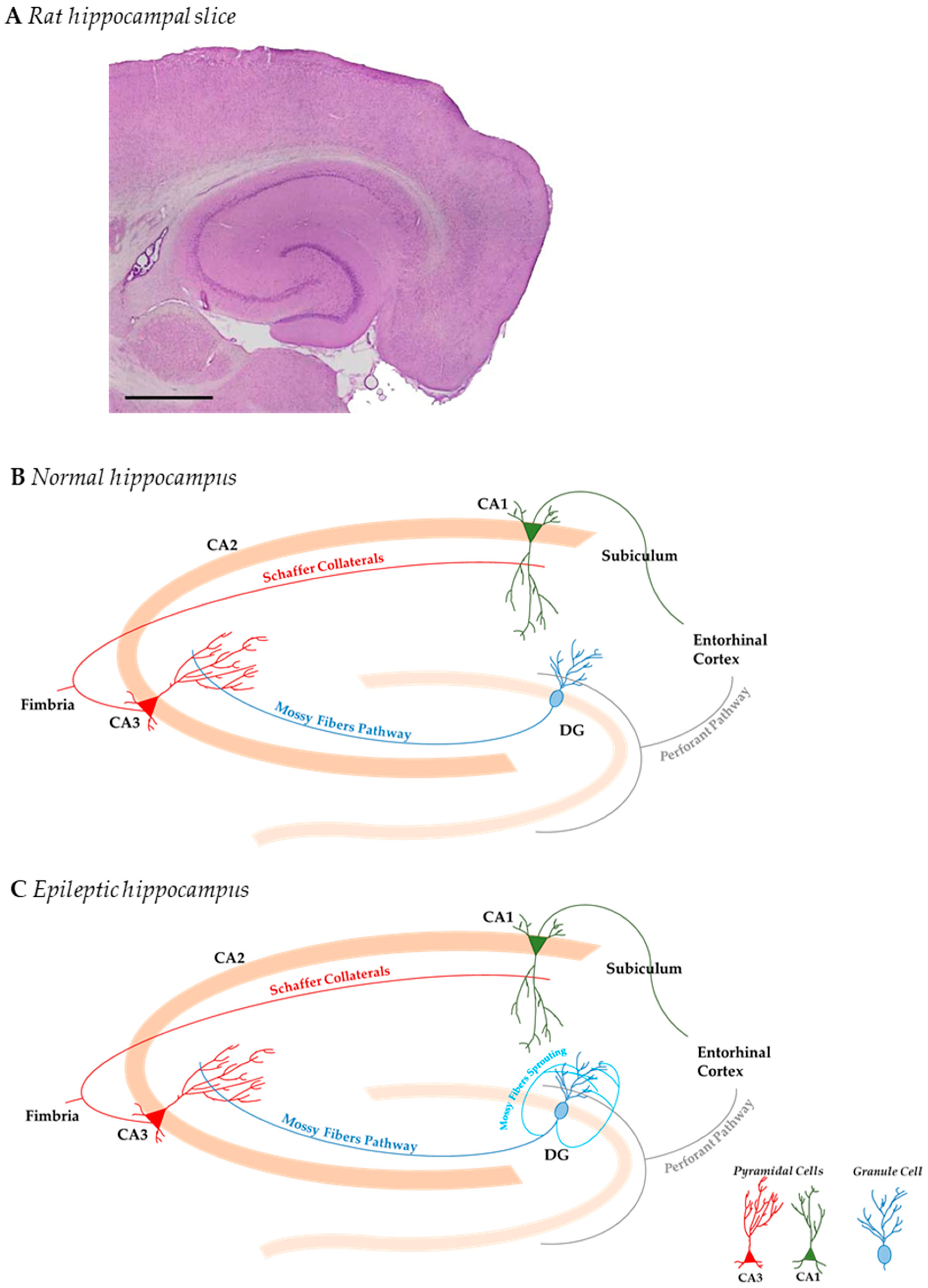
1. Introduction
2. Chemical Synapses Features in Temporal Lobe Epilepsy
2.1. Glutamatergic Excitatory Synapses
2.1.1. AMPA Receptors
2.1.2. NMDA Receptors
2.1.3. Kainic Receptors
2.1.4. Glutamatergic Metabotropic Receptors
2.2. GABAergic Inhibitory Synapses
2.2.1. GABAA Receptors
2.2.2. GABAB Receptors
3. Electrical Synapses Features in Temporal Lobe Epilepsy
3.1. Astrocytic Gap Junctions
3.2. Neuronal Gap Junctions
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AED | Antiepileptic drug |
| AMPA | α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid |
| CA | Cornu Ammonis |
| CNS | Central nervous system |
| CNTNAP4 | Protein contactin-associated protein-like 4 |
| Cx | Connexin |
| GABA | γ-Aminobutyric acid |
| GAT | GABA transporter |
| KA | Kainic acid |
| LTP | Long term potentiation |
| MAPK | Mitogen-activated protein kinase |
| mGluR | Metabotropic glutamate receptor |
| miRNA | Micro RNA |
| MRI | Magnetic resonance imaging |
| mTLE | Mesial temporal lobe epilepsy |
| NMDA | N-methyl-D-aspartate |
| PET | Positron emission tomography |
| SE | Status epilepticus |
| SRS | Spontaneous recurrent seizure |
| TLE | Temporal lobe epilepsy |
References
- Patel, D.C.; Tewari, B.P.; Chaunsali, L.; Sontheimer, H. Neuron–glia Interactions in the Pathophysiology of Epilepsy. Nat. Rev. Neurosci. 2019, 20, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Liu, S.; Qin, H.; Li, B.; Zhang, X. Drug-Resistant Epilepsy and Surgery. Curr. Neuropharmacol. 2018, 16, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Stewart, G.R.; Abrams, D.J.; Sharan, A. Prevalence and Incidence of Drug-Resistant Mesial Temporal Lobe Epilepsy in the United States. World Neurosurg. 2017, 99, 662–666. [Google Scholar] [CrossRef]
- D’Alessio, L.; Konopka, H.; Solís, P.; Scévola, L.; Lima, M.F.; Nuñez, C.; Seoane, E.; Oddo, S.; Kochen, S. Depression and Temporal Lobe Epilepsy: Expression Pattern of Calbindin Immunoreactivity in Hippocampal Dentate Gyrus of Patients Who Underwent Epilepsy Surgery with and without Comorbid Depression. Behav. Neurol. 2019, 2019, 7396793. [Google Scholar] [CrossRef]
- Mikulecká, A.; Druga, R.; Stuchlík, A.; Mareš, P.; Kubová, H. Comorbidities of Early-Onset Temporal Epilepsy: Cognitive, Social, Emotional, and Morphologic Dimensions. Exp. Neurol. 2019, 320, 113005. [Google Scholar] [CrossRef] [PubMed]
- Postma, T.S.; Cury, C.; Baxendale, S.; Thompson, P.J.; Cano-López, I.; de Tisi, J.; Burdett, J.L.; Sidhu, M.K.; Caciagli, L.; Winston, G.P.; et al. Hippocampal Shape Is Associated with Memory Deficits in Temporal Lobe Epilepsy. Ann. Neurol. 2020, 88, 170–182. [Google Scholar] [CrossRef]
- Capizzano, A.A.; Kawasaki, H.; Sainju, R.K.; Kirby, P.; Kim, J.; Moritani, T. Amygdala Enlargement in Mesial Temporal Lobe Epilepsy: An Alternative Imaging Presentation of Limbic Epilepsy. Neuroradiology 2019, 61, 119–127. [Google Scholar] [CrossRef]
- Deshpande, T.; Li, T.; Herde, M.K.; Becker, A.; Vatter, H.; Schwarz, M.K.; Henneberger, C.; Steinhäuser, C.; Bedner, P. Subcellular Reorganization and Altered Phosphorylation of the Astrocytic Gap Junction Protein connexin43 in Human and Experimental Temporal Lobe Epilepsy. Glia 2017, 65, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, N.; Bernasconi, A.; Caramanos, Z.; Antel, S.B.; Andermann, F.; Arnold, D.L. Mesial Temporal Damage in Temporal Lobe Epilepsy: A Volumetric MRI Study of the Hippocampus, Amygdala and Parahippocampal Region. Brain 2003, 126, 462–469. [Google Scholar] [CrossRef]
- Jber, M.; Habibabadi, J.M.; Sharifpour, R.; Marzbani, H.; Hassanpour, M.; Seyfi, M.; Mobarakeh, N.M.; Keihani, A.; Hashemi-Fesharaki, S.S.; Ay, M.; et al. Temporal and Extratemporal Atrophic Manifestation of Temporal Lobe Epilepsy Using Voxel-Based Morphometry and Corticometry: Clinical Application in Lateralization of Epileptogenic Zone. Neurol. Sci. 2021, 1–21. [Google Scholar] [CrossRef]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Bartolomei, F.; Bernasconi, A.; Bernasconi, N.; Bien, C.G.; Cendes, F.; Coras, R.; et al. International Consensus Classification of Hippocampal Sclerosis in Temporal Lobe Epilepsy: A Task Force Report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013, 54. [Google Scholar] [CrossRef] [PubMed]
- Wyler, A.; Curtis Dohan, F.; Schweitzer, J.P.; Berry, A.D., III. A Grading System for Mesial Temporal Pathology (Hippocampal Sclerosis) from Anterior Temporal Lobectomy. J. Epilepsy 1992, 5, 220–225. [Google Scholar] [CrossRef]
- Curia, G.; Levitt, M.; Fender, J.S.; Miller, J.W.; Ojemann, J.; D’Ambrosio, R. Impact of Injury Location and Severity on Posttraumatic Epilepsy in the Rat: Role of Frontal Neocortex. Cereb. Cortex 2011, 21, 1574–1592. [Google Scholar] [CrossRef]
- Alhourani, A.; Fish, K.N.; Wozny, T.A.; Sudhakar, V.; Hamilton, R.L.; Richardson, R.M. GABA Bouton Subpopulations in the Human Dentate Gyrus Are Differentially Altered in Mesial Temporal Lobe Epilepsy. J. Neurophysiol 2020, 123, 392–406. [Google Scholar] [CrossRef]
- Bertoglio, D.; Amhaoul, H.; Van Eetveldt, A.; Houbrechts, R.; Van De Vijver, S.; Ali, I.; Dedeurwaerdere, S. Kainic Acid-Induced Post-Status Epilepticus Models of Temporal Lobe Epilepsy with Diverging Seizure Phenotype and Neuropathology. Front. Neurol. 2017, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Witter, M.P. The Three-Dimensional Organization of the Hippocampal Formation: A Review of Anatomical Data. Neuroscience 1989, 31. [Google Scholar] [CrossRef]
- Jones, M.W.; McHugh, T.J. Updating Hippocampal Representations: CA2 Joins the Circuit. Trends Neurosci. 2011, 34, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Krook-Magnuson, E.; Armstrong, C.; Bui, A.; Lew, S.; Oijala, M.; Soltesz, I. In Vivo Evaluation of the Dentate Gate Theory in Epilepsy. J. Physiol. 2015, 593, 2379–2388. [Google Scholar] [CrossRef]
- Vismer, M.S.; Forcelli, P.A.; Skopin, M.D.; Gale, K.; Koubeissi, M.Z. The Piriform, Perirhinal, and Entorhinal Cortex in Seizure Generation. Front. Neural Circuits 2015, 9. [Google Scholar] [CrossRef]
- Janz, P.; Savanthrapadian, S.; Häussler, U.; Kilias, A.; Nestel, S.; Kretz, O.; Kirsch, M.; Bartos, M.; Egert, U.; Haas, C.A. Synaptic Remodeling of Entorhinal Input Contributes to an Aberrant Hippocampal Network in Temporal Lobe Epilepsy. Cereb. Cortex 2017, 27, 2348–2364. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.-Q.; Jia, J.-N.; Liu, Z.-Q.; Zhou, H.-H.; Mao, X.-Y. Targeting Gap Junction in Epilepsy: Perspectives and Challenges. Biomed. Pharmacother. 2019, 109, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Alonso Vanegas, M.A.; Lew, S.M.; Morino, M.; Sarmento, S.A. Microsurgical Techniques in Temporal Lobe Epilepsy. Epilepsia 2017, 58, 10–18. [Google Scholar] [CrossRef]
- Loddenkemper, T.; Talos, D.M.; Cleary, R.; Joseph, A.; Sánchez Fernández, I.; Alexopoulos, A.; Kotagal, P.; Najm, I.; Jensen, F.E. Subunit Composition of Glutamate and Gamma-Aminobutyric Acid Receptors in Status epilepticus. Epilepsy Res. 2014, 108, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Huberfeld, G.; Blauwblomme, T.; Miles, R. Hippocampus and Epilepsy: Findings from Human Tissues. Rev. Neurol. Paris 2015, 171, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; DuBois, J.M.; Rowley, J.; González-Otárula, K.A.; Soucy, J.-P.; Massarweh, G.; Hall, J.A.; Guiot, M.-C.; Rosa-Neto, P.; Kobayashi, E. In Vivo Metabotropic Glutamate Receptor Type 5 Abnormalities Localize the Epileptogenic Zone in Mesial Temporal Lobe Epilepsy. Ann. Neurol. 2019, 85, 218–228. [Google Scholar] [CrossRef]
- McGinnity, C.J.; Koepp, M.J.; Hammers, A.; Riaño Barros, D.A.; Pressler, R.M.; Luthra, S.; Jones, P.A.; Trigg, W.; Micallef, C.; Symms, M.R.; et al. NMDA Receptor Binding in Focal Epilepsies. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1150–1157. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakajima, W.; Hatano, M.; Shibata, Y.; Kuroki, Y.; Arisawa, T.; Serizawa, A.; Sano, A.; Kogami, S.; Yamanoue, T.; et al. Visualization of AMPA Receptors in Living Human Brain with Positron Emission Tomography. Nat. Med. 2020, 26, 281–288. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.G.; Avoli, M. The Pilocarpine Model of Temporal Lobe Epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Avoli, M. The Kainic Acid Model of Temporal Lobe Epilepsy. Neurosci. Biobehav. Rev. 2013, 37, 2887–2899. [Google Scholar] [CrossRef]
- Song, H.; Tufa, U.; Chow, J.; Sivanenthiran, N.; Cheng, C.; Lim, S.; Wu, C.; Feng, J.; Eubanks, J.H.; Zhang, L. Effects of Antiepileptic Drugs on Spontaneous Recurrent Seizures in a Novel Model of Extended Hippocampal Kindling in Mice. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Juvale, I.I.; Che Has, A.T. The Evolution of the Pilocarpine Animal Model of Status epilepticus. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Turski, W.A.; Cavalheiro, E.A.; Schwarz, M.; Czuczwar, S.J.; Kleinrok, Z.; Turski, L. Limbic Seizures Produced by Pilocarpine in Rats: Behavioural, Electroencephalographic and Neuropathological Study. Behav. Brain Res. 1983, 9. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Limbic Seizure and Brain Damage Produced by Kainic Acid: Mechanisms and Relevance to Human Temporal Lobe Epilepsy. Neuroscience 1985, 14, 375–403. [Google Scholar] [CrossRef]
- Goddard, G.V.; McIntyre, D.C.; Leech, C.K. A Permanent Change in Brain Function Resulting from Daily Electrical Stimulation. Exp. Neurol. 1969, 25, 295–330. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Liang, S.; Shaw, F. Rapid Amygdala Kindling Causes Motor Seizure and Comorbidity of Anxiety- and Depression-like Behaviors in Rats. Front. Behav. Neurosci. 2016, 10, 129. [Google Scholar] [CrossRef]
- Rocha, L.; Alonso-Vanegas, M.; Martínez-Juárez, I.E.; Orozco-Suárez, S.; Escalante-Santiago, D.; Feria-Romero, I.A.; Zavala-Tecuapetla, C.; Cisneros-Franco, J.M.; Buentello-García, R.M.; Cienfuegos, J. GABAergic Alterations in Neocortex of Patients with Pharmacoresistant Temporal Lobe Epilepsy Can Explain the Comorbidity of Anxiety and Depression: The Potential Impact of Clinical Factors. Front. Cell. Neurosci. 2015, 8, 442. [Google Scholar] [CrossRef]
- Bonansco, C.; Fuenzalida, M. Plasticity of Hippocampal Excitatory-Inhibitory Balance: Missing the Synaptic Control in the Epileptic Brain. Neural Plast. 2016, 2016, 8607038. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Frattaroli, D.; Venturini, A.; Passalacqua, M.; Nobile, M.; Alloisio, S.; Tacchetti, C.; Maura, G.; Agnati, L.F.; Marcoli, M. Calcium-Permeable AMPA Receptors Trigger Vescicular Glutamate Release from Bergmann Gliosomes. Neuropharmacology 2015, 99, 396–407. [Google Scholar] [CrossRef]
- Pirttimaki, T.M.; Sims, R.E.; Saunders, G.; Antonio, S.A.; Codadu, N.K.; Parri, H.R. Astrocyte-Mediated Neuronal Synchronization Properties Revealed by False Gliotransmitter Release. J. Neurosci. 2017, 37, 9859–9870. [Google Scholar] [CrossRef]
- Barker-Haliski, M.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Gruenbaum, S.E.; Dhaher, R.; Lee, T.-S.W.; Zhou, Y.; Danbolt, N.C. The Glutamate-Glutamine Cycle in Epilepsy. Adv. Neurobiol. 2016, 13, 351–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bramham, C.R. Bidirectional Dysregulation of AMPA Receptor-Mediated Synaptic Transmission and Plasticity in Brain Disorders. Front. Synaptic Neurosci. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Breese, C.R.; Logel, J.; Adams, C.; Leonard, S.S. Regional Gene Expression of the Glutamate Receptor Subtypes GluR1, GluR2, and GluR3 in Human Postmortem Brain. J. Mol. Neurosci. 1996, 7, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Egbenya, D.L.; Hussain, S.; Lai, Y.-C.; Xia, J.; Anderson, A.E. Changes in Synaptic AMPA Receptor Concentration and Composition in Chronic Temporal Lobe Epilepsy. Mol. Cell. Neurosci. 2018, 92, 93–103. [Google Scholar] [CrossRef]
- Graebenitz, S.; Kedo, O.; Speckmann, E.-J.; Gorji, A.; Panneck, H.; Hans, V.; Palomero-Gallagher, N.; Schleicher, A.; Zilles, K.; Pape, H.-C. Interictal-like Network Activity and Receptor Expression in the Epileptic Human Lateral Amygdala. Brain 2011, 134, 2929–2947. [Google Scholar] [CrossRef]
- Mathern, G.W.; Pretorius, J.K.; Leite, J.P.; Kornblum, H.I.; Mendoza, D.; Lozada, A.; Bertram, E.H. Hippocampal AMPA and NMDA mRNA Levels and Subunit Immunoreactivity in Human Temporal Lobe Epilepsy Patients and a Rodent Model of Chronic Mesial Limbic Epilepsy. Epilepsy Res. 1998, 32, 154–171. [Google Scholar] [CrossRef]
- Zilles, K.; Qü, M.S.; Köhling, R.; Speckmann, E.J. Ionotropic Glutamate and GABA Receptors in Human Epileptic Neocortical Tissue: Quantitative in Vitro Receptor Autoradiography. Neuroscience 1999, 94, 1051–1061. [Google Scholar] [CrossRef]
- Babb, T.L.; Mathern, G.W.; Leite, J.P.; Pretorius, J.K.; Yeoman, K.M.; Kuhlman, P.A. Glutamate AMPA Receptors in the Fascia Dentata of Human and Kainate Rat Hippocampal Epilepsy. Epilepsy Res. 1996, 26, 193–205. [Google Scholar] [CrossRef]
- Das, A.; Wallace, G.C., IV; Holmes, C.; McDowell, M.L.; Smith, J.A.; Marshall, J.D.; Bonilha, L.; Edwards, J.C.; Glazier, S.S.; Ray, S.K.; et al. Hippocampal Tissue of Patients with Refractory TLE Is Associated with Astrocyte Activation, Inflammation, and Altered Expression of Channels and Receptors. Neuroscience 2012, 220, 237–246. [Google Scholar] [CrossRef]
- Mathern, G.W.; Pretorius, J.K.; Kornblum, H.I.; Mendoza, D.; Lozada, A.; Leite, J.P.; Chimelli, L.M.; Fried, I.; Sakamoto, A.C.; Assirati, J.A.; et al. Human Hippocampal AMPA and NMDA mRNA Levels in Temporal Lobe Epilepsy Patients. Brain 1997, 120, 1937–1959. [Google Scholar] [CrossRef]
- Needs, H.I.; Henley, B.S.; Cavallo, D.; Gurung, S.; Modebadze, T.; Woodhall, G.; Henley, J.M. Changes in Excitatory and Inhibitory Receptor Expression and Network Activity during Induction and Establishment of Epilepsy in the Rat Reduced Intensity Status epilepticus (RISE) Model. Neuropharmacology 2019, 158, 107728. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, O.; Tirapelli, D.P.D.C.; Lizarte Neto, F.S.; Freitas-Lima, P.; Saggioro, F.P.; Cirino, M.L.A.; Assirati, J.A.J.; Serafini, L.N.; Velasco, T.R.; Sakamoto, A.C.; et al. Modulation of NMDA Receptor by miR-219 in the Amygdala and Hippocampus of Patients with Mesial Temporal Lobe Epilepsy. J. Clin. Neurosci. 2020, 74, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Jafarian, M.; Modarres Mousavi, S.M.; Alipour, F.; Aligholi, H.; Noorbakhsh, F.; Ghadipasha, M.; Gharehdaghi, J.; Kellinghaus, C.; Kovac, S.; Khaleghi Ghadiri, M.; et al. Cell Injury and Receptor Expression in the Epileptic Human Amygdala. Neurobiol. Dis. 2019, 124, 416–427. [Google Scholar] [CrossRef]
- Hanada, T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules 2020, 10, 464. [Google Scholar] [CrossRef]
- Seifert, G.; Hüttmann, K.; Schramm, J.; Steinhäuser, C. Enhanced Relative Expression of Glutamate Receptor 1 Flip AMPA Receptor Subunits in Hippocampal Astrocytes of Epilepsy Patients with Ammon’s Horn Sclerosis. J. Neurosci. 2004, 24, 1996–2003. [Google Scholar] [CrossRef]
- Ceprian, M.; Fulton, D. Glial Cell AMPA Receptors in Nervous System Health, Injury and Disease. Int. J. Mol. Sci. 2019, 20, 2450. [Google Scholar] [CrossRef]
- Wen, W.; Lin, C.-Y.; Niu, L. R/G Editing in GluA2R Flop Modulates the Functional Difference between GluA1 Flip and Flop Variants in GluA1/2R Heteromeric Channels. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, Function, and Allosteric Modulation of NMDA Receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Beesley, S.; Sullenberger, T.; Pilli, J.; Abbasi, S.; Gunjan, A.; Kumar, S.S. Colocalization of Distinct NMDA Receptor Subtypes at Excitatory Synapses in the Entorhinal Cortex. J. Neurophysiol. 2019, 121, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Neame, S.; Safory, H.; Radzishevsky, I.; Touitou, A.; Marchesani, F.; Marchetti, M.; Kellner, S.; Berlin, S.; Foltyn, V.N.; Engelender, S.; et al. The NMDA Receptor Activation by D-Serine and Glycine Is Controlled by an Astrocytic Phgdh-Dependent Serine Shuttle. Proc. Natl. Acad. Sci. USA 2019, 116, 20736–20742. [Google Scholar] [CrossRef]
- Beesley, S.; Sullenberger, T.; Kumar, S.S. The GluN3 Subunit Regulates Ion Selectivity within Native N-Methyl-D-Aspartate Receptors. IBRO Rep. 2020, 9, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Franck, J.E.; Pokorny, J.; Kunkel, D.D.; Schwartzkroin, P.A. Physiologic and Morphologic Characteristics of Granule Cell Circuitry in Human Epileptic Hippocampus. Epilepsia 1995, 36, 543–558. [Google Scholar] [CrossRef] [PubMed]
- de Moura, J.C.; Tirapelli, D.P.C.; Neder, L.; Saggioro, F.P.; Sakamoto, A.C.; Velasco, T.R.; Panepucci, R.A.; Leite, J.P.; Assirati Junior, J.A.; Colli, B.O.; et al. Amygdala Gene Expression of NMDA and GABAA Receptors in Patients with Mesial Temporal Lobe Epilepsy. Hippocampus 2012, 22, 92–97. [Google Scholar] [CrossRef]
- Alsharafi, W.A.; Xiao, B.; Li, J. MicroRNA-139-5p Negatively Regulates NR2A-Containing NMDA Receptor in the Rat Pilocarpine Model and Patients with Temporal Lobe Epilepsy. Epilepsia 2016, 57, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Tokay, T.; Porath, K.; Köhling, R.; Kirschstein, T. Enhanced NMDA Receptor-Dependent LTP in the Epileptic CA1 Area via Upregulation of NR2B. Neurobiol. Dis. 2013, 54, 183–193. [Google Scholar] [CrossRef]
- Peng, W.-F.; Ding, J.; Li, X.; Fan, F.; Zhang, Q.-Q.; Wang, X. N-Methyl-D-Aspartate Receptor NR2B Subunit Involved in Depression-like Behaviours in Lithium Chloride-Pilocarpine Chronic Rat Epilepsy Model. Epilepsy Res. 2016, 119, 77–85. [Google Scholar] [CrossRef]
- Beesley, S.; Sullenberger, T.; Ailani, R.; D’Orio, C.; Crockett, M.S.; Kumar, S.S. D-Serine Intervention in the Medial Entorhinal Area Alters TLE-Related Pathology in CA1 Hippocampus via the Temporoammonic Pathway. Neuroscience 2021, 453, 168–186. [Google Scholar] [CrossRef]
- Beesley, S.; Sullenberger, T.; Crotty, K.; Ailani, R.; D’Orio, C.; Evans, K.; Ogunkunle, E.O.; Roper, M.G.; Kumar, S.S. D-Serine Mitigates Cell Loss Associated with Temporal Lobe Epilepsy. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Crépel, V.; Mulle, C. Physiopathology of Kainate Receptors in Epilepsy. Curr. Opin. Pharmacol. 2015, 20, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ryazantseva, M.; Englund, J.; Shintyapina, A.; Huupponen, J.; Pitkänen, A.; Partanen, J.M.; Lauri, S.E. Kainate-Type of Glutamate Receptors Regulate Wiring of Intrinsic Glutamatergic Connectivity in the Amygdala. eLife 2020, 9, e52798. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Cortical Kainate Receptors and Behavioral Anxiety. Mol. Brain 2017, 10, 16. [Google Scholar] [CrossRef]
- Marques, J.M.; Rodrigues, R.J.; Valbuena, S.; Rozas, J.L.; Selak, S.; Marin, P.; Aller, M.I.; Lerma, J. CRMP2 Tethers Kainate Receptor Activity to Cytoskeleton Dynamics during Neuronal Maturation. J. Neurosci. 2013, 33, 18298–18310. [Google Scholar] [CrossRef]
- Perrais, D.; Veran, J.; Mulle, C. Gating and Permeation of Kainate Receptors: Differences Unveiled. Trends Pharmacol. Sci. 2010, 31, 516–522. [Google Scholar] [CrossRef]
- Falcón-Moya, R.; Sihra, T.S.; Rodríguez-Moreno, A. Kainate Receptors: Role in Epilepsy. Front. Mol. Neurosci. 2018, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Iida, I.; Konno, K.; Akashi, K.; Abe, M.; Natsume, R.; Watanabe, M.; Sakimura, K. Determination of Kainate Receptor Subunit Ratios in Mouse Brain Using Novel Chimeric Protein Standards. J. Neurochem. 2016, 136, 295–305. [Google Scholar] [CrossRef]
- Valbuena, S.; Lerma, J. Kainate Receptors, Homeostatic Gatekeepers of Synaptic Plasticity. Neuroscience 2019. [Google Scholar] [CrossRef]
- Mathern, G.W.; Pretorius, J.K.; Kornblum, H.I.; Mendoza, D.; Leita, J.P.; Chimelli, L.; Born, D.E.; Fried, I.; Sakamoto, A.C.; Assirati, J.A.; et al. Altered Hippocampal Kainate-Receptor mRNA Levels in Temporal Lobe Epilepsy Patients. Neurobiol. Dis. 1998, 5, 151–176. [Google Scholar] [CrossRef]
- Wenzel, H.J.; Woolley, C.S.; Robbins, C.A.; Schwartzkroin, P.A. Kainic Acid-induced Mossy Fiber Sprouting and Synapse Formation in the Dentate Gyrus of Rats. Hippocampus 2000, 10, 244–260. [Google Scholar] [CrossRef]
- Epsztein, J.; Represa, A.; Jorquera, I.; Ben-Ari, Y.; Crépel, V. Recurrent Mossy Fibers Establish Aberrant Kainate Receptor-Operated Synapses on Granule Cells from Epileptic Rats. J. Neurosci. 2005, 25, 8229–8239. [Google Scholar] [CrossRef] [PubMed]
- Artinian, J.; Peret, A.; Mircheva, Y.; Marti, G.; Crépel, V. Impaired neuronal operation through aberrant intrinsic plasticity in epilepsy. Ann. Neurol. 2015, 77, 592–606. [Google Scholar] [CrossRef]
- Li, J.-M.; Zeng, Y.; Peng, F.; Li, L.; Yang, T.; Hong, Z.; Lei, D.; Chen, Z.; Zhou, D. Aberrant Glutamate Receptor 5 Expression in Temporal Lobe Epilepsy Lesions. Brain Res. 2010, 1311, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Peret, A.; Christie, L.A.; Ouedraogo, D.W.; Gorlewicz, A.; Epsztein, J.; Mulle, C.; Crépel, V. Contribution of Aberrant GluK2-Containing Kainate Receptors to Chronic Seizures in Temporal Lobe Epilepsy. Cell Rep. 2014, 8, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.R.; Takahasi, K.; Thomson, K.E.; Wilcox, K. The Expression of Kainate Receptor Subunits in Hippocampal Astrocytes after Experimentally Induced Status epilepticus. J. Neuropathol Exp. Neurol 2013, 72, 919–932. [Google Scholar] [CrossRef]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef]
- Notenboom, R.G.E.; Hampson, D.R.; Jansen, G.H.; van Rijen, P.C.; van Veelen, C.W.M.; van Nieuwenhuizen, O.; de Graan, P.N.E. Up-Regulation of Hippocampal Metabotropic Glutamate Receptor 5 in Temporal Lobe Epilepsy Patients. Brain 2006, 129, 96–107. [Google Scholar] [CrossRef]
- Blümcke, I.; Becker, A.J.; Klein, C.; Scheiwe, C.; Lie, A.A.; Beck, H.; Waha, A.; Friedl, M.G.; Kuhn, R.; Emson, P.; et al. Temporal Lobe Epilepsy Associated Up-Regulation of Metabotropic Glutamate Receptors: Correlated Changes in mGluR1 mRNA and Protein Expression in Experimental Animals and Human Patients. J. Neuropathol. Exp. Neurol. 2000, 59, 1–10. [Google Scholar] [CrossRef]
- Umpierre, A.D.; West, P.J.; White, J.A.; Wilcox, K.S. Conditional Knock-out of mGluR5 from Astrocytes during Epilepsy Development Impairs High-Frequency Glutamate Uptake. J. Neurosci. 2019, 39, 727–742. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Rosa-Neto, P.; Monteiro, M.R.; Guiot, M.-C.; Assirati, J.A.; Carlotti, C.G.; Kobayashi, E.; Leite, J.P. Distinct Increased Metabotropic Glutamate Receptor Type 5 (mGluR5) in Temporal Lobe Epilepsy With and Without Hippocampal Sclerosis. Hippocampus 2013, 23, 1212–1230. [Google Scholar] [CrossRef]
- Tang, F.R.; Lee, W.R. Expression of the Group II and III Metabotropic Glutamate Receptors in the Hippocampus of Patients with Mesial Temporal Lobe Epilepsy. J. Neurocytol. 2001, 30, 137–143. [Google Scholar] [CrossRef]
- Lie, A.A.; Becker, A.; Behle, K.; Beck, H.; Malitschek, B.; Conn, P.J.; Kuhn, R.; Nitsch, R.; Plaschke, M.; Schramm, J.; et al. Up-Regulation of the Metabotropic Glutamate Receptor mGluR4 in Hippocampal Neurons with Reduced Seizure Vulnerability. Ann. Neurol. 2000, 47, 26–35. [Google Scholar] [CrossRef]
- Kral, T.; Erdmann, E.; Sochivko, D.; Clusmann, H.; Schramm, J.; Dietrich, D. Down-regulation of mGluR8 in Pilocarpine Epileptic Rats. Synapse 2003, 47, 278–284. [Google Scholar] [CrossRef]
- Sun, W.; McConnell, E.; Pare, J.-F.; Xu, Q.; Chen, M.; Peng, W.; Lovatt, D.; Han, X.; Smith, Y.; Nedergaard, M. Glutamate-Dependent Neuroglial Calcium Signaling Differs Between Young and Adult Brain. Science 2013, 339, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Lee, W.; Yeo, T.T. Expression of the Group I Metabotropic Glutamate Receptor in the Hippocampus of Patients with Mesial Temporal Lobe Epilepsy. J. Neurocytol. 2002, 30, 403–411. [Google Scholar] [CrossRef]
- Schijns, O.; Karaca, Ü.; Andrade, P.; de Nijs, L.; Küsters, B.; Peeters, A.; Dings, J.; Pannek, H.; Ebner, A.; Rijkers, K.; et al. Hippocampal GABA Transporter Distribution in Patients with Temporal Lobe Epilepsy and Hippocampal Sclerosis. J. Chem. Neuroanat. 2015, 68, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Castelli, P.; Gessa, G.L. Distribution and Localization of the GABAB Receptor. In GABAB Receptor; Colombo, G., Ed.; Springer Link: Berlin/Heidelberg, Germany, 2016; pp. 75–92. [Google Scholar]
- Schipper, S.; Aalbers, M.W.; Rijkers, K.; Swijsen, A.; Rigo, J.M.; Hoogland, G.; Vles, J.S.H. Tonic GABAA Receptors as Potential Target for the Treatment of Temporal Lobe Epilepsy. Mol. Neurobiol. 2016, 53, 5252–5265. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W. GABAA Receptor: Positive and Negative Allosteric Modulators. Neuropharmacology 2018, 136, 10–22. [Google Scholar] [CrossRef]
- Pan, G.; Chen, Z.; Zheng, H.; Zhang, Y.; Xu, H.; Bu, G.; Zheng, H.; Li, Y. Compensatory Mechanisms Modulate the Neuronal Excitability in a Kainic Acid-Induced Epilepsy Mouse Model. Front. Neural Circuits 2018, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Drexel, M.; Kirchmair, E.; Sperk, G. Changes in the Expression of GABAA Receptor Subunit mRNAs in Parahippocampal Areas after Kainic Acid Induced Seizures. Front. Neural Circuits 2013, 7, 142. [Google Scholar] [CrossRef]
- Loup, F.; Wieser, H.-G.; Yonekawa, Y.; Aguzzi, A.; Fritschy, J.-M. Selective Alterations in GABA(A) Receptor Subtypes in Human Temporal Lobe Epilepsy. J. Neurosci. 2000, 20, 5401–5419. [Google Scholar] [CrossRef]
- Pirker, S.; Schwarzer, C.; Czech, T.; Baumgartner, C.; Pockberger, H.; Maier, H.; Hauer, B.; Sieghart, W.; Furtinger, S.; Sperk, G. Increased Expression of GABAA Receptor β-Subunits in the Hippocampus of Patients with Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2003, 62, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Stefanits, H.; Milenkovic, I.; Mahr, N.; Pataraia, E.; Baumgartner, C.; Hainfellner, J.A.; Kovacs, G.G.; Kasprian, G.; Sieghart, W.; Yilmazer-Hanke, D.; et al. Alterations in GABAA Receptor Subunit Expression in the Amygdala and Entorhinal Cortex in Human Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2019, 78, 1022–1048. [Google Scholar] [CrossRef]
- Palma, E.; Spinelli, G.; Torchia, G.; Martinez-Torres, A.; Ragozzino, D.; Miledi, R.; Eusebi, F. Abnormal GABAA Receptors from the Human Epileptic Hippocampal Subiculum Microtransplanted to Xenopus Oocytes. Proc. Acad. Natl. Sci. USA 2005, 102, 2514–2518. [Google Scholar] [CrossRef]
- Ragozzino, D.; Palma, E.; Di Angelantonio, S.; Amici, M.; Mascia, A.; Arcella, A.; Giangaspero, F.; Cantore, G.; Di Gennaro, G.; Manfredi, M.; et al. Rundown of GABA Type A Receptors Is a Dysfunction Associated with Human Drug-Resistant Mesial Temporal Lobe Epilepsy. Proc. Acad. Natl. Sci. USA 2005, 102, 15219–15223. [Google Scholar] [CrossRef]
- Loup, F.; Picard, F.; André, V.M.; Kehrli, P.; Yonekawa, Y.; Wieser, H.-G.; Fritschy, J.-M. Altered Expression of α3-Containing GABAA Receptors in the Neocortex of Patients with Focal Epilepsy. Brain 2006, 129, 3277–3289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yilmazer-Hanke, D.; O’Loughlin, E.; McDermott, K. Contribution of Amygdala Pathology to Comorbid Emotional Disturbances in Temporal Lobe Epilepsy. J. Neurosci. Res. 2016, 94, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Galanopoulou, A.S. Dissociated Gender-Specific Effects of Recurrent Seizures on GABA Signaling in CA1 Pyramidal Neurons: Role of GABAA Receptors. J. Neurosci. 2008, 28, 1557–1567. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Galanopoulou, A.S.; Moshé, S.L. Sex Dimorphism in Seizure-Controlling Networks. Neurobiol. Dis. 2014, 72PB, 144–152. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Liang, S.; Zhang, G.; Yang, X. Role of NKCC1 and KCC2 in Epilepsy: From Expression to Function. Front. Neurol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Chen, Z. Double-Edged GABAergic Synaptic Transmission in Seizures: The Importance of Chloride Plasticity. Brain Res. 2018, 1701, 126–136. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, P.; Inaba, Y.; Biagini, G.; Baldelli, E.; Mollinari, C.; Merlo, D.; Avoli, M. Subiculum Network Excitability Is Increased in a Rodent Model of Temporal Lobe Epilepsy. Hippocampus 2006, 16, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, C.; Xu, Z.; Ji, C.; Liang, J.; Wang, J.; Wang, Y.; Chen, B.; Wu, X.; Gao, F.; et al. Depolarized GABAergic Signaling in Subicular Microcircuits Mediates Generalized Seizure in Temporal Lobe Epilepsy. Neuron 2017, 95, 92–105. [Google Scholar] [CrossRef]
- Barros-Barbosa, A.R.; Fonseca, A.L.; Guerra-Gomes, S.; Ferreirinha, F.; Santos, A.; Rangel, R.; Lobo, M.G.; Correia-de-Sá, P.; Cordeiro, J.M. Up-Regulation of P2 × 7 Receptor–mediated Inhibition of GABA Uptake by Nerve Terminals of the Human Epileptic Neocortex. Epilepsia 2016, 57, 99–110. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.; Zhou, R.; Li, Y.; Yang, Y.; Wang, X. Protrudin Modulates Seizure Activity through GABA A Receptor Regulation. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Y.; Xu, X.; Ganbat, B.; Li, Y.; Wang, W.; Yang, Y.; Lu, X.; Du, C.; Tian, X.; Wang, X. CNTNAP4 Impacts Epilepsy through GABAA Receptors Regulation: Evidence From Temporal Lobe Epilepsy Patients and Mouse Models. Cereb. Cortex 2018, 28, 3491–3504. [Google Scholar] [CrossRef]
- Roseti, C.; van Vliet, E.A.; Cifelli, P.; Ruffolo, G.; Baayen, J.C.; Di Castro, M.A.; Bertollini, C.; Limatola, C.; Aronica, E.; Vezzani, A.; et al. GABAA Currents Are Decreased by IL-1β in Epileptogenic Tissue of Patients with Temporal Lobe Epilepsy: Implications for Ictogenesis. Neurobiol. Dis. 2015, 82, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Sheilabi, M.A.; Battacharyya, D.; Caetano, L.; Thom, M.; Reuber, M.; Duncan, J.S.; Princivalle, A.P. Quantitative Expression and Localization of GABAB Receptor Protein Subunits in Hippocampi from Patients with Refractory Temporal Lobe Epilepsy. Neuropharmacology 2018, 136, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Straessle, A.; Loup, F.; Arabadzisz, D.; Ohning, G.V.; Fritschy, J.-M. Rapid and Long-Term Alterations of Hippocampal GABAB Receptors in a Mouse Model of Temporal Lobe Epilepsy. Eur. J. Neurosci. 2003, 18, 2213–2226. [Google Scholar] [CrossRef]
- Evenseth, L.S.M.; Gabrielsen, M.; Sylte, I. The GABAB Receptor—structure, Ligand Binding and Drug Development. Molecules 2020, 25, 3093. [Google Scholar] [CrossRef]
- Princivalle, A.P.; Duncan, J.S.; Thom, M.; Bowery, N.G. GABAB1a, GABAB1b AND GABAB2 mRNA Variants Expression in Hippocampus Resected from Patients with Temporal Lobe Epilepsy. Neuroscience 2003, 122, 975–984. [Google Scholar] [CrossRef]
- Billinton, A.; Baird, V.; Thom, M.; Duncan, J.S.; Upton, N.; Bowery, N.G. GABAB(1) mRNA Expression in Hippocampal Sclerosis Associated with Human Temporal Lobe Epilepsy. Mol. Brain Res. 2001, 86, 84–89. [Google Scholar] [CrossRef]
- Furtinger, S.; Bettler, B.; Sperka, G. Altered Expression of GABAB Receptors in the Hippocampus after Kainic-Acid-Induced Seizures in Rats. Mol. Brain Res. 2003, 113, 107–115. [Google Scholar] [CrossRef]
- Teichgräber, L.A.; Lehmann, T.-N.; Meencke, H.-J.; Weiss, T.; Nitsch, R.; Deisz, R.A. Impaired Function of GABAB Receptors in Tissues from Pharmacoresistant Epilepsy Patients. Epilepsia 2009, 50, 1697–1716. [Google Scholar] [CrossRef] [PubMed]
- Martinello, K.; Sciaccaluga, M.; Morace, R.; Mascia, A.; Arcella, A.; Esposito, V.; Fucile, S. Loss of Constitutive Functional γ-Aminobutyric Acid Type A-B Receptor Crosstalk in Layer 5 Pyramidal Neurons of Human Epileptic Temporal Cortex. Epilepsia 2018, 59, 449–459. [Google Scholar] [CrossRef]
- Papaneophytou, C.; Georgiou, E.; Kleopa, K.A. The Role of Oligodendrocyte Gap Junctions in Neuroinflammation. Channels 2019, 13, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Rackauskas, M.; Neverauskas, V.; Skeberdis, V.A. Diversity and Properties of Connexin Gap Junction Channels. Medicina 2010, 46, 1–12. [Google Scholar] [CrossRef]
- Sáez, J.C.; Martínez, A.D.; Brañes, M.C.; González, H.E. Regulation of Gap Junctions by Protein Phosphorylation. Braz. J. Med. Biol. Res. 1998, 31, 593–600. [Google Scholar] [CrossRef]
- Scott, C.A.; Tattersall, D.; O’Toole, E.A.; Kelsell, D.P. Connexins in Epidermal Homeostasis and Skin Disease. Biochim. Biophys. Acta BBA Biomembr. 2012, 1818, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Rash, J.E. Connexins and Gap Junctions of Astrocytes and Oligodendrocytes in the CNS. Brain Res. Rev. 2000, 32, 29–44. [Google Scholar] [CrossRef]
- Medina-Ceja, L.; Salazar-Sánchez, J.C.; Ortega-Ibarra, J.; Morales-Villagrán, A. Connexins-Based Hemichannels/Channels and Their Relationship with Inflammation, Seizures and Epilepsy. Int. J. Mol. Sci. 2019, 20, 5976. [Google Scholar] [CrossRef]
- Lapato, A.S.; Tiwari-Woodruff, S.K. Connexins & Pannexins: At the Junction of Neuro-Glial Homeostasis & Disease. J. Neurosci. Res. 2018, 96, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Pannasch, U.; Derangeon, M.; Chever, O.; Rouach, N. Astroglial Gap Junctions Shape Neuronal Network Activity. Commun. Integr. Biol. 2012, 5, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pannasch, U.; Rouach, N. Emerging Role for Astroglial Networks in Information Processing: From Synapse to Behavior. Trends Neurosci. 2013, 36, 405–417. [Google Scholar] [CrossRef]
- Collignon, F.; Wetjen, N.M.; Cohen-Gadol, A.A.; Cascino, G.D.; Parisi, J.; Meyer, F.B.; Marsh, W.R.; Roche, P.; Weigand, S.D. Altered Expression of Connexin Subtypes in Mesial Temporal Lobe Epilepsy in Humans. J. Neurosurg. 2006, 105, 77–87. [Google Scholar] [CrossRef]
- Fonseca, C.G.; Green, C.R.; Nicholson, L.F.B. Upregulation in Astrocytic Connexin 43 Gap Junction Levels May Exacerbate Generalized Seizures in Mesial Temporal Lobe Epilepsy. Brain Res. 2002, 929, 105–116. [Google Scholar] [CrossRef]
- Naus, C.C.G.; Bechberger, J.F.; Paul, D.L. Gap Junction Gene Expression in Human Seizure Disorder. Exp. Neurol. 1991, 111, 198–203. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L.; Feng, J.; Qiu, J.; Lin, W. Expression of Connexin 32 and Connexin 43 in the Cerebral Cortex of Patients with Refractory Epilepsy. J. Lab. Med. 2017, 41, 33–40. [Google Scholar] [CrossRef]
- Wu, X.L.; Tang, Y.C.; Lu, Q.Y.; Xiao, X.L.; Song, T.B.; Tang, F.R. Astrocytic Cx 43 and Cx 40 in the Mouse Hippocampus during and after Pilocarpine-Induced Status epilepticus. Exp. Brain Res. 2015, 233, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, S.; Sayyah, M.; Golkar, M.; Sepehri, H.; Babaie, J.; Vaziri, B. Changes in Hippocampal Connexin 36 mRNA and Protein Levels during Epileptogenesis in the Kindling Model of Epilepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Motaghi, S.; Sayyah, M.; Babapour, V.; Mahdian, R. Hippocampal Expression of Connexin36 and Connexin43 during Epileptogenesis in Pilocarpine Model of Epilepsy. Iran. Biomed. J. 2017, 21, 167–173. [Google Scholar] [CrossRef]
- Söhl, G.; Güldenagel, M.; Beck, H.; Teubner, B.; Traub, O.; Gutiérrez, R.; Heinemann, U.; Willecke, K. Expression of Connexin Genes in Hippocampus of Kainate-Treated and Kindled Rats under Conditions of Experimental Epilepsy. Mol. Brain Res. 2000, 83, 44–51. [Google Scholar] [CrossRef]
- Bedner, P.; Dupper, A.; Hüttmann, K.; Müller, J.; Herde, M.K.; Dublin, P.; Deshpande, T.; Schramm, J.; Häussler, U.; Haas, C.A.; et al. Astrocyte Uncoupling as a Cause of Human Temporal Lobe Epilepsy. Brain 2015, 138, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- van der Hel, W.S.; Notenboom, R.G.E.; Bos, I.W.M.; van Rijen, P.C.; van Veelen, C.W.M.; de Graan, P.N.E. Reduced Glutamine Synthetase in Hippocampal Areas with Neuron Loss in Temporal Lobe Epilepsy. Neurology 2005, 64, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Cavus, I.; Kasoff, W.S.; Cassaday, M.P.; Jacob, R.; Gueorguieva, R.; Sherwin, R.S.; Krystal, J.H.; Spencer, D.D.; Abi-Saab, W.M. Extracellular Metabolites in the Cortex and Hippocampus of Epileptic Patients. Ann. Neurol. 2005, 57, 226–235. [Google Scholar] [CrossRef]
- De Lanerolle, N.C.; Lee, T.-S. New Facets of the Neuropathology and Molecular Profile of Human Temporal Lobe Epilepsy. Epilepsy Behav. EB 2005, 7, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M. Direct Signaling from Astrocytes to Neurons in Cultures of Mammalian Brain Cells. Science 1994, 263, 1768–1771. [Google Scholar] [CrossRef]
- O’Connor, E.R.; Sontheimer, H.; Spencer, D.D.; de Lanerolle, N.C. Astrocytes from Human Hippocampal Epileptogenic Foci Exhibit Action Potential-like Responses. Epilepsia 1998, 39, 347–354. [Google Scholar] [CrossRef]
- Cottrell, G.T.; Lin, R.; Warn-Cramer, B.J.; Lau, A.F.; Burt, J.M. Mechanism of v-Src- and Mitogen-Activated Protein Kinase-Induced Reduction of Gap Junction Communication. Am. J. Physiol. Cell Physiol. 2003, 284, C511–C530. [Google Scholar] [CrossRef]
- Warn-Cramer, B.J.; Cottrell, G.T.; Burt, J.M.; Lau, A.F. Regulation of Connexin-43 Gap Junctional Intercellular Communication by Mitogen-Activated Protein Kinase. J. Biol. Chem. 1998, 273, 9188–9196. [Google Scholar] [CrossRef]
- Lampe, P.D.; TenBroek, E.M.; Burt, J.M.; Kurata, W.E.; Johnson, R.G.; Lau, A.F. Phosphorylation of connexin43 on serine368 by Protein Kinase C Regulates Gap Junctional Communication. J. Cell Biol. 2000, 149, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Même, W.; Calvo, C.-F.; Froger, N.; Ezan, P.; Amigou, E.; Koulakoff, A.; Giaume, C. Proinflammatory Cytokines Released from Microglia Inhibit Gap Junctions in Astrocytes: Potentiation by Beta-Amyloid. FASEB J. 2006, 20, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Granata, T.; Ghosh, C.; Janigro, D. Blood–brain Barrier Dysfunction and Epilepsy: Pathophysiologic Role and Therapeutic Approaches. Epilepsia 2012, 53, 1877–1886. [Google Scholar] [CrossRef]
- Janigro, D. Are you in or out? Leukocyte, Ion, and Neurotransmitter Permeability across the Epileptic Blood–brain Barrier. Epilepsia 2012, 53, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Friedman, A. Structural, Molecular, and Functional Alterations of the Blood-Brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int. J. Mol. Sci. 2020, 21, 591. [Google Scholar] [CrossRef]
- Ivens, S.; Kaufer, D.; Flores, L.P.; Bechmann, I.; Zumsteg, D.; Tomkins, O.; Seiffert, E.; Heinemann, U.; Friedman, A. TGF-Beta Receptor-Mediated Albumin Uptake into Astrocytes Is Involved in Neocortical Epileptogenesis. Brain 2007, 130, 535–547. [Google Scholar] [CrossRef]
- Turecek, J.; Yuen, G.S.; Han, V.Z.; Zeng, X.-H.; Bayer, K.U.; Welsh, J.P. NMDA Receptor Activation Strengthens Weak Electrical Coupling in Mammalian Brain. Neuron 2014, 81, 1375–1388. [Google Scholar] [CrossRef][Green Version]
- Wu, X.L.; Ma, D.M.; Zhang, M.; Zhou, J.S.; Huo, Y.W.; Lu, M.; Tang, F.R. Cx36 in the Mouse Hippocampus during and after Pilocarpine-Induced Status epilepticus. Epilepsy Res. 2018, 141, 64–72. [Google Scholar] [CrossRef]
- Scherer, S.; Deschenes, S.; Xu, Y.; Grinspan, J.; Fischbeck, K.; Paul, D. Connexin32 Is a Myelin-Related Protein in the PNS and CNS. J. Neurosci. 1995, 15, 8281–8294. [Google Scholar] [CrossRef] [PubMed]
- Menichella, D.M.; Majdan, M.; Awatramani, R.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Genetic and Physiological Evidence That Oligodendrocyte Gap Junctions Contribute to Spatial Buffering of Potassium Released during Neuronal Activity. J. Neurosci. 2006, 26, 10984–10991. [Google Scholar] [CrossRef]
- Ferrer, I.; Santpere, G.; Arzberger, T.; Bell, J.; Blanco, R.; Boluda, S.; Budka, H.; Carmona, M.; Giaccone, G.; Krebs, B.; et al. Brain Protein Preservation Largely Depends on the Postmortem Storage Temperature: Implications for Study of Proteins in Human Neurologic Diseases and Management of Brain Banks: A Brainnet Europe Study. J. Neuropathol. Exp. Neurol. 2007, 66, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Maheu, M.; Lopez, J.P.; Vaillancourt, K.; Cruceanu, C.; Gross, J.A.; Arnovitz, M.; Mechawar, N.; Turecki, G. Effects of Postmortem Interval on Biomolecule Integrity in the Brain. J. Neuropathol. Exp. Neurol. 2015, 74, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; TesFaye, E.; Yasuda, R.P.; Mash, D.C.; Armstrong, D.A.; Wolfe, B.B. Effects of Post-Mortem Delay on Subunits of Ionotropic Glutamate Receptors in Human Brain. Mol. Brain Res. 2000, 80, 123–131. [Google Scholar] [CrossRef]
- Patino, C.M.; Ferreira, J.C. Inclusion and Exclusion Criteria in Research Studies: Definitions and Why They Matter. J. Bras. Pneumol. 2018, 44, 84. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.T.; Jackowski, A.P.; Britto Fdos, S.; Sandim, G.B.; Caboclo, L.O.; Centeno, R.S.; Carrete, H.J.; Yacubian, E.M. Gender and Hemispheric Differences in Temporal Lobe Epilepsy: A VBM Study. Seizure 2014, 23, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; MacLusky, N.J. Sex Differences in the Neurobiology of Epilepsy: A Preclinical Perspective. Neurobiol. Dis. 2014, 72, 180–192. [Google Scholar] [CrossRef]
- Biskup, E.; Martinkova, J.; Ferretti, M.T. Gender Medicine: Towards a Gender-Specific Treatment of Neuropsychiatric Disorders. Handb. Clin. Neurol. 2020, 175, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.T.; Galea, L.A.M. Improving Pharmacological Treatment in Brain and Mental Health Disorders: The Need for Gender and Sex Analyses. Front. Neuroendocrinol. 2018, 50, 1–2. [Google Scholar] [CrossRef]
| Hippocampus | DG | Amg | Temp ctx | TLE Model | Refs | |
|---|---|---|---|---|---|---|
| AMPA | ↑ | Human | [27] | |||
| ↑(La) | Human | [47] | ||||
| ↑ | Human | [49] | ||||
| GluA1 | =(BLA and CeM) | Human | [55] | |||
| ↑ | Human | [51] | ||||
| ↑/↑ | ↑/↑ | Human | [48] | |||
| ↑/↑ | ↑/↑ | Human | [52] | |||
| ↑ | Human and Rat KA | [50] | ||||
| ↓ | Rat PILO | [53] | ||||
| ↓ | Rat KA | [46] | ||||
| GluA2 | = | ↑ | Human | [54] | ||
| =(BLA and CeM) | Human | [55] | ||||
| ↓ | Human | [51] | ||||
| ↑/↑ | ↑/↑ | Human | [48] | |||
| =/↑ | ↑/↑ | Human | [52] | |||
| ↓ | Rat PILO | [53] | ||||
| ↓ | Rat KA | [46] | ||||
| GluA3 | ↑/↑ | ↑/↑ | Human | [48] | ||
| =/= | =/= | Human | [52] | |||
| ↓ | Rat PILO | [53] | ||||
| NMDA | =(La) | Human | [47] | |||
| ↑ | Human | [64] | ||||
| GluN1 | ↓ | ↑ | Human | [54] | ||
| =(BLA and CeM) | Human | [55] | ||||
| ↑ | Human | [65] | ||||
| ↑/↑ | ↑/↑ | Human | [48] | |||
| =/= | =/↑ | Human | [52] | |||
| = | Rat PILO | [53] | ||||
| GluN2A | =(BLA) | Human | [65] | |||
| ↑ | Human and Rat PILO | [66] | ||||
| ↓ | Rat PILO | [53] | ||||
| =(CA1) | Rat PILO | [67] | ||||
| GluN2B | =(BLA and CeM) | Human | [55] | |||
| =(BLA) | Human | [65] | ||||
| ↑ | Human and Rat PILO | [66] | ||||
| ↑/↑ | ↑/↑ | Human | [48] | |||
| = | Rat PILO | [53] | ||||
| ↓ | Rat KA | [46] | ||||
| ↑(CA1) | Rat PILO | [67] | ||||
| KA | ↑(La) | Human | [47] | |||
| GluK1 | ↑ | = | Human | [83] | ||
| ↓(CA2–4)/= | =/↑ | Human | [79] | |||
| GluK2 | ↓(CA2-4)/↓(CA2-4) | =/= | Human | [79] | ||
| = | Rat PILO | [53] | ||||
| = | Rat KA | [46] | ||||
| GluK3 | =/= | =/= | Human | [79] | ||
| GluK4 | ↑ | Human | [51] | |||
| =/= | =/= | Human | [79] | |||
| GluK5 | ↑ | Human | [51] | |||
| =/= | =/↑ | Human | [79] | |||
| = | Rat PILO | [53] |
| Hippocampus | DG | Sub | Amg | Temp ctx | TLE Model | Refs | |
|---|---|---|---|---|---|---|---|
| Group I | |||||||
| mGluR1α | =(BLA and CeM) | Human | [55] | ||||
| =/= | =/= | Human | [87] | ||||
| ↑ | Human, Rat KA, and Rat Kindling | [88] | |||||
| = | Rat PILO | [53] | |||||
| mGluR5 | ↓(head) | ↓ | ↓ | Human | [25] | ||
| ↑ | ↑ | ↑ | ↑(Ent) | Human | [90] | ||
| ↑ | Human | [51] | |||||
| ↑/↑ | ↑/↑ | Human | [87] | ||||
| = | Rat PILO | [53] | |||||
| Group II | |||||||
| mGluR2/3 | ↑ | Human | [51] | ||||
| ↑(La) | Human | [47] | |||||
| Group III | |||||||
| mGluR4 | ↑(CA4) =(CA1–3)/↑(CA4) =(CA1–2–3) | ↑/↑ | Human | [92] | |||
| mGluR8 | ↓ | Rat PILO | [93] |
| Hippocampus | DG | Hilus | Sub | Amg | Temp ctx | TLE Model | Refs | |
|---|---|---|---|---|---|---|---|---|
| GABAA | ↓(La) | Human | [47] | |||||
| α1 | ↓(BLA and CeM) | Human | [55] | |||||
| =(BLA) ↓(La, BM, and CeM) | ↓(Ent) | Human | [104] | |||||
| =(BLA) | Human | [65] | ||||||
| =/= | Human | [107] | ||||||
| =/↓(CA1) =(CA2–3) | =/= | =/↓ | =/= | Human | [103] | |||
| =(CA2)/↓(CA2) | =/↑ | Human | [102] | |||||
| ↑(CA1-3) | ↑ | = | ↑(Ent Lyr II and Per) | Rat KA | [101] | |||
| α2 | ↓ | = | Human | [54] | ||||
| ↓(La, BLA and BM) =(CeM) | =(Ent) | Human | [104] | |||||
| = | Human | [107] | ||||||
| =(CA2)/↑(CA2) | =/↑ | Human | [102] | |||||
| =(CA1–3) | = | ↓ | =(Ent and Per) | Rat KA | [101] | |||
| α3 | ↓ | ↓(Ent) | Human | [104] | ||||
| ↓(Lyr I–III)/↓(Lyr I–III) | Human | [107] | ||||||
| =/↓(CA1) =(CA2–3) | ↑/= | =/= | ↑/= | Human | [103] | |||
| =(CA2)/↓(CA2) | =/= | Human | [102] | |||||
| ↑(CA1) =(CA3) | = | = | =(Ent and Per) | Rat KA | [101] | |||
| α4 | ↑ | Human | [36] | |||||
| =(CA1–3) | ↑ | ↑(Proximal) | =(Ent and Per) | Rat KA | [101] | |||
| α5 | ↓(La, BLA, BM, and CeM) | =(Ent) | Human | [104] | ||||
| ↓(CA1–3) | ↓ | ↓ | ↓(Ent and Per) | Rat KA | [101] | |||
| β1 | =(BLA) | Human | [65] | |||||
| ↑(CA3) =(CA1–2)/↑(CA2) =(CA1–3) | ↑/↑ | ↑/↑ | ↑/↑ | Human | [103] | |||
| ↓(CA3) =(CA1) | = | ↓(Distal) | ↓(Ent Lyr V–VI and Per) | Rat KA | [101] | |||
| β2 | ↓(La) ↑(CeM) =(BLA and BM) | ↓(Ent) | Human | [104] | ||||
| =(BLA) | Human | [65] | ||||||
| ↑(CA1–3) =(CA2)/↑(CA1–2–3) | ↑/↑ | =/↑ | ↑/↑ | Human | [103] | |||
| ↓(CA3) =(CA1) | = | ↓ | ↓(Ent Lyr V–VI and Per) | Rat KA | [101] | |||
| β3 | = | = | Human | [54] | ||||
| ↓(BLA and CeM) | Human | [55] | ||||||
| ↑(CA1–2–3)/↓(CA1)↑(CA2) =(CA3) | ↑/↑ | =/= | ↑/↑ | Human | [103] | |||
| ↓ | Rat PILO | [53] | ||||||
| =(CA1–3) | = | ↓ | ↓(Ent and Per) | Rat KA | [101] | |||
| γ2 | = | ↑ | Human | [54] | ||||
| ↓(BLA and CeM) | Human | [55] | ||||||
| ↑ | =(Ent) | Human | [104] | |||||
| ↑ | Human | [36] | ||||||
| =/= | Human | [107] | ||||||
| =/↓(CA1) =(CA2–3) | =/↑ | =/= | =/↑ | Human | [103] | |||
| γ2 | =(CA2)/=(CA2) | =/↑ | Human | [102] | ||||
| =(CA1–3) | ↑ | = | ↑(Ent Lyr II and Per) | Rat KA | [101] | |||
| δ | ↓(CA1) =(CA3) | = | ↓ | ↓(Ent and Per) | Rat KA | [101] |
| Hippocampus | DG | Hilus | Sub | Amg | Temp ctx | TLE Model | Refs | |
|---|---|---|---|---|---|---|---|---|
| GABAB | ↓ | Human | [36] | |||||
| =(La) | Human | [47] | ||||||
| ↓ | Human | [125] | ||||||
| GABAB1 | ↓(BLA and CeM) | Human | [55] | |||||
| ↑ | Human | [119] | ||||||
| ↑(CA1) =(CA2–3) | ↑ | ↑ | = | Human | [122] | |||
| ↑(CA1) =(CA2–3) | ↑ | ↑ | Human | [123] | ||||
| ↓(CA1–2–3) | = | Rat KA | [124] | |||||
| ↓(CA1–3) | ↑ | ↓ | Mouse KA | [120] | ||||
| GABAB2 | ↓(BLA and CeM) | Human | [55] | |||||
| ↑ | Human | [119] | ||||||
| ↑(CA1–3) =(CA2) | ↑ | ↑ | = | Human | [122] | |||
| ↓(CA1–2–3) | = | Rat KA | [124] | |||||
| ↓(CA1–3) | ↑ | ↓ | Mouse KA | [120] |
| Hippocampus | DG | Sub | Temp ctx | TLE Model | Refs | |
|---|---|---|---|---|---|---|
| Cx30 | = | Rat KA, Rat Kindling | [143] | |||
| Cx32 | ↑ | ↑ | Human | [139] | ||
| ↓ | = | ↓ | Human | [136] | ||
| = | Human | [138] | ||||
| = | Rat KA and Rat Kindling | [143] | ||||
| Cx36 | = | = | = | Human | [136] | |
| ↓(CA1–3) | ↓ | Mouse PILO | [159] | |||
| = | Rat PILO | [142] | ||||
| = | Rat Kindling | [141] | ||||
| = | Rat KA and Rat Kindling | [143] | ||||
| Cx40 | ↑(CA1–3) | ↑ | Mouse PILO | [140] | ||
| Cx43 | ↑ | ↑ | Human | [139] | ||
| ↑ | Human | [51] | ||||
| ↑ | ↑ | ↑ | Human | [136] | ||
| ↑(CA1–4) | Human | [137] | ||||
| ↑ | Human | [138] | ||||
| = | Rat PILO | [142] | ||||
| ↑(CA1–3) | ↑ | Mouse PILO | [140] | |||
| = | Rat Kindling | [141] | ||||
| = | Rat KA and Rat Kindling | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, E.; Curia, G. Synaptic Reshaping and Neuronal Outcomes in the Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2021, 22, 3860. https://doi.org/10.3390/ijms22083860
Ren E, Curia G. Synaptic Reshaping and Neuronal Outcomes in the Temporal Lobe Epilepsy. International Journal of Molecular Sciences. 2021; 22(8):3860. https://doi.org/10.3390/ijms22083860
Chicago/Turabian StyleRen, Elisa, and Giulia Curia. 2021. "Synaptic Reshaping and Neuronal Outcomes in the Temporal Lobe Epilepsy" International Journal of Molecular Sciences 22, no. 8: 3860. https://doi.org/10.3390/ijms22083860
APA StyleRen, E., & Curia, G. (2021). Synaptic Reshaping and Neuronal Outcomes in the Temporal Lobe Epilepsy. International Journal of Molecular Sciences, 22(8), 3860. https://doi.org/10.3390/ijms22083860






