PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis
Abstract
1. Introduction
2. Results
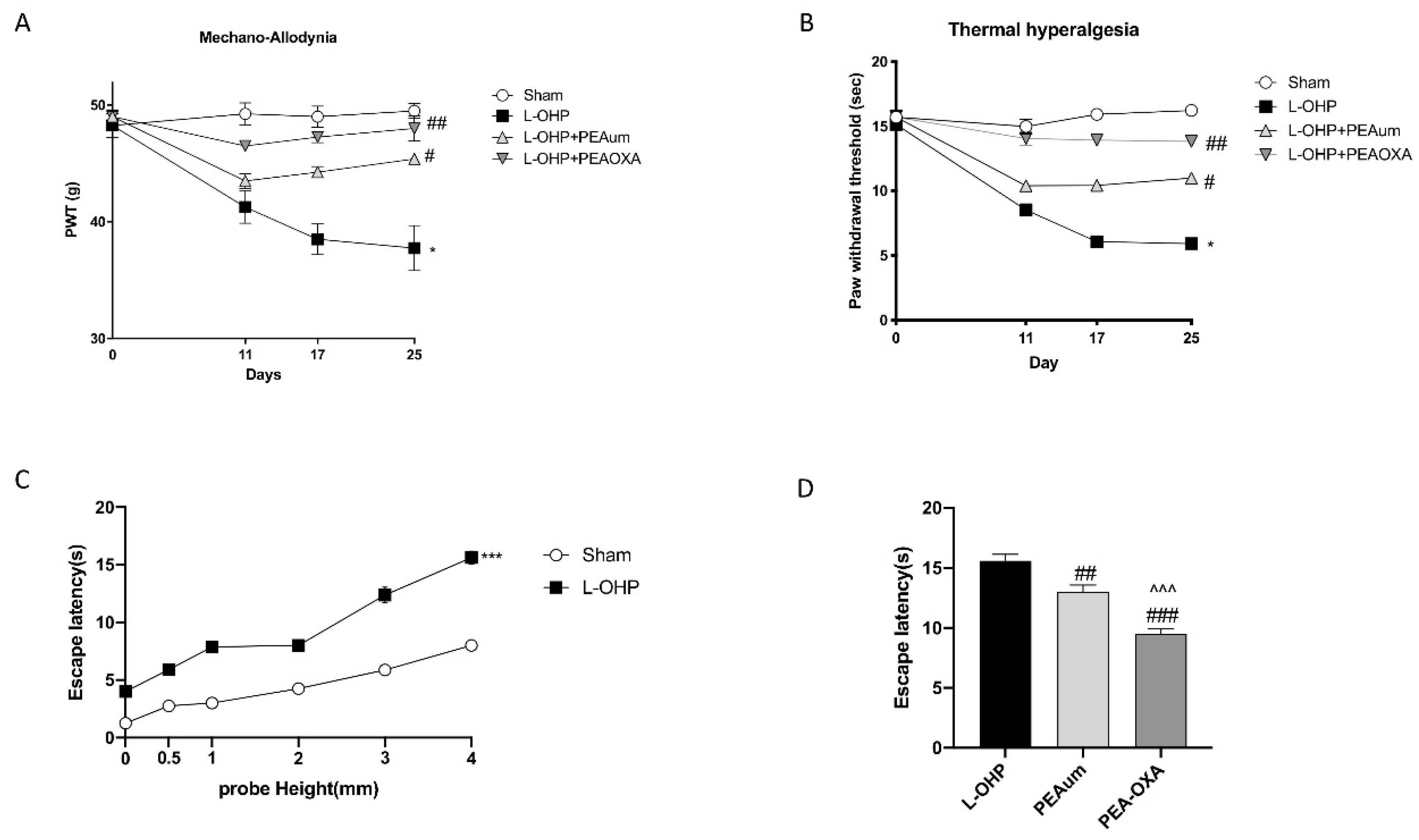
2.1. PEA-OXA Prevented Mechano-Allodynia and Thermal Hyperalgesia and Suppressed OIPN Symptoms in an Operant-Based Mechanical Conflict System (MCS)
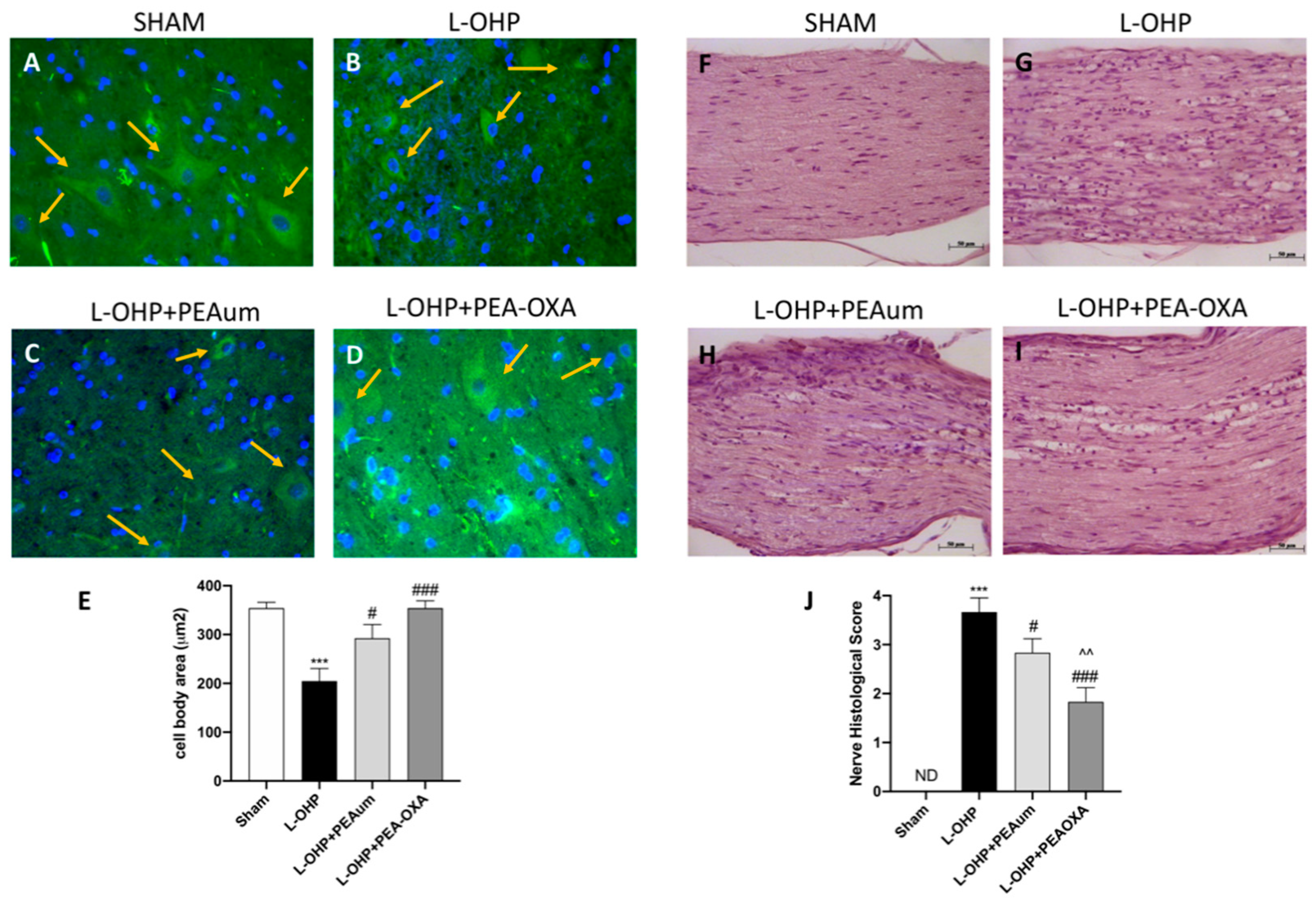
2.2. PEA-OXA Preserved Neuronal Morphological Change and Sciatic Nerve Damage Following L-OHP Injection
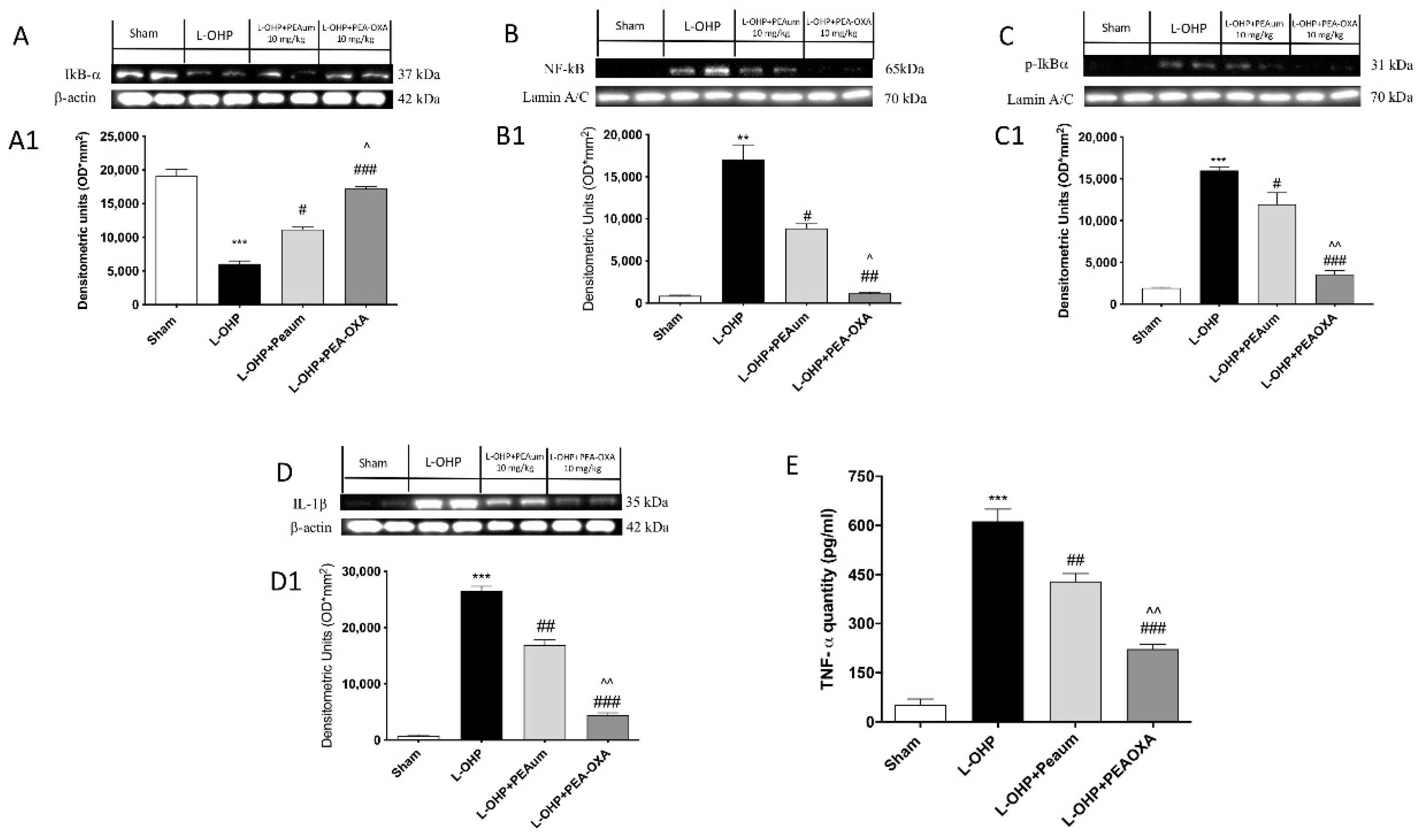
2.3. Effect of PEA-OXA on Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB) Pathway and Proinflammatory Cytokines Induced by L-OHP Injection
2.4. PEA-OXA Treatment Stimulated Antioxidant Response in the Lumbar Spinal Cord
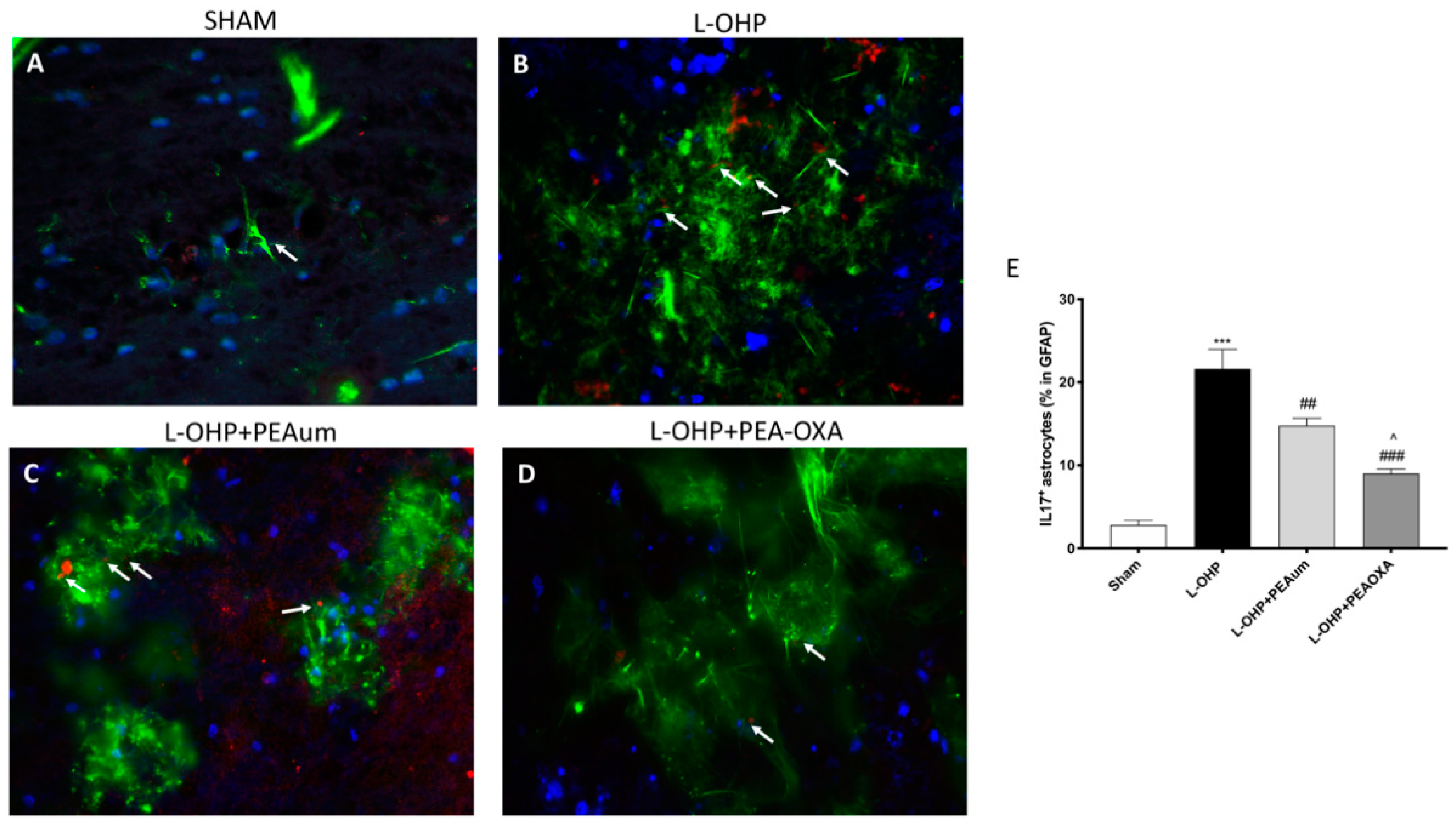
2.5. PEA-OXA Reduced Astrogliosis and IL-17 Production in the Lumbar Spinal Cord
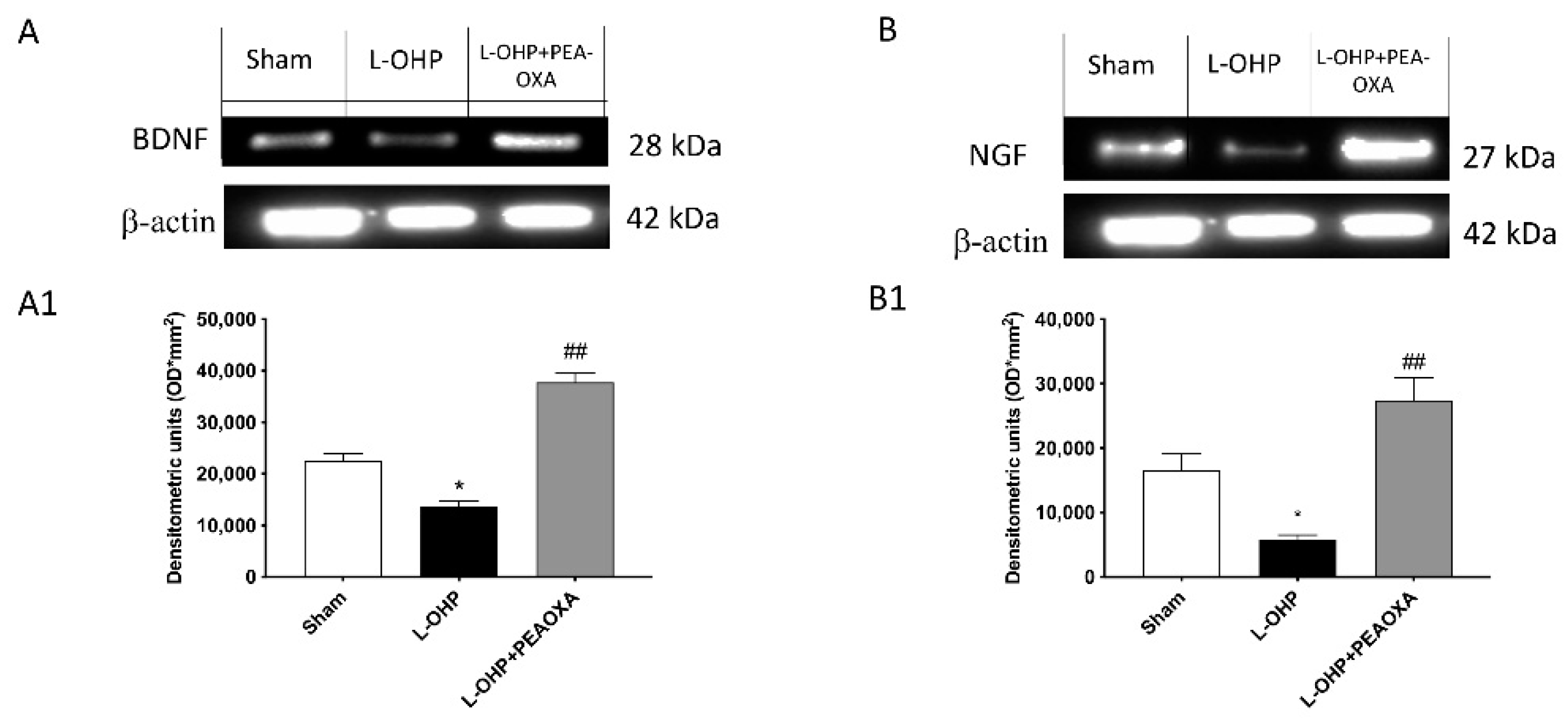
2.6. PEA-OXA Stimulates Growth Factor Expression in the DRG
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Animals
4.3. OIPN Model
Experimental Groups
- (1)
- Sham + vehicle (veh): rats received saline orally (n = 20);
- (2)
- Sham + PEAum: rats received PEAum (10 mg/kg) orally (n = 20);
- (3)
- Sham + PEA-OXA: rats received PEA-OXA (10 mg/kg) orally (n = 20);
- (4)
- L-OHP: rats received L-OHP (10 mg/kg) intraperitoneally (n = 20);
- (5)
- L-OHP + PEAum: rats received PEAum orally at the dose of 10 mg/kg 20 min before OXA injection (n = 20);
- (6)
- L-OHP + PEA-OXA: rats received PEA-OXA orally at the dose of 10 mg/kg 20 min before OXA injection (n = 20).
4.4. Synthesis of PEA and PEA-OXA
4.5. Behavioral Tests
Mechanical Conflict System (MCS) for Drug Assessment
4.6. Histology
4.7. Immunofluorescence of β-III Tubulin, GFAP, and IL-17
4.8. Western Blot Analysis for IκB-α, NF-κB, p-IκB α IL-1β, HO-1, MnSOD, Nrf-2, BDNF, and NGF
4.9. DRG Dissection
4.10. ELISA Kit for TNF-α
4.11. Measurement of GSH
4.12. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutierrez-Gutierrez, G.; Sereno, M.; Miralles, A.; Casado-Saenz, E.; Gutierrez-Rivas, E. Chemotherapy-induced peripheral neuropathy: Clinical features, diagnosis, prevention and treatment strategies. Clin. Transl. Oncol. 2010, 12, 81–91. [Google Scholar] [CrossRef]
- Ferrier, J.; Pereira, V.; Busserolles, J.; Authier, N.; Balayssac, D. Emerging trends in understanding chemotherapy-induced peripheral neuropathy. Curr. Pain Headache Rep. 2013, 17, 364. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Kocot-Kepska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Donvito, G.; Wilkerson, J.L.; Damaj, M.I.; Lichtman, A.H. Palmitoylethanolamide Reverses Paclitaxel-Induced Allodynia in Mice. J. Pharmacol. Exp. Ther. 2016, 359, 310–318. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol. Neurobiol. 2013, 48, 340–352. [Google Scholar] [CrossRef]
- Nestmann, E.R. Safety of micronized palmitoylethanolamide (microPEA): Lack of toxicity and genotoxic potential. Food Sci. Nutr. 2017, 5, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.K.; Kopsky, D.J. Palmitoylethanolamide, a neutraceutical, in nerve compression syndromes: Efficacy and safety in sciatic pain and carpal tunnel syndrome. J. Pain Res. 2015, 8, 729–734. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Crupi, R.; Pascali, J.; Alfonsi, D.; Marcolongo, G.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline: Identification in coffee, synthesis and activity in a rat model of carrageenan-induced hindpaw inflammation. Pharmacol. Res. 2016, 108, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Takezaki, N.; Ueda, N. The N-acylethanolamine-hydrolyzing acid amidase (NAAA). Chem. Biodivers. 2007, 4, 1914–1925. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 264. [Google Scholar] [CrossRef]
- Petrosino, S.; Campolo, M.; Impellizzeri, D.; Paterniti, I.; Allara, M.; Gugliandolo, E.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Esposito, E.; et al. 2-Pentadecyl-2-Oxazoline, the Oxazoline of Pea, Modulates Carrageenan-Induced Acute Inflammation. Front. Pharmacol. 2017, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Draberova, E.; Lukas, Z.; Ivanyi, D.; Viklicky, V.; Draber, P. Expression of class III beta-tubulin in normal and neoplastic human tissues. Histochem. Cell Biol. 1998, 109, 231–239. [Google Scholar] [CrossRef]
- Katsetos, C.D.; Legido, A.; Perentes, E.; Mörk, S.J. Class III β-tubulin isotype: A key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J. Child Neurol. 2003, 18, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Cavaletti, G.; Briani, C.; Velasco, R.; Bruna, J.; Campagnolo, M.; Alberti, P.; Bergamo, F.; Cortinovis, D.; Cazzaniga, M.; et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: A prospective study in 170 patients with colorectal cancer. Cancer 2013, 119, 438–444. [Google Scholar] [CrossRef]
- Kober, K.M.; Olshen, A.; Conley, Y.P.; Schumacher, M.; Topp, K.; Smoot, B.; Mazor, M.; Chesney, M.; Hammer, M.; Paul, S.M.; et al. Expression of mitochondrial dysfunction-related genes and pathways in paclitaxel-induced peripheral neuropathy in breast cancer survivors. Mol. Pain 2018, 14, 1744806918816462. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-induced neuropathy: Oxidative stress as pathological mechanism. Protective effect of silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Su, Z.; Wang, H.; Pang, X. Involvement of pro-inflammation signal pathway in inhibitory effects of rapamycin on oxaliplatin-induced neuropathic pain. Mol. Pain 2018, 14, 1744806918769426. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Pacini, A.; Corti, F.; Boccella, S.; Luongo, L.; Esposito, E.; Cuzzocrea, S.; Maione, S.; Calignano, A.; Ghelardini, C. Antineuropathic profile of N-palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity. PLoS ONE 2015, 10, e0128080. [Google Scholar] [CrossRef]
- Boccella, S.; Guida, F.; Iannotta, M.; Iannotti, F.A.; Infantino, R.; Ricciardi, F.; Cristiano, C.; Vitale, R.M.; Amodeo, P.; Marabese, I.; et al. 2-Pentadecyl-2-oxazoline ameliorates memory impairment and depression-like behaviour in neuropathic mice: Possible role of adrenergic alpha2- and H3 histamine autoreceptors. Mol. Brain 2021, 14, 28. [Google Scholar] [CrossRef]
- Iannotta, M.; Belardo, C.; Trotta, M.C.; Iannotti, F.A.; Vitale, R.M.; Maisto, R.; Boccella, S.; Infantino, R.; Ricciardi, F.; Mirto, B.F.; et al. N-palmitoyl-D-glucosamine, a Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice. Int. J. Mol. Sci. 2021, 22, 1491. [Google Scholar] [CrossRef]
- Seol, T.K.; Lee, W.; Park, S.; Kim, K.N.; Kim, T.Y.; Oh, Y.N.; Jun, J.H. Effect of palmitoylethanolamide on inflammatory and neuropathic pain in rats. Korean J. Anesthesiol. 2017, 70, 561–566. [Google Scholar] [CrossRef]
- Zhou, P.; Xiang, L.; Yang, Y.; Wu, Y.; Hu, T.; Liu, X.; Lin, F.; Xiu, Y.; Wu, K.; Lu, C.; et al. N-Acylethanolamine acid amidase (NAAA) inhibitor F215 as a novel therapeutic agent for osteoarthritis. Pharmacol. Res. 2019, 145, 104264. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. N-Palmitoylethanolamide-Oxazoline Protects against Middle Cerebral Artery Occlusion Injury in Diabetic Rats by Regulating the SIRT1 Pathway. Int. J. Mol. Sci. 2019, 20, 4845. [Google Scholar] [CrossRef]
- Boccella, S.; Iannotta, M.; Cristiano, C.; Iannotti, F.A.; Bello, F.D.; Guida, F.; Belardo, C.; Infantino, R.; Ricciardi, F.; Giannella, M.; et al. Treatment With 2-Pentadecyl-2-Oxazoline Restores Mild Traumatic Brain Injury-Induced Sensorial and Neuropsychiatric Dysfunctions. Front. Pharmacol. 2020, 11, 91. [Google Scholar] [CrossRef]
- Janes, K.; Little, J.W.; Li, C.; Bryant, L.; Chen, C.; Chen, Z.; Kamocki, K.; Doyle, T.; Snider, A.; Esposito, E.; et al. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J. Biol. Chem. 2014, 289, 21082–21097. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Chemosensory changes experienced by patients undergoing cancer chemotherapy: A qualitative interview study. J. Pain Symptom Manag. 2007, 34, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.; Hekker, T.A. Therapeutic utility of palmitoylethanolamide in the treatment of neuropathic pain associated with various pathological conditions: A case series. J. Pain Res. 2012, 5, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, A.; Meregalli, C.; Rodriguez-Menendez, V.; Nicolini, G. Chemotherapy-Induced Peripheral Neuropathy and Changes in Cytoskeleton. Int. J. Mol. Sci. 2019, 20, 2287. [Google Scholar] [CrossRef] [PubMed]
- Lebok, P.; Ozturk, M.; Heilenkotter, U.; Jaenicke, F.; Muller, V.; Paluchowski, P.; Geist, S.; Wilke, C.; Burandt, E.; Lebeau, A.; et al. High levels of class III beta-tubulin expression are associated with aggressive tumor features in breast cancer. Oncol. Lett. 2016, 11, 1987–1994. [Google Scholar] [CrossRef]
- Renn, C.L.; Carozzi, V.A.; Rhee, P.; Gallop, D.; Dorsey, S.G.; Cavaletti, G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Mol. Pain 2011, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Wafai, L.; Taher, M.; Jovanovska, V.; Bornstein, J.C.; Dass, C.R.; Nurgali, K. Effects of oxaliplatin on mouse myenteric neurons and colonic motility. Front. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.A.; Walters, E.T.; Dougherty, P.M. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015, 5, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.; Saleh, S.; El Abhar, H.; Saad, A.S.; Schaalan, M. L-Carnosine protects against Oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: A perspective on targeting Nrf-2 and NF-kappaB pathways. Toxicol. Appl. Pharmacol. 2019, 365, 41–50. [Google Scholar] [CrossRef]
- Kroigard, T.; Metaxas, A.; Wirenfeldt, M.; Finsen, B. Protective effect of ibuprofen in a rat model of chronic oxaliplatin-induced peripheral neuropathy. Exp. Brain Res. 2019, 237, 2645–2651. [Google Scholar] [CrossRef]
- Stubgen, J.P. Tumor necrosis factor-alpha antagonists and neuropathy. Muscle Nerve 2008, 37, 281–292. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. N-Palmitoylethanolamine-Oxazoline as a New Therapeutic Strategy to Control Neuroinflammation: Neuroprotective Effects in Experimental Models of Spinal Cord and Brain Injury. J. Neurotrauma 2017, 34, 2609–2623. [Google Scholar] [CrossRef]
- Janes, K.; Wahlman, C.; Little, J.W.; Doyle, T.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav. Immun. 2015, 44, 91–99. [Google Scholar] [CrossRef]
- Xiao, W.H.; Zheng, H.; Bennett, G.J. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 2012, 203, 194–206. [Google Scholar] [CrossRef]
- McDonald, E.S.; Windebank, A.J. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol. Dis. 2002, 9, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free. Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Schmelzer, J.D.; Bieber, A.J.; Loprinzi, C.L.; Sieck, G.C.; Brederson, J.D.; Low, P.A.; Windebank, A.J. A novel and selective poly (ADP-ribose) polymerase inhibitor ameliorates chemotherapy-induced painful neuropathy. PLoS ONE 2013, 8, e54161. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Nair, S.; Doh, S.T.; Chan, J.Y.; Kong, A.N.; Cai, L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br. J. Cancer 2008, 99, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Kumar, A.; Sharma, S.S. Nrf2 and NF-kappaB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovascular Res. 2011, 8, 294–304. [Google Scholar] [CrossRef]
- Cascinu, S.; Catalano, V.; Cordella, L.; Labianca, R.; Giordani, P.; Baldelli, A.M.; Beretta, G.D.; Ubiali, E.; Catalano, G. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: A randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2002, 20, 3478–3483. [Google Scholar] [CrossRef]
- Hamers, F.P.; Brakkee, J.H.; Cavalletti, E.; Tedeschi, M.; Marmonti, L.; Pezzoni, G.; Neijt, J.P.; Gispen, W.H. Reduced glutathione protects against cisplatin-induced neurotoxicity in rats. Cancer Res. 1993, 53, 544–549. [Google Scholar]
- Noma, N.; Khan, J.; Chen, I.F.; Markman, S.; Benoliel, R.; Hadlaq, E.; Imamura, Y.; Eliav, E. Interleukin-17 levels in rat models of nerve damage and neuropathic pain. Neurosci. Lett. 2011, 493, 86–91. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Lao, L.; Saito, R.; Li, A.; Backman, C.M.; Berman, B.M.; Ren, K.; Wei, P.K.; Zhang, R.X. Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain 2013, 154, 294–305. [Google Scholar] [CrossRef]
- Luo, H.; Liu, H.Z.; Zhang, W.W.; Matsuda, M.; Lv, N.; Chen, G.; Xu, Z.Z.; Zhang, Y.Q. Interleukin-17 Regulates Neuron-Glial Communications, Synaptic Transmission, and Neuropathic Pain after Chemotherapy. Cell Rep. 2019, 29, 2384–2397. [Google Scholar] [CrossRef]
- Cavaletti, G.; Bogliun, G.; Marzorati, L.; Zincone, A.; Piatti, M.; Colombo, N.; Franchi, D.; La Presa, M.T.; Lissoni, A.; Buda, A.; et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann. Oncol. 2004, 15, 1439–1442. [Google Scholar] [CrossRef]
- Yan, M.; Li, Y.; Zeng, H.; Zhao, X.; Wu, H.; Qian, W.; Guo, X. The effect of rat nerve growth factor combined with vitamin B on peripheral neuropathy in multiple myeloma patients. Hematology 2020, 25, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, Y.; Kamiya, Y.; Funakoshi, K.; Miyazaki, T.; Uchimoto, K.; Tojo, K.; Ogawa, K.; Fukuoka, T.; Goto, T. Role of nerve growth factor-tyrosine kinase receptor A signaling in paclitaxel-induced peripheral neuropathy in rats. Biochem. Biophys. Res. Commun. 2014, 444, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Petruccioli, M.G.; Marmiroli, P.; Rigolio, R.; Galbiati, S.; Zoia, C.; Ferrarese, C.; Tagliabue, E.; Dolci, C.; Bayssas, M.; et al. Circulating nerve growth factor level changes during oxaliplatin treatment-induced neurotoxicity in the rat. Anticancer Res. 2002, 22, 4199–4204. [Google Scholar]
- Szudy-Szczyrek, A.; Mlak, R.; Bury-Kaminska, M.; Mielnik, M.; Podgajna, M.; Kusmierczuk, K.; Mazurek, M.; Homa-Mlak, I.; Szczyrek, M.; Krawczyk, J.; et al. Serum brain-derived neurotrophic factor (BDNF) concentration predicts polyneuropathy and overall survival in multiple myeloma patients. Br. J. Haematol. 2020, 191, 77–89. [Google Scholar] [CrossRef]
- Azoulay, D.; Horowitz, N.A. Brain-derived neurotrophic factor as a potential biomarker of chemotherapy-induced peripheral neuropathy and prognosis in haematological malignancies; what we have learned, the challenges and a need for global standardization. Br. J. Haematol. 2020, 191, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cordaro, M.; Verde, R.; Moriello, A.S.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Ahmad, N.; Subhan, F.; Islam, N.U.; Shahid, M.; Rahman, F.U.; Sewell, R.D.E. Gabapentin and its salicylaldehyde derivative alleviate allodynia and hypoalgesia in a cisplatin-induced neuropathic pain model. Eur. J. Pharmacol. 2017, 814, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Allard, D.E.; Wang, Y.; Howard, J.F., Jr.; Montgomery, S.A.; Su, M.A. IL-10 Paradoxically Promotes Autoimmune Neuropathy through S1PR1-Dependent CD4(+) T Cell Migration. J. Immunol. 2018, 200, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campolo, M.; Lanza, M.; Paterniti, I.; Filippone, A.; Ardizzone, A.; Casili, G.; Scuderi, S.A.; Puglisi, C.; Mare, M.; Memeo, L.; et al. PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis. Int. J. Mol. Sci. 2021, 22, 3927. https://doi.org/10.3390/ijms22083927
Campolo M, Lanza M, Paterniti I, Filippone A, Ardizzone A, Casili G, Scuderi SA, Puglisi C, Mare M, Memeo L, et al. PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis. International Journal of Molecular Sciences. 2021; 22(8):3927. https://doi.org/10.3390/ijms22083927
Chicago/Turabian StyleCampolo, Michela, Marika Lanza, Irene Paterniti, Alessia Filippone, Alessio Ardizzone, Giovanna Casili, Sarah A. Scuderi, Caterina Puglisi, Marzia Mare, Lorenzo Memeo, and et al. 2021. "PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis" International Journal of Molecular Sciences 22, no. 8: 3927. https://doi.org/10.3390/ijms22083927
APA StyleCampolo, M., Lanza, M., Paterniti, I., Filippone, A., Ardizzone, A., Casili, G., Scuderi, S. A., Puglisi, C., Mare, M., Memeo, L., Cuzzocrea, S., & Esposito, E. (2021). PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis. International Journal of Molecular Sciences, 22(8), 3927. https://doi.org/10.3390/ijms22083927











