Targeting the Stromal Pro-Tumoral Hyaluronan-CD44 Pathway in Pancreatic Cancer
Abstract
:1. Introduction
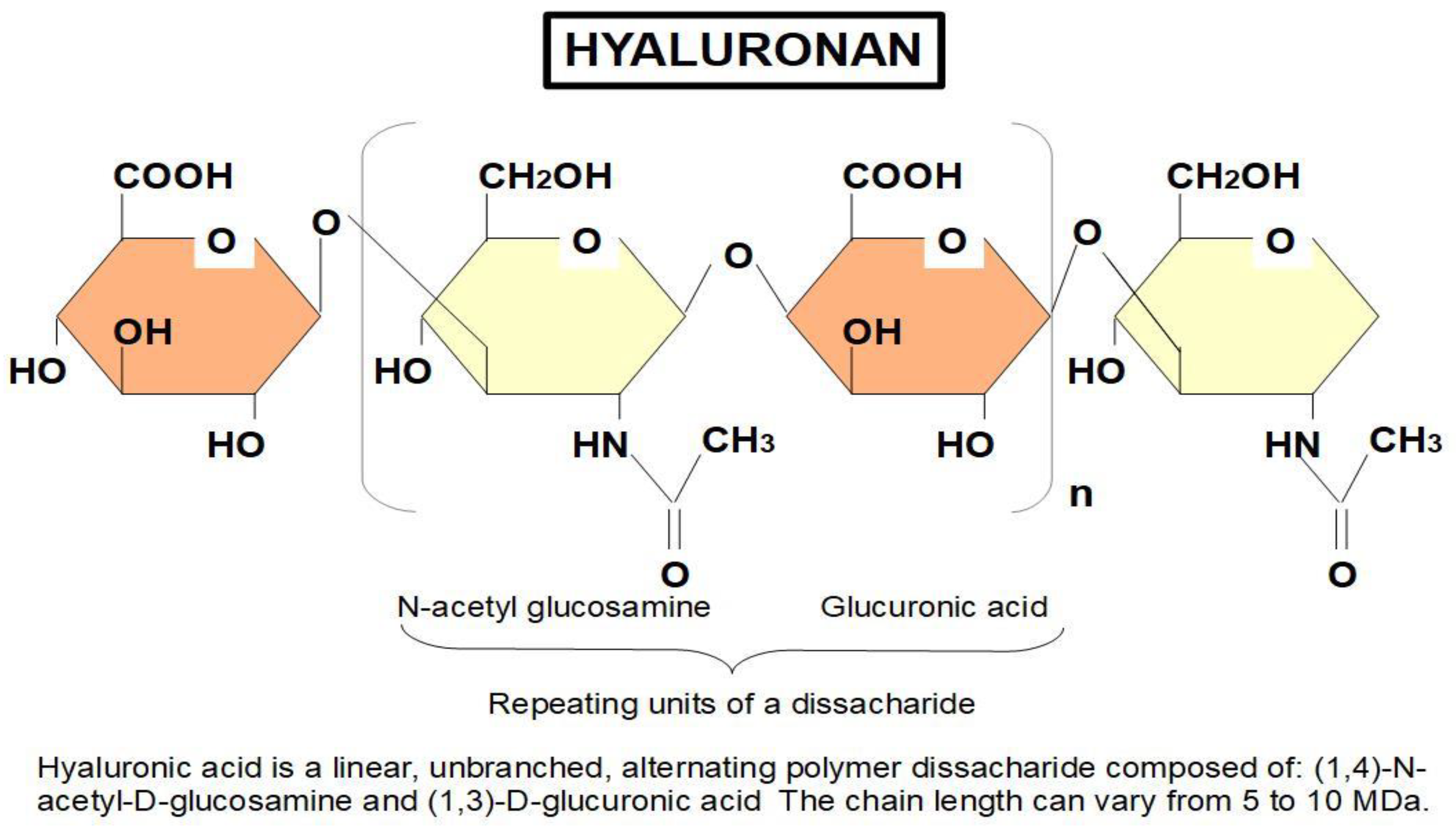
2. Hyaluronan
- (1)
- Structural
- (2)
- Signaling
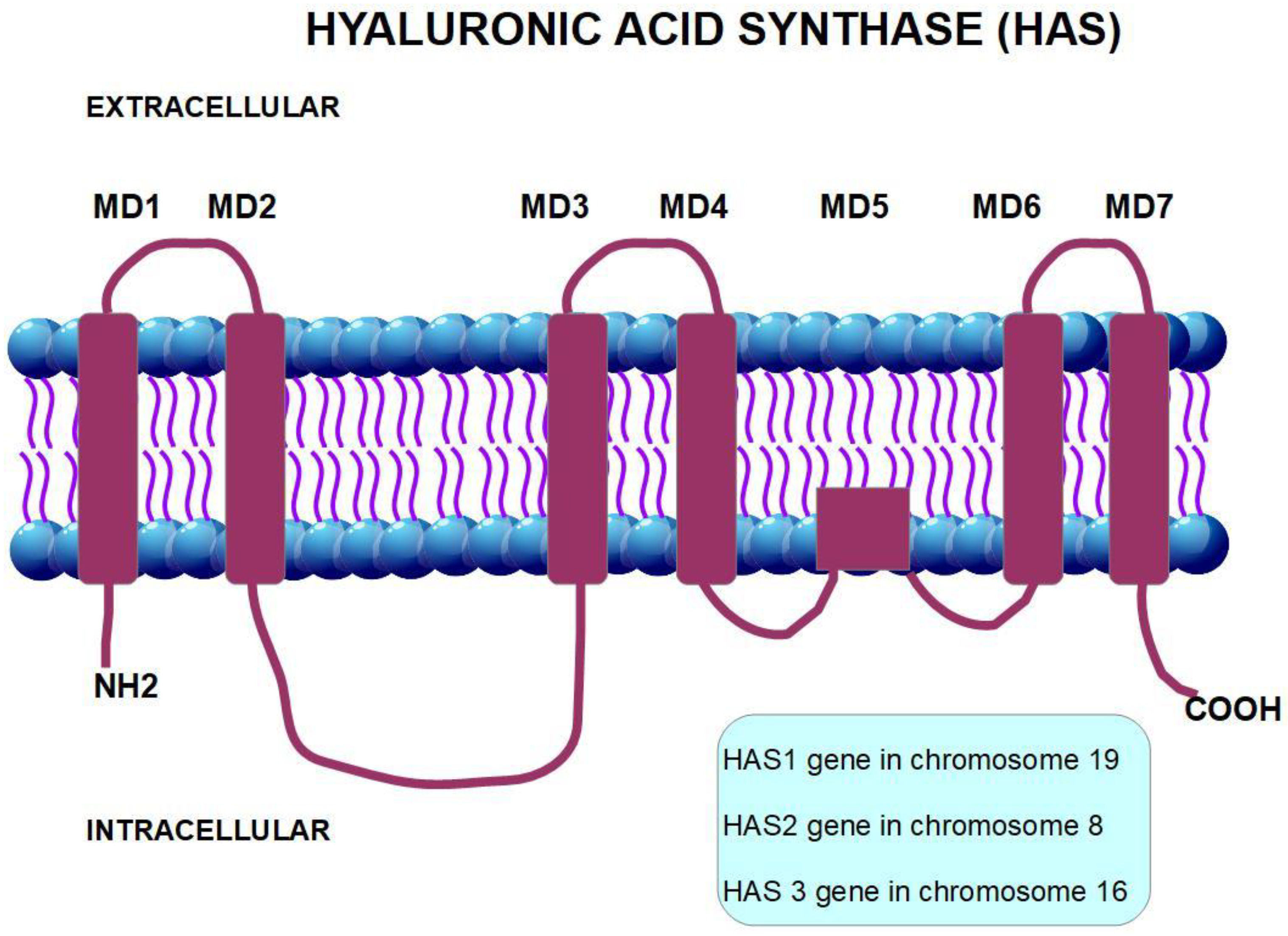
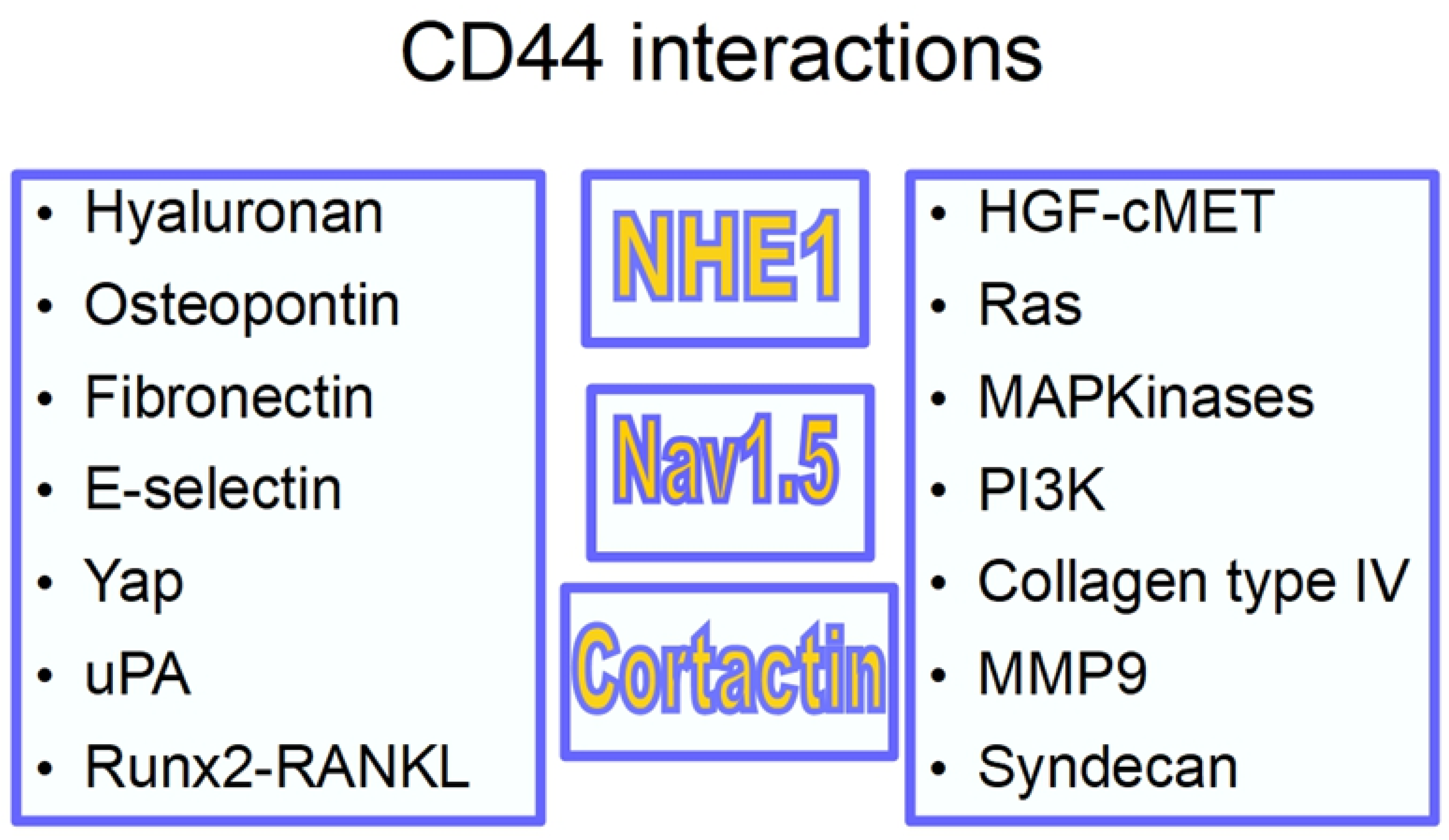
3. The CD44 Antigen
- (1)
- An extracellular amino-terminal domain to which hyaluronan binds activating signaling. The extracellular domain is also a binding site for other adhesion molecules such as E-selectin and fibronectin.
- (2)
- A transmembrane single spanning domain.
- (3)
- A short carboxy-terminal intracellular domain responsible for the signaling activity.
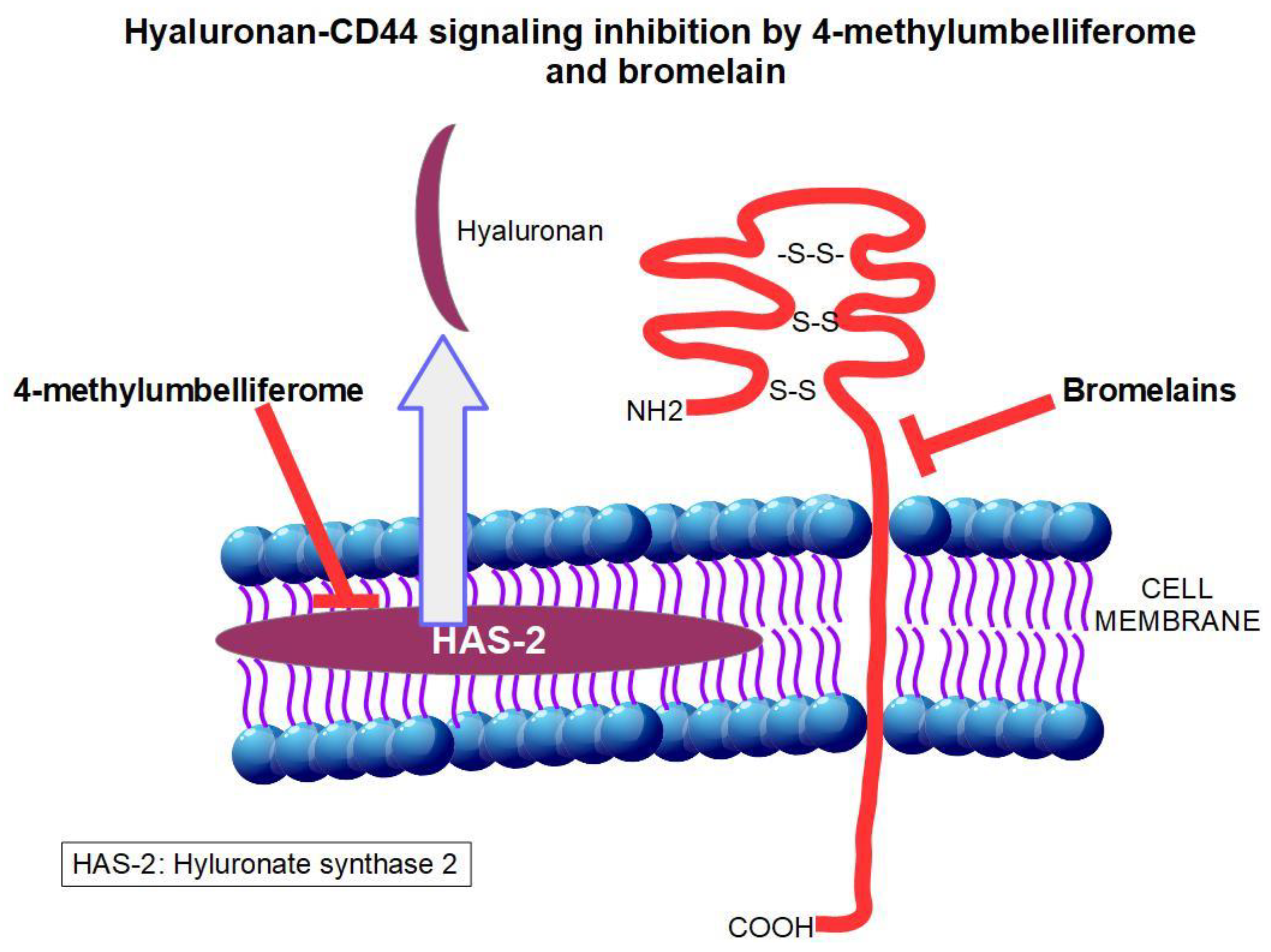
3.1. Bromelain (BRO)
Bioavailability
3.2. 4-Methylumbelliferone (Hymecromone)
3.3. Pirfenidone
- increased hypoxia with its pro-tumoral consequences and;
- inability of chemotherapeutic drugs to reach their target cells.
- (a)
- inhibits collagen fibrils assembly [200];
- (b)
- down-regulates the intercellular adhesion molecule-1 (ICAM1) [201];
- (c)
- (d)
- down-regulates the pro-fibrotic hedgehog signaling pathway [204];
- (e)
- decreases fibroblast proliferation [205];
- (f)
- blocks myofibroblast differentiation [206];
- (g)
- suppresses tumor necrosis factor alpha (TNFα) [207];
- (h)
- decreases cell migration-inducing and hyaluronan-binding protein [208];
- (i)
- inhibits MUC1 [209].
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Provenzano, P.P.; Hingorani, S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibi-tion of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, J.; Korc, M. Pancreatic Cancer Stroma: Friend or Foe? Cancer Cell 2014, 25, 711–712. [Google Scholar] [CrossRef] [Green Version]
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Wilson, J.S. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Apte, M.V.; Wilson, J.S.; Lugea, A.; Pandol, S.J. A starring role for stellate cells in the pancreatic cancer microen-vironment. Gastroenterology 2013, 144, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Pandol, S.; Gukovskaya, A.; Edderkaoui, M.; Dawson, D.; Eibl, G.; Lugea, A. Epidemiology, risk factors, and the promotion of pancreatic cancer: Role of the stellate cell. J. Gastroenterol. Hepatol. 2012, 27, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangi-Garimella, S.; Krantz, S.B.; Barron, M.R.; Shields, M.A.; Heiferman, M.J.; Grippo, P.J.; Bentrem, D.J.; Munshi, H.G. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA. Cancer Res. 2011, 71, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Mollenhauer, J.; Roether, I.; Kern, H.F. Distribution of extracellular matrix proteins in pancreatic ductal adenocar-cinoma and its influence on tumor cell proliferation in vitro. Pancreas 1987, 2, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.F.; Moore, T.; Arumugam, T.; Ramachandran, V.; Amos, K.D.; Rivera, A.; Ji, B.; Evans, D.B.; Logsdon, C.D. Cancer-Associated Stromal Fibroblasts Promote Pancreatic Tumor Progression. Cancer Res. 2008, 68, 918–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procacci, P.; Moscheni, C.; Sartori, P.; Sommariva, M.; Gagliano, N. Tumor–Stroma Cross-Talk in Human Pancreatic Ductal Adenocarcinoma: A Focus on the Effect of the Extracellular Matrix on Tumor Cell Phenotype and Invasive Potential. Cells 2018, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Chen, K.; Jiang, Z.; Chen, X.; Sun, L.; Li, J.; Lei, J.; Xu, Q.; Ma, J.; Li, X.; et al. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett. 2017, 385, 225–233. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef] [Green Version]
- Biondani, G.; Zeeberg, K.; Greco, M.R.; Cannone, S.; Dando, I.; Pozza, E.D.; Mastrodonato, M.; Forciniti, S.; Casavola, V.; Palmieri, M.; et al. Extracellular matrix composition modulates PDAC parenchymal and stem cell plasticity and behavior through the secretome. FEBS J. 2018, 285, 2104–2124. [Google Scholar] [CrossRef] [Green Version]
- Begum, A.; Ewachiw, T.; Jung, C.; Huang, A.; Norberg, K.J.; Marchionni, L.; McMillan, R.; Penchev, V.; RajeshKumar, N.V.; Maitra, A.; et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS ONE 2017, 12, e0180181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancato, V.; Comunanza, V.; Imparato, G.; Corà, D.; Urciuolo, F.; Noghero, A.; Netti, P.A. Bioengineered tu-moral microtissues recapitulate desmoplastic reaction of pancreatic cancer. Acta Biomater. 2017, 49, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.J.; Keshishian, V.; Law, J.J.; Ho, J.C.; Favela, C.A.; Rees, P.; Smith, B.; Mohammad, S.; Hwang, R.F.; Rajapakshe, K.; et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials 2016, 108, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahlbacher, V.; Sewing, A.; Elsässer, H.P.; Kern, H.F. Hyaluronan is a secretory product of human pancreatic adenocarcinoma cells. Eur. J. Cell Biol. 1992, 58, 28–34. [Google Scholar]
- Mantoni, T.S.; Lunardi, S.; Al-Assar, O.; Masamune, A.; Brunner, T.B. Pancreatic stellate cells radioprotect pan-creatic cancer cells through β1-integrin signaling. Cancer Res. 2011, 71, 3453–3458. [Google Scholar] [CrossRef] [Green Version]
- Vonlaufen, A.; Phillips, P.A.; Xu, Z.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Pancreatic Stellate Cells and Pancreatic Cancer Cells: An Unholy Alliance: Figure 1. Cancer Res. 2008, 68, 7707–7710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, Y.; Masugi, Y.; Abe, T.; Yamazaki, K.; Ueno, A.; Fujii-Nishimura, Y.; Hori, S.; Yagi, H.; Abe, Y.; Kitago, M.; et al. Three Distinct Stroma Types in Human Pancreatic Cancer Identified by Image Analysis of Fibroblast Subpopulations and Collagen. Clin. Cancer Res. 2021, 27, 107–119. [Google Scholar] [CrossRef]
- Helms, E.; Onate, M.K.; Sherman, M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020, 10, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, P.E.; Adoumie, M.; Kim, E.C.; Zhang, Y.; Scales, M.K.; El-Tawil, Y.S.; Shaikh, A.Z.; Wen, H.-J.; Bednar, F.; Allen, B.L.; et al. Differential Contribution of Pancreatic Fibroblast Subsets to the Pancreatic Cancer Stroma. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Drug Approval Package: Esbriet (Pirfenidone) Capsules NDA Nº022535 U.S.; Food and Drug Administration: Silver Spring, MD, USA, 1999.
- Shepard, H.M. Breaching the Castle Walls: Hyaluronan Depletion as a Therapeutic Approach to Cancer Therapy. Front. Oncol. 2015, 5, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Ma, Q.; Xu, Q.; Duan, W.; Lei, J.; Wu, E. Targeting the Cancer-Stroma Interaction: A Potential Approach for Pancreatic Cancer Treatment. Curr. Pharm. Des. 2012, 18, 2404–2415. [Google Scholar] [CrossRef] [Green Version]
- Kota, J.; Hancock, J.; Kwon, J.; Korc, M. Pancreatic cancer: Stroma and its current and emerging targeted therapies. Cancer Lett. 2017, 391, 38–49. [Google Scholar] [CrossRef]
- Rucki, A.A.; Zheng, L. Pancreatic cancer stroma: Understanding biology leads to new therapeutic strategies. World J. Gastroenterol. 2014, 20, 2237–2246. [Google Scholar] [CrossRef]
- Bahrami, A.; Khazaei, M.; Bagherieh, F.; Ghayour-Mobarhan, M.; Maftouh, M.; Hassanian, S.M.; Avan, A. Target-ing stroma in pancreatic cancer: Promises and failures of targeted therapies. J. Cell. Physiol. 2017, 232, 2931–2937. [Google Scholar] [CrossRef]
- Neesse, A.; Krug, S.; Gress, T.M.; Tuveson, D.A.; Michl, P. Emerging concepts in pancreatic cancer medicine: Tar-geting the tumor stroma. OncoTargets Ther. 2014, 7, 33. [Google Scholar]
- Waghray, M.; Yalamanchili, M.; Di Magliano, M.P.; Simeone, D.M. Deciphering the role of stroma in pancreatic cancer. Curr. Opin. Gastroenterol. 2013, 29, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Hou, Y.; Chen, X.; Zhang, P.; Kang, M.; Jin, Q.; Gao, M. Metformin-induced stromal depletion to en-hance the penetration of gemcitabine-loaded magnetic nanoparticles for pancreatic cancer targeted therapy. J. Am. Chem. Soc. 2020, 142, 4944–4954. [Google Scholar] [CrossRef]
- Kocher, H.M.; Basu, B.; Froeling, F.E.; Sarker, D.; Slater, S.; Carlin, D.; Propper, D.J. Phase I clinical trial repur-posing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Li, W.; Sun, L.; Lei, J.; Wu, Z.; Ma, Q.; Wang, Z. Curcumin inhibits pancreatic cancer cell invasion and EMT by interfering with tumor stromal crosstalk under hypoxic conditions via the IL 6/ERK/NF κB axis. Oncol. Rep. 2020, 44, 382–392. [Google Scholar] [CrossRef]
- Sharma, N.S.; Gupta, V.K.; Garrido, V.T.; Hadad, R.; Durden, B.C.; Kesh, K.; Giri, B.; Ferrantella, A.; Dudeja, V.; Saluja, A.; et al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J. Clin. Investig. 2019, 130, 451–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudson, C.B.; Knudson, W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 2001, 12, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Blundell, C.D.; Seyfried, N.T.; Day, A.J. Structural and Functional Diversity of Hyaluronan-Binding Proteins; Elsevier BV: Amsterdam, The Netherlands, 2004; pp. 189–204. [Google Scholar]
- McCarthy, J.B.; El-Ashry, D.; Turley, E.A. Hyaluronan, cancer-associated fibroblasts and the tumor microenvi-ronment in malignant progression. Front. Cell Dev. Biol. 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N.; Bollyky, P.L. 4-Methylumbelliferone Treatment and Hyaluronan Inhibition as a Therapeutic Strategy in Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Kohi, S.; Hirata, K.; Goggins, M. Role of hyaluronan in pancreatic cancer biology and therapy: Once again in the spotlight. Cancer Sci. 2016, 107, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P.; Hascall, V.C. Hyaluronan and Tumor Growth. Am. J. Pathol. 2002, 161, 745–747. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.-B.; Kohi, S.; Koga, A.; Hirata, K.; Sato, N. Hyaluronan stimulates pancreatic cancer cell motility. Oncotarget 2015, 7, 4829–4840. [Google Scholar] [CrossRef]
- Sironen, R.K.; Tammi, M.; Tammi, R.; Auvinen, P.K.; Anttila, M.; Kosma, V.M. Hyaluronan in human malignan-cies. Exp. Cell Res. 2011, 317, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Underhill, C.B.; Chen, L. Hyaluronan on the surface of tumor cells is correlated with metastatic behav-ior. Cancer Res. 1995, 55, 428–433. [Google Scholar]
- Toole, B.P. Hyaluronan promotes the malignant phenotype. Glycobiology 2002, 12, 37R–42R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in Tissue Injury and Repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef] [Green Version]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, T.C.; Laurent, U.B.; E. Fraser, J.R. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 1996, 74, a1–a7. [Google Scholar] [CrossRef] [PubMed]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Hall, C.L.; Wang, C.; Lange, L.; Turley, E. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J. Cell Biol. 1994, 126, 575–588. [Google Scholar] [CrossRef] [PubMed]
- McCourt, P.; Ek, B.; Forsberg, N.; Gustafson, S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J. Biol. Chem. 1994, 269, 30081–30084. [Google Scholar] [CrossRef]
- Hardwick, C.; Hoare, K.; Owens, R.; Hohn, H.P.; Hook, M.; Moore, D.; Cripps, V.; Austen, L.; Nance, D.M.; Turley, E. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J. Cell Biol. 1992, 117, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Ricciardelli, C.; Russell, D.L.; Ween, M.P.; Mayne, K.; Suwiwat, S.; Byers, S.; Horsfall, D.J. Formation of hyalu-ronan-and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J. Biol. Chem. 2007, 282, 10814–10825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collis, L.; Hall, C.; Lange, L.; Ziebell, M.; Prestwich, R.; Turley, E. Rapid hyaluronan uptake is associated with enhanced motility: Implications for an intracellular mode of action. FEBS Lett. 1998, 440, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Meran, S.; Thomas, D.W.; Stephens, P.; Enoch, S.; Martin, J.; Steadman, R.; Phillips, A.O. Hyaluronan Facilitates Transforming Growth Factor-β1-mediated Fibroblast Proliferation. J. Biol. Chem. 2008, 283, 6530–6545. [Google Scholar] [CrossRef] [Green Version]
- Slevin, M.; Krupinski, J.; Kumar, S.; Gaffney, J. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab. Investig. 1998, 78, 987–1003. [Google Scholar] [PubMed]
- Toole, B.P. Proteoglycans and Hyaluronan in Morphogenesis and Differentiation. In Cell Biology of Extracellular Matrix; Springer: Boston, MA, USA, 1991; pp. 305–341. [Google Scholar]
- Assmann, V.; Marshall, J.F.; Fieber, C.; Hofmann, M.; Hart, I.R. The human hyaluronan receptor RHAMM is ex-pressed as an intracellular protein in breast cancer cells. J. Cell Sci. 1998, 111, 1685–1694. [Google Scholar] [PubMed]
- Wang, C.; Thor, A.D.; Moore, D.H.; Zhao, Y.; Kerschmann, R.; Stern, R.; Turley, E.A. The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated pro-tein kinase and is a significant parameter in breast cancer progression. Clin. Cancer Res. 1998, 4, 567–576. [Google Scholar] [PubMed]
- Weigel, P.H.; Hascall, V.C.; Tammi, M. Hyaluronan Synthases. J. Biol. Chem. 1997, 272, 13997–14000. [Google Scholar] [CrossRef] [Green Version]
- Itano, N.; Kimata, K. Mammalian Hyaluronan Synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef]
- Price, Z.K.; Lokman, N.A.; Ricciardelli, C. Differing Roles of Hyaluronan Molecular Weight on Cancer Cell Behavior and Chemotherapy Resistance. Cancers 2018, 10, 482. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Liu, G.; Liu, H.; Han, J.; Liu, Z.; Ma, H. Molecular weight impact on the mechanical forces between hya-luronan and its receptor. Carbohydr. Polym. 2018, 197, 326–336. [Google Scholar] [CrossRef]
- Wolny, P.M.; Banerji, S.; Gounou, C.; Brisson, A.R.; Day, A.J.; Jackson, D.G.; Richter, R.P. Analysis of CD44-hyaluronan interactions in an artificial membrane system insights into the distinct binding properties of high and low molecu-lar weight hyaluronan. J. Biol. Chem. 2010, 285, 30170–30180. [Google Scholar] [CrossRef] [Green Version]
- Garg, H.G.; Hales, C.A. (Eds.) Methods for Analysis of Hyaluronan and Its Fragments; Chapter Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Mauri, P.; Scarpa, A.; Nascimbeni, A.C.; Benazzi, L.; Parmagnani, E.; Mafficini, A.; Della Peruta, M.; Bassi, C.; Miyazaki, K.; Sorio, C. Identification of proteins released by pancreatic cancer cells by multidimensional protein identification technology: A strategy for identification of novel cancer markers. FASEB J. 2005, 19, 1125–1127. [Google Scholar] [CrossRef]
- Yeung, T.L.; Leung, C.S.; Wong, K.K.; Samimi, G.; Thompson, M.S.; Liu, J.; Mok, S.C. TGF-β modulates ovari-an cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013, 73, 5016–5028. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; He, G.; Xu, G.; Wen, J.; Yu, X. miRNA-34a inhibits cell adhesion by targeting CD44 in human renal epi-thelial cells: Implications for renal stone disease. Urolithiasis 2019, 48, 1–8. [Google Scholar]
- Merzak, A.; Koocheckpour, S.; Pilkington, G.J. CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res. 1994, 54, 3988–3992. [Google Scholar]
- Hill, A.; McFarlane, S.; Mulligan, K.; Gillespie, H.; Draffin, J.; Trimble, A.; Ouhtit, A.; Johnston, P.G.; Harkin, D.P.; McCormick, D.; et al. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene 2006, 25, 6079–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohadwala, M.; Luo, J.; Zhu, L.; Lin, Y.; Dougherty, G.J.; Sharma, S.; Huang, M.; Põld, M.; Batra, R.K.; Dubinett, S.M. Non-small Cell Lung Cancer Cyclooxygenase-2-dependent Invasion Is Mediated by CD44. J. Biol. Chem. 2001, 276, 20809–20812. [Google Scholar] [CrossRef] [Green Version]
- Jothy, S. CD44 and its partners in metastasis. Clin. Exp. Metastasis 2003, 20, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Rutnam, Z.J.; Yang, B.B. The non-coding 3′ UTR of CD44 induces metastasis by regulating extracellular matrix functions. J. Cell Sci. 2012, 125, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Troness, B.; Spartz, A.; Sharma, U.; Miller, P.; Saenz, K.M.; Lippman, M.; El-Ashry, D. CD44 facilitates metasta-sis by promoting co-clustering of breast cancer cells and cancer associated fibroblasts. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Thorne, R.F.; Legg, J.W.; Isacke, C.M. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J. Cell Sci. 2019, 117, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Cao, W.; Chellaiah, M.A. Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-κB ligand signaling axis. Mol. Cancer 2012, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Suzuki, M.; Kanayama, N.; Nishida, T.; Takigawa, M.; Terao, T. CD44 stimulation by fragmented hyaluronic acid induces upregulation of urokinase-type plasminogen activator and its receptor and subsequently facilitates invasion of human chondrosarcoma cells. Int. J. Cancer 2002, 102, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Osada, H.; Murakami-Tonami, Y.; Horio, Y.; Hida, T.; Sekido, Y. Statin suppresses Hippo pathway-inactivated malignant mesothelioma cells and blocks the YAP/CD44 growth stimulatory axis. Cancer Lett. 2017, 385, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Ashkar, S.; Glimcher, M.J.; Cantor, H. Receptor-Ligand Interaction between CD44 and Osteopontin (Eta-1). Science 1996, 271, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Furger, K.A.; Menon, R.K.; Tuck, A.B.; Bramwell, V.H.; Chambers, A.F. The functional and clinical roles of oste-opontin in cancer and metastasis. Curr. Mol. Med. 2001, 1, 621–632. [Google Scholar] [CrossRef]
- Rittling, S.R.; Chambers, A.F. Role of osteopontin in tumour progression. Br. J. Cancer 2004, 90, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Guo, Y. Osteopontin induces angiogenesis through activa-tion of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Akutagawa, S.; Fukumoto, H.; Tsukiyama, S.; Ohe, Y.; Takahashi, K.; Nishio, K. Osteopontin in-duces angiogenesis of murine neuroblastoma cells in mice. Int. J. Cancer 2002, 98, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Knutson, J.R.; Iida, J.; Fields, G.B.; McCarthy, J.B. CD44/chondroitin sulfate proteoglycan and alpha 2 beta 1 in-tegrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol. Biol. Cell 1996, 7, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Jalkanen, M.; Elenius, K.; Salmivirta, M. Syndecan—A cell surface proteoglycan that selectively binds extracellular effector molecules. Heparin Relat. Polysacch. 1992, 313, 79–85. [Google Scholar]
- Sayyad, M.R.; Puchalapalli, M.; Vergara, N.G.; Wangensteen, S.M.; Moore, M.; Mu, L.; Edwards, C.; Anderson, A.; Kall, S.; Sullivan, M.; et al. Syndecan-1 facilitates breast cancer metastasis to the brain. Breast Cancer Res. Treat. 2019, 178, 35–49. [Google Scholar] [CrossRef]
- Conejo, J.R.; Kleeff, J.; Koliopanos, A.; Matsuda, K.; Zhu, Z.W.; Goecke, H.; Büchler, M.W. Syndecan-1 expres-sion is up-regulated in pancreatic but not in other gastrointestinal cancers. Int. J. Cancer 2000, 88, 12–20. [Google Scholar] [CrossRef]
- Yao, W.; Rose, J.L.; Wang, W.; Seth, S.; Jiang, H.; Taguchi, A.; Liu, J.; Yan, L.; Kapoor, A.; Hou, P.; et al. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nat. Cell Biol. 2019, 568, 410–414. [Google Scholar] [CrossRef]
- Miletti-González, K.E.; Murphy, K.; Kumaran, M.N.; Ravindranath, A.K.; Wernyj, R.P.; Kaur, S.; Rodríguez-Rodríguez, L. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): Modulation of matrix metal-loproteinase 9 (MMP-9) transcription via novel promoter response element. J. Biol. Chem. 2012, 287, 18995–19007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-S.; Su, C.-H.; Kuo, C.-C.; Shaw, C.-F.; Peng, S.-T. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int. J. Oncol. 2007, 31, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, L.Y.; Singleton, P.A.; Diedrich, F.; Stern, R.; Gilad, E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell inva-sion. J. Biol. Chem. 2004, 279, 26991–27007. [Google Scholar] [CrossRef]
- Suleiman, M.; Abdulrahman, N.; Yalcin, H.; Mraiche, F. The role of CD44, hyaluronan and NHE1 in cardiac remodeling. Life Sci. 2018, 209, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Wang, J.; Zhang, H.; Zhang, Y.; Wang, C.; Xu, H.; Pang, T. CD44 targets Na(+)/H(+) exchanger 1 to mediate MDA-MB-231 cells’ metastasis via the regulation of ERK1/2. Br. J. Cancer 2014, 110, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Stock, C.; Cardone, R.A.; Busco, G.; Krähling, H.; Schwab, A.; Reshkin, S.J. Protons extruded by NHE1: Digestive or glue? Eur. J. Cell Biol. 2008, 87, 591–599. [Google Scholar] [CrossRef]
- Nelson, M.; Yang, M.; Millican-Slater, R.; Brackenbury, W.J. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget 2015, 6, 32914–32929. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; James, A.D.; Suman, R.; Kasprowicz, R.; Nelson, M.; O’Toole, P.J.; Brackenbury, W.J. Volt-age-dependent activation of Rac1 by Nav1. 5 channels promotes cell migration. J. Cell. Physiol. 2020, 235, 3950–3972. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Xu, Y.; Wei, Y.; Qiu, Q.; Chew, T.-L.; Kang, Y.; Cheng, C. The CD44s splice isoform is a central mediator for invadopodia activity. J. Cell Sci. 2016, 129, 1355–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, A.M. Invadopodia: Specialized Cell Structures for Cancer Invasion. Clin. Exp. Metastasis 2006, 23, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, C.; Guichet, P.-O.; Masliantsev, K.; Wager, M.; Karayan-Tapon, L. Functional invadopodia formed in glioblastoma stem cells are important regulators of tumor angiogenesis. Oncotarget 2018, 9, 20640–20657. [Google Scholar] [CrossRef] [Green Version]
- Gould, C.M.; Courtneidge, S. Regulation of invadopodia by the tumor microenvironment. Cell Adhes. Migr. 2014, 8, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisson, L.; Driffort, V.; Benoist, L.; Poet, M.; Counillon, L.; Antelmi, E.; Roger, S. NaV1. 5 Na+ channels allosteri-cally regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013, 126, 4835–4842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddy, R.J.; Weidmann, M.D.; Sharma, V.P.; Condeelis, J.S. Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends Cell Biol. 2017, 27, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wu, T.; Wu, W.; Chen, G.; Luo, X.; Jiang, L.; Tao, H.; Rong, M.; Kang, S.; Deng, M. The Functional Role of Voltage-Gated Sodium Channel Nav1.5 in Metastatic Breast Cancer. Front. Pharmacol. 2020, 11, 1111. [Google Scholar] [CrossRef]
- Grass, G.D.; Tolliver, L.B.; Bratoeva, M.; Toole, B.P. CD147, CD44, and the Epidermal Growth Factor Receptor (EGFR) Signaling Pathway Cooperate to Regulate Breast Epithelial Cell Invasiveness. J. Biol. Chem. 2013, 288, 26089–26104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellaiah, M. CD44-Src signaling promotes invadopodia formation in prostate cancer (PC3) cells. OA Cancer 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of CD44 and Cancer Stem Cells. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; pp. 31–42. [Google Scholar]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. STEM CELLS Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, M. CD44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Frontiers Immunol. 2015, 6, 235. [Google Scholar]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Wu, G.D. The role of the hyalu-ronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Motiani, K.; Giridhar, P.V.; Kasper, S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp. Biol. Med. 2013, 238, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Hope, K.J.; Zhai, Q.; Smadja-Joffe, F.; Dick, J.E. Targeting of CD44 eradicates human acute myeloid leuke-mic stem cells. Nat. Med. 2006, 12, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Wen, J.; Bang, S.; Park, S.; Song, S.Y. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer 2009, 125, 2323–2331. [Google Scholar] [CrossRef]
- Li, X.-P.; Zhang, X.-W.; Zheng, L.-Z.; Guo, W.-J. Expression of CD44 in pancreatic cancer and its significance. Int. J. Clin. Exp. Pathol. 2015, 8, 6724–6731. [Google Scholar]
- Tsukita, S.; Oishi, K.; Sato, N.; Sagara, J.; Kawai, A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 1994, 126, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Yonemura, S.; Tsukita, S. ERM proteins: Head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem. Sci. 1997, 22, 53–58. [Google Scholar] [CrossRef]
- Aziz, K.A.; Till, K.J.; Zuzel, M.; Cawley, J.C. Involvement of CD44-hyaluronan interaction in malignant cell hom-ing and fibronectin synthesis in hairy cell leukemia. Blood J. Am. Soc. Hematol. 2000, 96, 3161–3167. [Google Scholar]
- Ichikawa, T.; Itano, N.; Sawai, T.; Kimata, K.; Koganehira, Y.; Saida, T.; Taniguchi, S.I. Increased synthesis of hya-luronate enhances motility of human melanoma cells. J. Investig. Dermatol. 1999, 113, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Favia, M.; Guerra, L.; Fanelli, T.; Cardone, R.A.; Monterisi, S.; Di Sole, F.; Casavola, V. Na+/H+ exchanger regu-latory factor 1 overexpression-dependent increase of cytoskeleton organization is fundamental in the rescue of F508del cystic fibrosis transmembrane conductance regulator in human airway CFBE41o-cells. Mol. Biol. Cell 2010, 21, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belizzi, A.; Greco, M.R.; Rubino, R.; Paradiso, A.; Forciniti, S.; Zeeberg, K.; Cardone, R.A.; Reshkin, S.J. The scaffolding protein NHERF1 sensitizes EGFR-dependent tumor growth, motility and invadopodia function to gefitinib treatment in breast cancer cells. Int. J. Oncol. 2014, 46, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Reshkin, S.J.; Harguindey, S. An Innovative Approach to Understanding and Treating Cancer: Targeting ph: From Etiopathogenesis to New Therapeutic Avenues; Chapter 11; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wharton, C.W. The structure and mechanism of stem bromelain. Evaluation of the homogeneity of purified stem bromelain, determination of the molecular weight and kinetic analysis of the bromelain-catalysed hydrolysis of N-benzyloxycarbonyl-l-phenylalanyl-l-serine methyl ester. Biochem. J. 1974, 143, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Gerard, G. Anticancer treatment and bromelains. Agressol. Revue Int. Physio-Biologie Pharma-Cologie Appl. Eff. L’agression 1972, 13, 261. [Google Scholar]
- Taussig, S.J.; Szekerczes, J.; Batkin, S. Inhibition of tumour growth in vitro by bromelain, an extract of the pineap-ple plant (Ananas comosus). Planta Med. 1985, 51, 538–539. [Google Scholar] [CrossRef]
- Batkin, S.; Taussig, S.J.; Szekerezes, J. Antimetastatic effect of bromelain with or without its proteolytic and antico-agulant activity. J. Cancer Res. Clin. Oncol. 1988, 114, 507–508. [Google Scholar] [CrossRef]
- Harrach, T.; Gebauer, F.; Eckert, K.; Kunze, R.; Maurer, H.R. Bromelain proteinases modulate the cd44 expression on human molt-4/8 leukemia and sk-mel-28 melanoma-cells in-vitro. Int. J. Oncol. 1994, 5, 485–488. [Google Scholar] [CrossRef]
- Garbin, F.; Harrach, T.; Eckert, K.; Maurer, H. Bromelain proteinase-f9 augments human lymphocyte-mediated growth-inhibition of various tumor-cells in-vitro. Int. J. Oncol. 1994, 5, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, E.; Eckert, K.; Fichtner, I.; Schulzeforster, K.; Maurer, H. Bromelain proteases suppress growth, inva-sion and lung metastasis of B16F10 mouse melanoma cells. Int. J. Oncol. 1997, 11, 243–248. [Google Scholar] [PubMed]
- Tysnes, B.B.; Maurert, H.R.; Porwol, T.; Probst, B.; Bjerkvig, R.; Hoover, F. Bromelain Reversibly Inhibits Invasive Properties of Glioma Cells. Neoplasia 2001, 3, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Baez, R.; Lopes, M.T.; Salas, C.E.; Hernandez, M. In vivo antitumoral activity of stem pineapple (Ananas como-sus) bromelain. Planta Med. 2007, 73, 1377–1383. [Google Scholar] [CrossRef]
- Bhui, K.; Prasad, S.; George, J.; Shukla, Y. Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett. 2009, 282, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Paroulek, A.F.; Jaffe, M.; Rathinavelu, A. The Effects of the Herbal Enzyme Bromelain against Breast Cancer Cell Line Gi-101a. Ph.D. Thesis, Nova Southeastern University, Fort Lauderdale, FL, USA, 2010. [Google Scholar]
- Dhandayuthapani, S.; Perez, H.D.; Paroulek, A.; Chinnakkannu, P.; Kandalam, U.; Jaffe, M.; Rathinavelu, A. Bromelain-Induced Apoptosis in GI-101A Breast Cancer Cells. J. Med. Food 2010, 15, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bhui, K.; Tyagi, S.; Srivastava, A.K.; Singh, M.; Roy, P.; Singh, R.; Shukla, Y. Bromelain inhibits nuclear factor kappa-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G2/M arrest to apop-tosis. Mol. Carcinog. 2012, 51, 231–243. [Google Scholar] [CrossRef]
- Amini, A.; Ehteda, A.; Moghaddam, S.M.; Akhter, J.; Pillai, K.; Morris, D.L. Cytotoxic effects of bromelain in hu-man gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21). OncoTargets Ther. 2013, 6, 403. [Google Scholar]
- Mohr, T.; Desser, L. Plant proteolytic enzyme papain abrogates angiogenic activation of human umbilical vein en-dothelial cells (HUVEC) in vitro. BMC Complementary Altern. Med. 2013, 13, 231. [Google Scholar] [CrossRef] [Green Version]
- Pillai, K.; Ehteda, A.; Akhter, J.; Chua, T.C.; Morris, D.L. Anticancer effect of bromelain with and without cispla-tin or 5-FU on malignant peritoneal mesothelioma cells. Anti-Cancer Drugs 2014, 25, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Masoumi-Moghaddam, S.; Ehteda, A.; Liauw, W.; Akhter, J.; Pilai, K.; Morris, D.L. Abstract LB-007: Synergistic inhibition of human gastric and colorectal cancers by Bromelain and N-acetylcysteine: An in vivo study. Exp. Mol. Ther. 2015, 75. [Google Scholar] [CrossRef]
- Amini, A.; Masoumi-Moghaddam, S.; Morris, D.L. Utility of Bromelain and N-acetylcysteine in Treatment of Peri-Toneal Dissemination of Gastrointestinal Mucin-Producing Malignancies; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Plentz, R.; Müller, A.; Barat, S.; Chen, X.; Bui, C.; Bozko, P.; Malek, N. Treatment of Cholangiocarcinoma by Bro-melain and Papain. J. Hepatol. 2016, 64, S577. [Google Scholar] [CrossRef]
- Debnath, R.; Majumder, D.; Singha, A.K.; Ghosh, D.; Maiti, D. Bromelain plus peroxidase from pine-apple induces apoptosis via mitochondrial dependent pathway in lymphoma cells. Int. J. Pharm. Sci. Res. 2018, 9, 4610–4618. [Google Scholar]
- Lee, J.H.; Lee, J.T.; Park, H.R.; Kim, J.B. The potential use of bromelain as a natural oral medicine having anticar-cinogenic activities. Food Sci. Nutr. 2019, 7, 1656–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, R.; Majumder, D.; Nath, P.; Ghosh, D.; Maiti, D. Bromelain plus peroxidase reduces non-Hodgkin lym-phoma progression in invivo via up-regulation of antioxidant enzymes and modulating apoptotic protein expression. Nutr. Cancer 2019, 72, 1–11. [Google Scholar]
- Hale, L.P.; Haynes, B.F. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J. Immunol. 1992, 149, 3809–3816. [Google Scholar] [PubMed]
- Munzig, E.; Eckert, K.; Harrach, T.; Graf, H.; Maurer, H.R. Bromelain protease F9 reduces the CD44 mediated ad-hesion of human peripheral blood lymphocytes to human umbilical vein endothelial cells. FEBS Lett. 1994, 351, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Kleef, R.; Delohery, T.M.; Bovbjerg, D.H. Selective modulation of cell adhesion molecules on lymphocytes by bro-melain protease. Pathobiology 1996, 64, 339–346. [Google Scholar] [CrossRef]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Fouz, N.; Amid, A.; Hashim, Y.Z.H.Y. Gene expression analysis in MCF-7 breast cancer cells treated with recom-binant bromelain. Appl. Biochem. Biotechnol. 2014, 173, 1618–1639. [Google Scholar] [CrossRef]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.R., Jr.; Carson, W.F., IV; Cloutier, M.M.; Guernsey, L.A.; Schramm, C.M.; Wu, C.A.; Thrall, R.S. Brome-lain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell. Immunol. 2005, 237, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Secor, E.R.; Szczepanek, S.M.; Castater, C.A.; Adami, A.J.; Matson, A.P.; Rafti, E.T.; Guernsey, L.; Natarajan, P.; McNamara, J.T.; Schramm, C.M.; et al. Bromelain Inhibits Allergic Sensitization and Murine Asthma via Modulation of Dendritic Cells. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Andrew, D.; Murphy, M.; Mynott, T.L. Bromelain Activates Murine Macrophages and Natural Killer Cells in Vitro. Cell. Immunol. 2001, 210, 5–10. [Google Scholar] [CrossRef] [PubMed]
- White, R.R.; Crawley, F.E.H.; Vellini, M.; Rovati, L.A. Bioavailability of125I bromelain after oral administration to rats. Biopharm. Drug Dispos. 1988, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Friedrich, G.; Kuhn, C.S.; Poppe, G.E. Intestinal absorption of undegraded proteins in men: Presence of bromelain in plasma after oral intake. Am. J. Physiol. Liver Physiol. 1997, 273, G139–G146. [Google Scholar] [CrossRef] [PubMed]
- Heinicke, R.M.; Van Der Wal, L.; Yokoyama, M. Effect of bromelain (ananase®) on human platelet aggregation. Cell. Mol. Life Sci. 1972, 28, 844–845. [Google Scholar] [CrossRef]
- Metzig, C.; Grabowska, E.; Eckert, K.; Rehse, K.; Maurer, H.R. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. In Vivo 1999, 13, 7–12. [Google Scholar]
- Pirotta, F.; de Giuli-Morghen, C. Bromelain: Anti-inflammatory and serum fibronolytic activity after oral administration in the rat. Drugs Exptl. Clin. Res. 1978, 4, 1–20. [Google Scholar]
- Pavan, R.; Jain, S.; Shraddha, K.A. Properties and Therapeutic Application of Bromelain: A Review. Biotechnol. Res. Int. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kudo, D.; Kon, A.; Yoshihara, S.; Kakizaki, I.; Sasaki, M.; Endo, M.; Takagaki, K. Effect of a hyaluronan synthase suppressor, 4-methylumbelliferone, on B16F-10 melanoma cell adhesion and locomotion. Biochem. Biophys. Res. Commun. 2004, 321, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Ghatak, S.; Zoltan-Jones, A.; Toole, B.P. Regulation of Multidrug Resistance in Cancer Cells by Hyaluronan. J. Biol. Chem. 2003, 278, 25285–25288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toole, B.P.; Slomiany, M.G. Hyaluronan: A constitutive regulator of chemoresistance and malignancy in cancer cells. Semin. Cancer Biol. 2008, 18, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kultti, A.; Zhao, C.; Singha, N.C.; Zimmerman, S.; Osgood, R.J.; Symons, R.; Jacobetz, M.A. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvi-ronment. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipponen, P.; Aaltomaa, S.; Tammi, R.; Tammi, M.; Ågren, U.; Kosma, V.M. High stromal hyaluronan level is as-sociated with poor differentiation and metastasis in prostate cancer. Eur. J. Cancer 2001, 37, 849–856. [Google Scholar] [CrossRef]
- Setälä, L.P.; Tammi, M.I.; Tammi, R.H.; Eskelinen, M.J.; Lipponen, P.K.; Ågren, U.M.; Kosma, V.M. Hyalu-ronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br. J. Cancer 1999, 79, 1133. [Google Scholar] [CrossRef] [Green Version]
- Anttila, M.A.; Tammi, R.H.; Tammi, M.I.; Syrjänen, K.J.; Saarikoski, S.V.; Kosma, V.M. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000, 60, 150–155. [Google Scholar]
- Bharadwaj, A.G.; Kovar, J.L.; Loughman, E.; Elowsky, C.; Oakley, G.G.; Simpson, M.A. Spontaneous Metastasis of Prostate Cancer Is Promoted by Excess Hyaluronan Synthesis and Processing. Am. J. Pathol. 2009, 174, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Brichkina, A.; Bertero, T.; Loh, H.M.; Nguyen, N.T.M.; Emelyanov, A.; Rigade, S.; Ilie, M.; Hofman, P.; Gaggioli, C.; Bulavin, D.V. p38MAPK builds a hyaluronan cancer niche to drive lung tumorigenesis. Genes Dev. 2016, 30, 2623–2636. [Google Scholar] [CrossRef]
- Nakazawa, H.; Yoshihara, S.; Kudo, D.; Morohashi, H.; Kakizaki, I.; Kon, A.; Takagaki, K.; Sasaki, M. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother. Pharmacol. 2006, 57, 165–170. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Lopez, L.E.; Munoz, D.; Chi, A.; Shirodkar, S.P.; Lokeshwar, S.D.; Escudero, D.O.; Dhir, N.; Altman, N. Antitumor Activity of Hyaluronic Acid Synthesis Inhibitor 4-Methylumbelliferone in Prostate Cancer Cells. Cancer Res. 2010, 70, 2613–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccioni, F.; Malvicini, M.; Garcia, M.G.; Rodriguez, A.; Atorrasagasti, C.; Kippes, N.; Viola, M. Antitumor ef-fects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 2011, 22, 400–410. [Google Scholar] [CrossRef]
- Arai, E.; Nishida, Y.; Wasa, J.; Urakawa, H.; Zhuo, L.; Kimata, K.; Ishiguro, N. Inhibition of hyaluronan reten-tion by 4-methylumbelliferone suppresses osteosarcoma cells in vitro and lung metastasis in vivo. Br. J. Cancer 2011, 105, 1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urakawa, H.; Nishida, Y.; Wasa, J.; Arai, E.; Zhuo, L.; Kimata, K.; Ishiguro, N. Inhibition of hyaluronan synthe-sis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int. J. Cancer 2012, 130, 454–466. [Google Scholar] [CrossRef]
- Saito, T.; Dai, T.; Asano, R. The hyaluronan synthesis inhibitor 4-methylumbelliferone exhibits antitumor effects against mesenchymal-like canine mammary tumor cells. Oncol. Lett. 2013, 5, 1068–1074. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Quesada, A.R.; Medina, M. Ángel 4-Methylumbelliferone Inhibits Angiogenesis in Vitro and in Vivo. J. Agric. Food Chem. 2013, 61, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vilas, J.A.; Rodriguez Quesada, A.; Medina Torres, M.A. 4-Methylumbelliferone, an inhibitor of hya-luronic acid biosynthesis, inhibits key steps of angiogenesis. Angiogenesis 2014, 17, 753–754. [Google Scholar]
- Tamura, R.; Yokoyama, Y.; Yoshida, H.; Imaizumi, T.; Mizunuma, H. 4-Methylumbelliferone inhibits ovarian can-cer growth by suppressing thymidine phosphorylase expression. J. Ovuarian Res. 2014, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Kon, A.; Kudo, D.; Nakazawa, H.; Kakizaki, I.; Sasaki, M.; Endo, M.; Takagaki, K. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005, 579, 2722–2726. [Google Scholar] [CrossRef] [Green Version]
- Morohashi, H.; Kon, A.; Nakai, M.; Yamaguchi, M.; Kakizaki, I.; Yoshihara, S.; Sasaki, M.; Takagaki, K. Study of hyaluronan synthase inhibitor, 4-methylumbelliferone derivatives on human pancreatic cancer cell (KP1-NL). Biochem. Biophys. Res. Commun. 2006, 345, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Kudo, D.; Nagase, H.; Shimoda, H.; Suto, S.; Negishi, M.; Kakizaki, I.; Endo, M.; Hakamada, K. Antitumor effects of the hyaluronan inhibitor 4-methylumbelliferone on pancreatic cancer. Oncol. Lett. 2016, 12, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.N.; Alaniz, L.D.; Menger, M.D.; Barañao, R.I.; Laschke, M.W.; Meresman, G.F. Inhibition of hyalu-ronic acid synthesis suppresses angiogenesis in developing endometriotic lesions. PLoS ONE 2016, 11, e0152302. [Google Scholar] [CrossRef]
- Nagase, H.; Kudo, D.; Suto, A.; Yoshida, E.; Suto, S.; Negishi, M.; Hakamada, K. 4-Methylumbelliferone sup-presses hyaluronan synthesis and tumor progression in SCID mice intra-abdominally inoculated with pancreatic cancer cells. Pancreas 2017, 46, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, E.; Kudo, D.; Nagase, H.; Suto, A.; Shimoda, H.; Suto, S.; Hakamada, K. 4-Methylumbelliferone De-creases the Hyaluronan-rich Extracellular Matrix and Increases the Effectiveness of 5-Fluorouracil. Anticancer Res. 2018, 38, 5799–5804. [Google Scholar] [CrossRef]
- Cheng, X.; Sato, N.; Kohi, S.; Koga, A.; Hirata, K. 4-Methylumbelliferone inhibits enhanced hyaluronan synthesis and cell migration in pancreatic cancer cells in response to tumor-stromal interactions. Oncol. Lett. 2018, 15, 6297–6301. [Google Scholar] [CrossRef] [Green Version]
- Karalis, T.T.; Heldin, P.; Vynios, D.H.; Neill, T.; Buraschi, S.; Iozzo, R.V.; Skandalis, S.S. Tumor-suppressive functions of 4-MU on breast cancer cells of different ER status: Regulation of hyaluronan/HAS2/CD44 and specific matrix ef-fectors. Matrix Biol. 2019, 78, 118–138. [Google Scholar] [CrossRef]
- Lokman, N.A.; Price, Z.K.; Hawkins, E.K.; MacPherson, A.M.; Oehler, M.K.; Ricciardelli, C. 4-Methylumbelliferone Inhibits Cancer Stem Cell Activation and Overcomes Chemoresistance in Ovarian Cancer. Cancers 2019, 11, 1187. [Google Scholar] [CrossRef] [Green Version]
- Saga, R.; Hasegawa, K.; Murata, K.; Chiba, M.; Nakamura, T.; Okumura, K.; Hosokawa, Y. Regulation of radio-sensitivity by 4 methylumbelliferone via the suppression of interleukin 1 in fibrosarcoma cells. Oncol. Lett. 2019, 17, 3555–3561. [Google Scholar]
- Makkar, S.; Riehl, T.E.; Chen, B.; Yan, Y.; Alvarado, D.M.; Ciorba, M.A.; Stenson, W.F. Hyaluronic Acid Binding to TLR4 Promotes Proliferation and Blocks Apoptosis in Colon Cancer. Mol. Cancer Ther. 2019, 18, 2446–2456. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.E.; Santos, M.T.; Binkley, P.A.; Sultana, M.; Tekmal, R.R.; Schenken, R.S.; Knudtson, J.F. Inhibi-tion of Hyaluronic Acid Synthesis Decreases Endometrial Cell Attachment, Migration, and Invasion. Reprod. Sci. 2020, 27, 1058–1063. [Google Scholar] [CrossRef]
- Pibuel, M.A.; Díaz, M.; Molinari, Y.; Poodts, D.; Silvestroff, L.; Lompardía, S.L.; Hajos, S.E. 4-Methylumbelliferone as a potent and selective antitumor drug on a glioblastoma model. Glycobiology 2021, 31, 29–43. [Google Scholar] [CrossRef]
- Carter, N.J. Pirfenidone. Drugs 2011, 71, 1721–1732. [Google Scholar] [CrossRef]
- Taniguchi, H.; Ebina, M.; Kondoh, Y.; Ogura, T.; Azuma, A.; Suga, M.; Taguchi, Y.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2009, 35, 821–829. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.; Lancaster, L.A.; Sahn, S.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- Sharma, K.; Ix, J.H.; Mathew, A.V.; Cho, M.; Pflueger, A.; Dunn, S.R.; Francos, B.; Sharma, S.; Falkner, B.; McGowan, T.A.; et al. Pirfenidone for Diabetic Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Garcıa, L.; Hernández, I.; Sandoval, A.; Salazar, A.; Garcia, J.; Vera, J.; Armendariz-Borunda, J. Pirfenidone ef-fectively reverses experimental liver fibrosis. J. Hepatol. 2002, 37, 797–805. [Google Scholar] [CrossRef]
- Polydorou, C.; Mpekris, F.; Papageorgis, P.; Voutouri, C.; Stylianopoulos, T. Pirfenidone normalizes the tumor mi-croenvironment to improve chemotherapy. Oncotarget 2017, 8, 24506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stylianopoulos, T.; Martin, J.D.; Snuderl, M.; Mpekris, F.; Jain, S.R.; Jain, R.K. Coevolution of solid stress and in-terstitial fluid pressure in tumors during progression: Implications for vascular collapse. Cancer Res. 2013, 73, 3833–3841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozono, S.; Ohuchida, K.; Eguchi, D.; Ikenaga, N.; Fujiwara, K.; Cui, L.; Tanaka, M. Pirfenidone inhibits pancre-atic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013, 73, 2345–2356. [Google Scholar] [CrossRef] [Green Version]
- Knüppel, L.; Ishikawa, Y.; Aichler, M.; Heinzelmann, K.; Hatz, R.; Behr, J.; Staab-Weijnitz, C.A. A novel antifi-brotic mechanism of nintedanib and pirfenidone. Inhibition of collagen fibril assembly. Am. J. Respir. Cell Mol. Biol. 2017, 57, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Inoue, H.; Nakazawa, R.; Azuma, N.; Suzuki, M.; Yamauchi, S.; Saito, I. Pirfenidone induces inter-cellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin. Exp. Immunol. 1998, 113, 72–76. [Google Scholar] [CrossRef]
- Shihab, F.S.; Bennett, W.M.; Yi, H.; Andoh, T.F. Pirfenidone treatment decreases transforming growth factor-β1 and matrix proteins and ameliorates fibrosis in chronic cyclosporine nephrotoxicity. Am. J. Transplant. 2002, 2, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, H.; Oku, H.; Yamane, S.; Tsuruta, Y.; Suzuki, R. A novel anti-fibrotic agent pirfenidone suppresses tu-mor necrosis factor-α at the translational level. Eur. J. Pharmacol. 2002, 446, 177–185. [Google Scholar] [CrossRef]
- Didiasova, M.; Singh, R.; Wilhelm, J.; Kwapiszewska, G.; Wujak, L.; Zakrzewicz, D.; Schaefer, L.; Markart, P.; Seeger, W.; Lauth, M.; et al. Pirfenidone exerts antifibrotic effects through inhibition of GLI transcription factors. FASEB J. 2017, 31, 1916–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, E.; Gili, E.; Fagone, E.; Fruciano, M.; Iemmolo, M.; Vancheri, C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur. J. Pharm. Sci. 2014, 58, 13–19. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; Thomas, B.J.; Bardin, P.G. Pirfenidone: Molecular mechanisms and potential clinical applica-tions in lung disease. Am. J. Respir. Cell Mol. Biol. 2020, 62, 413–422. [Google Scholar] [CrossRef]
- Oku, H.; Nakazato, H.; Horikawa, T.; Tsuruta, Y.; Suzuki, R. Pirfenidone suppresses tumor necrosis factor-α, en-hances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharm. 2002, 446, 167–176. [Google Scholar] [CrossRef]
- Kwapiszewska, G.; Gungl, A.; Wilhelm, J.; Marsh, L.M.; Puthenparampil, H.T.; Sinn, K.; Wygrecka, M. Tran-scriptome profiling reveals the complexity of pirfenidone effects in idiopathic pulmonary fibrosis. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef] [Green Version]
- Ballester, B.; Milara, J.; Cortijo, J. Pirfenidone anti-fibrotic effects are partially mediated by the inhibition of MUC1 bioactivation. Oncotarget 2020, 11, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, F.; Granata, G.; Pelliccia, C.; La Porta, R.; Vitiello, A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2. Eur. J. Clin. Pharmacol. 2020, 76, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Hinoda, Y.; Ikematsu, Y.; Horinochi, M.; Sato, S.; Yamamoto, K.; Nakano, T.; Fukui, M.; Suehiro, Y.; Hamanaka, Y.; Nishikawa, Y.; et al. Increased expression of MUC1 in advanced pancreatic cancer. J. Gastroenterol. 2003, 38, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Mukherjee, P. MUC1 enhances in-vasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, R.K.; Lee, K.M.; McKolanis, J.; Hitbold, E.; Schraut, W.; Moser, A.J.; Finn, O.J. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pan-creatic cancer. Cancer Immunol. Immunother. 2005, 54, 254–264. [Google Scholar] [CrossRef]
- Shukla, S.K.; Purohit, V.; Mehla, K.; Gunda, V.; Chaika, N.V.; Vernucci, E.; Singh, P.K. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell 2017, 32, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumida, H.; Swanson, B.J.; Singh, P.K.; Caffrey, T.C.; Kitajima, S.; Goto, M.; Yonezawa, S.; Hollingsworth, M.A. RNA Interference Suppression of MUC1 Reduces the Growth Rate and Metastatic Phenotype of Human Pancreatic Cancer Cells. Clin. Cancer Res. 2006, 12, 2976–2987. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Daneshvar, K.; Roy, L.D.; Grover, P.; Kidiyoor, A.; Mosley, L.; Mukherjee, P. MUC1 induces drug re-sistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2013, 2, e51. [Google Scholar] [CrossRef] [Green Version]
- Chaika, N.V.; Gebregiworgis, T.; Lewallen, M.E.; Purohit, V.; Radhakrishnan, P.; Liu, X.; Zhang, B.; Mehla, K.; Brown, R.B.; Caffrey, T.; et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 13787–13792. [Google Scholar] [CrossRef] [Green Version]
- Usugi, E.; Ishii, K.; Hirokawa, Y.; Kanayama, K.; Matsuda, C.; Uchida, K.; Shiraishi, T.; Watanabe, M. Antifibrotic Agent Pirfenidone Suppresses Proliferation of Human Pancreatic Cancer Cells by Inducing G0/G1 Cell Cycle Arrest. Pharmacology 2019, 103, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla-Varela, M.; Boateng, K.; Noyes, D.; Antonia, S.J. The anti-fibrotic agent pirfenidone synergizes with cisplatin in killing tumor cells and cancer-associated fibroblasts. BMC Cancer 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, P.; Loh, W.M.; Gopinath, S.C.; Bonam, S.R.; Fareez, I.M.; Mac Guad, R.; Sim, M.S.; Wu, Y.S. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm. Sin. B 2020, 10, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Pillai, K.; Morris, D.L. Mucins in pancreatic cancer: Biological role, implications in carcinogenesis and ap-plications in diagnosis and therapy. Am. J. Cancer Res. 2017, 7, 1372. [Google Scholar]
- Sugahara, K.N.; Hirata, T.; Hayasaka, H.; Stern, R.; Murai, T.; Miyasaka, M. Tumor Cells Enhance Their Own CD44 Cleavage and Motility by Generating Hyaluronan Fragments. J. Biol. Chem. 2006, 281, 5861–5868. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, L.Y.; Xia, W.; Wong, G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates β-catenin signaling and NFκB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J. Biol. Chem. 2009, 284, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Ref. | Finding |
|---|---|
| [7] | Gemcitabine resistance was promoted by collagen I through a metalloproteases (MT1-MMP) expression that in turn increases high mobility group AT 2 (HMGA2) protein which is an indirect transcription promoter. |
| [8] | Extracellular matrix proteins showed pro-proliferative activity in PDAC in vitro. |
| [9] | Pancreatic stromal fibroblasts showed the ability to promote cancer progression. |
| [10] | Matrix collagen significantly increased the production of MMPs in PDAC impacting cell migration and invasive potential. |
| [11] | Suppressing the desmoplastic process with metformin inhibited pancreatic cancer progression. |
| [12] | Desmoplasia promoted pancreatic cancer progression. |
| [13] | Extracellular matrix composition modulated PDAC and stem cells. An increased in collagen I introduced a switch in rapidly growing CSCs to a slower-growing avascular niche endothelial-type of cells, creating a supportive mechanism for tumor progression and mainly for tumor invasion. An active cross-talk between PDAC cells, CSCs, and matrix was carried out through the secretome. This process led to less proliferation but more invasion of PDAC cells into the CSCs derived vascular network. |
| [14] | The extracellular matrix of PDAC and focal adhesion kinase (FAK) were found to regulate CSCs |
| [15] | When PDAC cells were co-cultured with fibroblast, the latter acquired a myofibroblastic morphology expressing desmoplastic markers. |
| [16] | Adding stellate cells to a PDAC 3D cell culture generated a rich stroma similar to that found in vivo. |
| Year/Ref. | Tissue/Animal | Finding |
|---|---|---|
| 1985 [124] | Lewis lung carcinoma, YC-8 lymphoma, MCA-1 ascitic tumor cells | BRO retarded tumor cell growth even when proteolytic and platelet aggregation effects were deactivated by heating when peroxidase activity was conserved. |
| 1988 [125] | Mice fed with BRO | Reduced lung metastasis of implanted Lewis lung carcinoma. |
| 1994 [126] | Human leukemia cells | BRO reduced CD44 expression on the cell surface, reducing adhesiveness to the endothelium. |
| 1994 [127] | Breast cancer, squamous carcinoma, and melanoma cells | Pre-treating lymphocytes with BRO inhibited the growth of the 3 tested cell types. BRO also had inhibitory effects when it was directly applied to the cells. |
| 1997 [128] | Mouse melanoma cells | In vitro treatment of the melanoma cells with BRO before i.v. injection into mice prevented lung colonization. |
| 2001 [129] | Glioma cells | BRO reduced migratory abilities by decreasing α3 and β1 integrin subunits and hyaluronan receptor CD44 protein levels. |
| 2007 [130] | Leukemia, sarcoma, Lewis lung carcinoma, Ehrich ascites, breast carcinoma, and melanoma cells. | These cells were injected into mice. Treatment with BRO started 24 hrs. later. With the exception of melanoma, the mice treated with BRO had significantly prolonged survival as compared with mice treated with 5FU used as controls. |
| 2009 [131] | Mouse skin tumorigenesis model | Pre-treatment with BRO reduced the total number of tumors and an average number of tumors per mouse. It inhibited COX2 expression and NF-kB activation. |
| 2010 [132] | GI-101A breast cancer cell | Dose-dependent apoptosis through the caspases pathway. |
| 2012 [133] | GI-101A breast cancer cell | Dose-dependent apoptosis through the caspases pathway. |
| 2012 [134] | Melanoma and epidermoid carcinoma cells | BRO induced apoptosis through inhibition of NF-kB nuclear translocation. BRO increased ROS by depleting intracellular glutathione. |
| 2013 [135] | Human gastrointestinal carcinoma cells | Cytotoxic effects of BRO were confirmed on these cells involving the caspases system, but also blocking Akt and attenuating Bcl2 and MUC1 oncoproteins. |
| 2013 [136] | Human umbilical vein endothelial cells (HUVEC) | BRO showed anti-angiogenic activity in vitro, inhibiting cell proliferation and tube formation. |
| 2014 [137] | Peritoneal Mesothelioma cells | Cells expressing MUC1 were treated with BRO. BRO induced cell death and increased cisplatin cytotoxicity. |
| 2015 [138] | Gastric and colorectal cancer | BRO and n-acetylcysteine showed synergistic inhibition of tumor growth in vivo. |
| 2016 [139] | Peritoneal dissemination of gastrointestinal mucin-producing cells | BRO and n-acetylcysteine reduced the dissemination and proliferation of gastrointestinal mucin-producing cancers. |
| 2016 [140] | Cholangiocarcinoma cells | BRO decreased proliferation, migration, and invasion. BRO inhibited NF-kB/AMPK signaling. |
| 2018 [141] | Lymphoma cells | BRO with peroxidases induced mitochondrial apoptosis. |
| 2019 [142] | Oral cancer cells | Viability was reduced in a dose-dependent manner. |
| 2019 [143] | Non-Hodgkin lymphoma | BRO with peroxidases reduced progression of non-Hodgkin lymphoma in vivo. |
| Year/Ref | Tissue | Findings |
|---|---|---|
| 1992 [144] | Human T cells | BRO removed CD44 from the cell surface |
| 1994 [145] | Human peripheral blood lymphocytes | BRO reduced the CD44 mediated adhesion of human lymphocytes to human umbilical vein endothelial cells. |
| 1994 [126] | Leukemia and melanoma cells | BRO modulated CD44 expression |
| 1996 [146] | Lymphocytes | BRO modulated CD44 expression and reduced lymphocyte adhesiveness. |
| 2001 [129] | Glioma cells | BRO reduced invasiveness of glioblastoma cells by downregulating CD44 |
| 2016 [147] | Human and mouse tumor cells | BRO reduced CD44 expression from the surface of tumor cells. |
| Year/Ref. | Tissue | Findings |
|---|---|---|
| 2006 [169] | Pancreatic cancer cells | 4MU increased the cytotoxicity of gemcitabine. |
| 2010 [170] | Prostate cancer cells | 4MU inhibited motility, invasion, and proliferation, inducing apoptosis of four different prostate cancer cell lines. It also inhibited NF-kB activity. |
| 2011 [171] | Hepatocarcinoma | In an orthotopic model of hepatocarcinoma, 4MU reduced proliferation and induced apoptosis. |
| 2011 [172] | Osteosarcoma | 4MU suppressed hyaluronan production and matrix formation in osteosarcoma cells, with reduction of proliferation in vitro and lung metastasis in vivo. |
| 2012 [173] | Breast cancer | 4MU inhibited tumorigenicity in vitro. It reduced tumor metastasis in vivo. |
| 2013 [174] | Canine mammary tumor cells | 4MU showed anti-tumoral actions in mesenchymal-like tumor cells. |
| 2013 [175] | Anti-angiogenic effects in vitro and in vivo | |
| 2014 [176] | Anti-angiogenic effects in vitro and in vivo | |
| 2014 [177] | Ovarian cancer cells | 4MU inhibited ovarian cancer cell proliferation in vitro, without affecting migration and invasion. In a peritoneal carcinomatosis model, 4MU inhibited tumor growth and prolonged survival. The authors suggest that thymidine phosphorylase inhibition is the reason for this anti-cancer activity. |
| 2015 [178] | Melanoma | 4MU reduced liver metastasis in a melanoma in vivo model. |
| 2016 [179] | Pancreatic cancer cells | 4MU derivatives were studied, showing a more powerful inhibition on the hyaluronan inhibition. |
| 2016 [180] | Pancreatic cancer cells | 4MU reduced hyaluronan production and suppressed cell proliferation in vitro and in vivo. |
| 2016 [181] | Non-malignant endometriotic lesions | 4MU reduced angiogenesis. |
| 2017 [182] | Implanted pancreatic cancer cells | SCID mice with cancer cells implanted in the peritoneum and treated with 4MU showed decreased progression, migration, and invasion. |
| 2018 [183] | Pancreatic cancer cells | 4MU decreased hyaluronan production and increased cytotoxicity of 5-fluorouracil. |
| 2018 [184] | Pancreatic cancer cells | 4MU decreased motility of pancreatic cancer cells. |
| 2019 [185] | Breast cancer cells | 4MU showed higher anti-tumoral activity in ERα negative tumors. |
| 2019 [186] | Ovarian cancer | 4MU decreased chemoresistance and inhibited cancer stem cell activation. |
| 2019 [187] | Fibrosarcoma cells | 4MU increased radiosensitivity by decreasing IL6 and IL8. |
| 2019 [188] | Colon cancer | Hyaluronan is a pro-tumoral molecule that also binds to TLR4 increasing proliferation and blocking apoptosis. Blockade of hyaluronan binding by any method, including decreased production with 4MU decreased proliferation. |
| 2020 [189] | Human normal endometrial cells | Reduced attachment, invasion, and invasion of endometrial epithelial and stromal cells. |
| 2021 [190] | Glioblastoma cells | 4MU diminished proliferation, cell migration, and metalloproteases inducing apoptosis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koltai, T.; Reshkin, S.J.; Carvalho, T.M.A.; Cardone, R.A. Targeting the Stromal Pro-Tumoral Hyaluronan-CD44 Pathway in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 3953. https://doi.org/10.3390/ijms22083953
Koltai T, Reshkin SJ, Carvalho TMA, Cardone RA. Targeting the Stromal Pro-Tumoral Hyaluronan-CD44 Pathway in Pancreatic Cancer. International Journal of Molecular Sciences. 2021; 22(8):3953. https://doi.org/10.3390/ijms22083953
Chicago/Turabian StyleKoltai, Tomas, Stephan Joel Reshkin, Tiago M. A. Carvalho, and Rosa A. Cardone. 2021. "Targeting the Stromal Pro-Tumoral Hyaluronan-CD44 Pathway in Pancreatic Cancer" International Journal of Molecular Sciences 22, no. 8: 3953. https://doi.org/10.3390/ijms22083953






