Hybrid Materials Based on Magnetic Iron Oxides with Benzothiazole Derivatives: A Plausible Potential Spectroscopy Probe
Abstract
1. Introduction
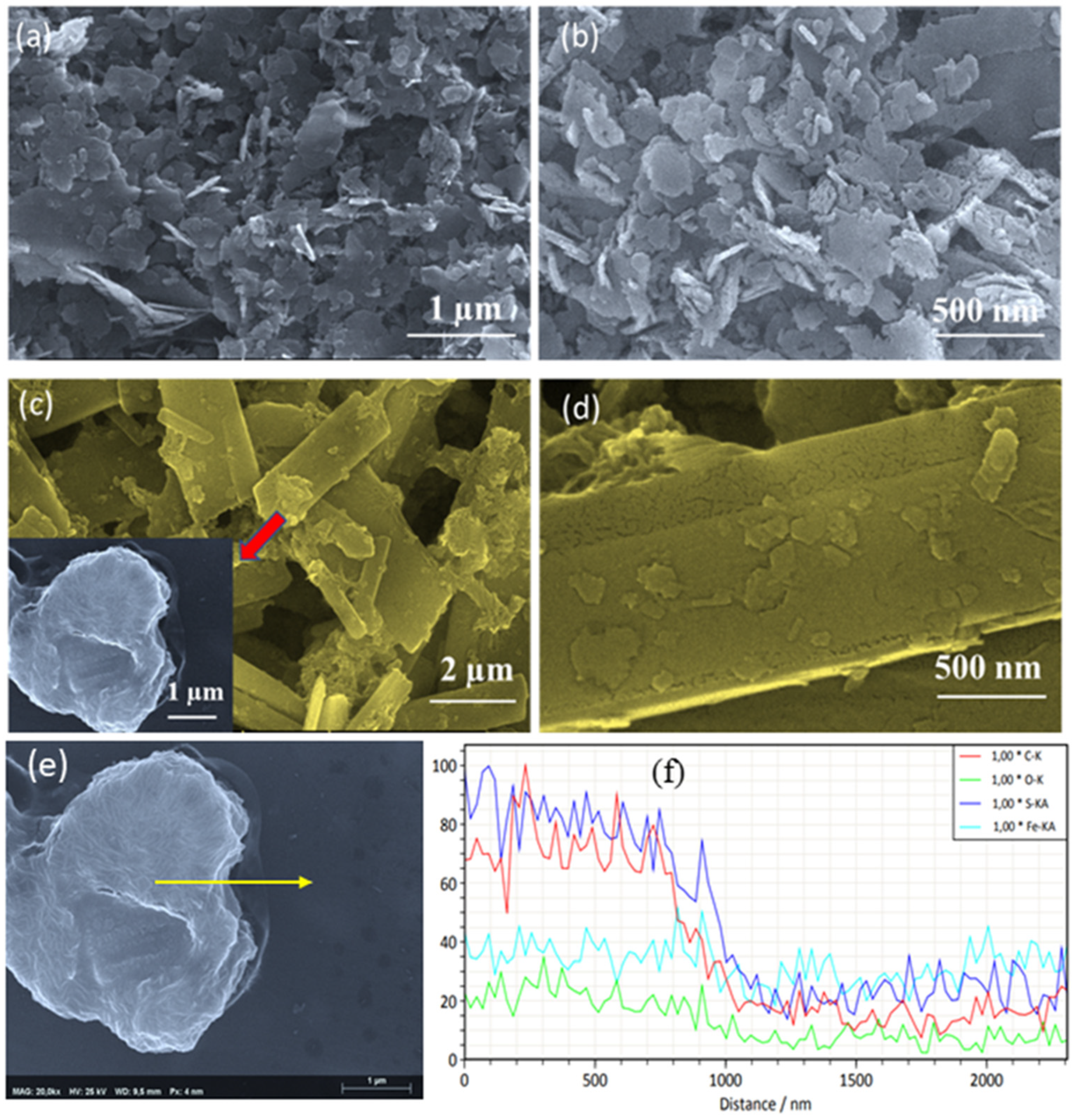
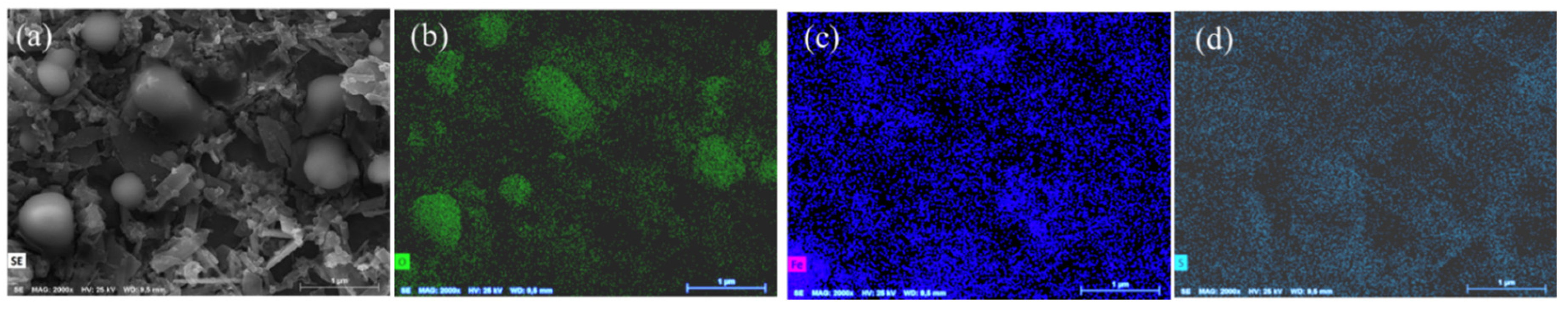
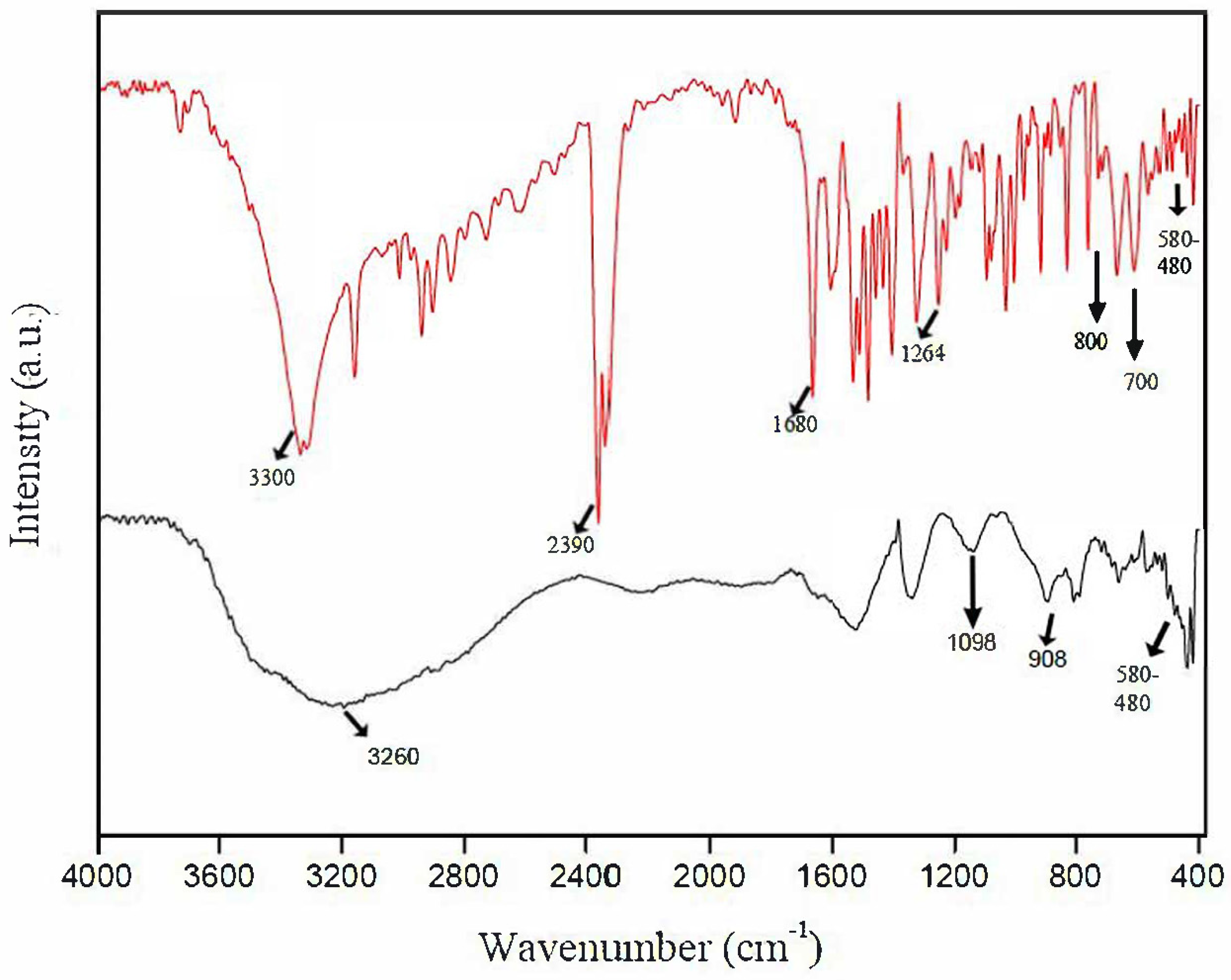
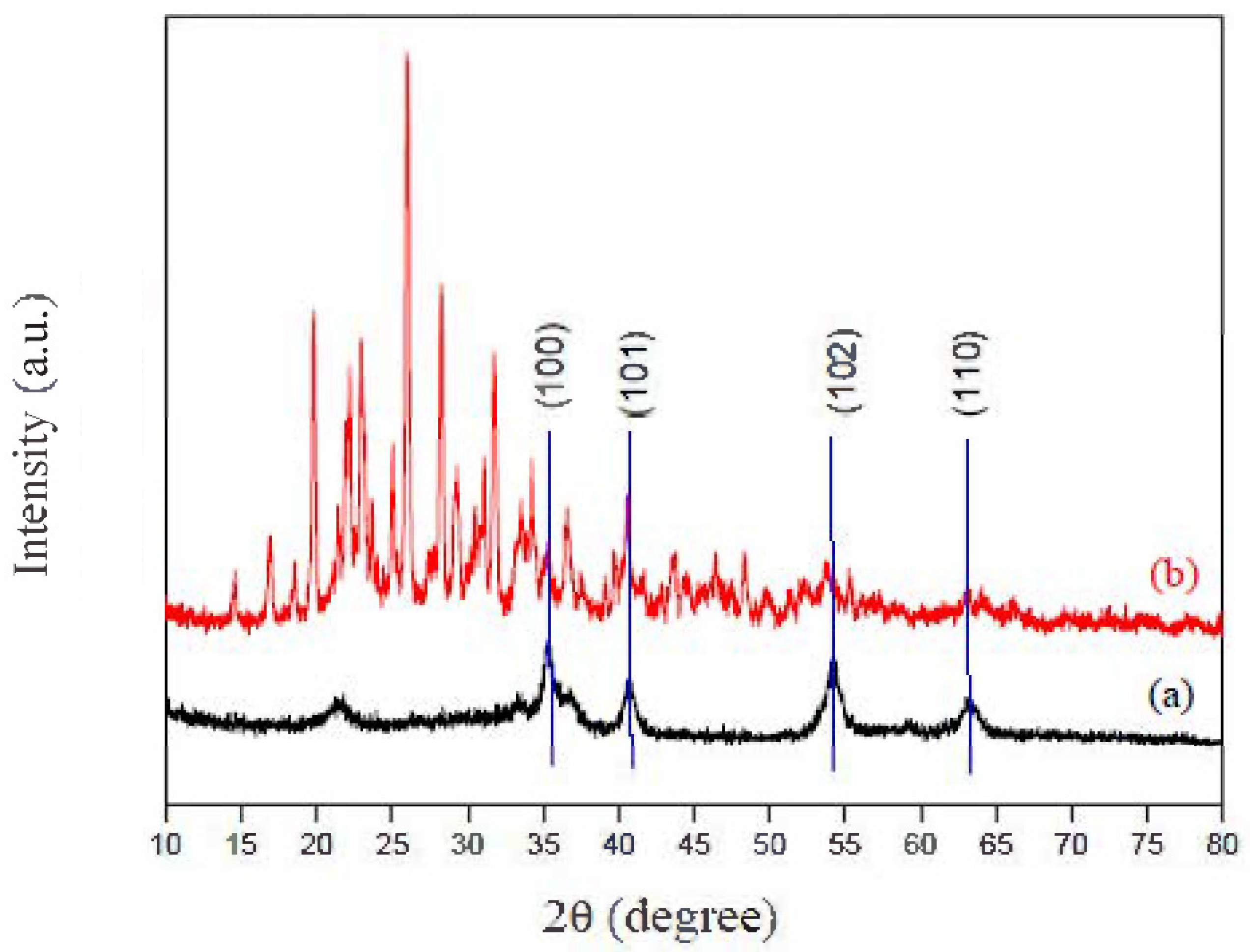
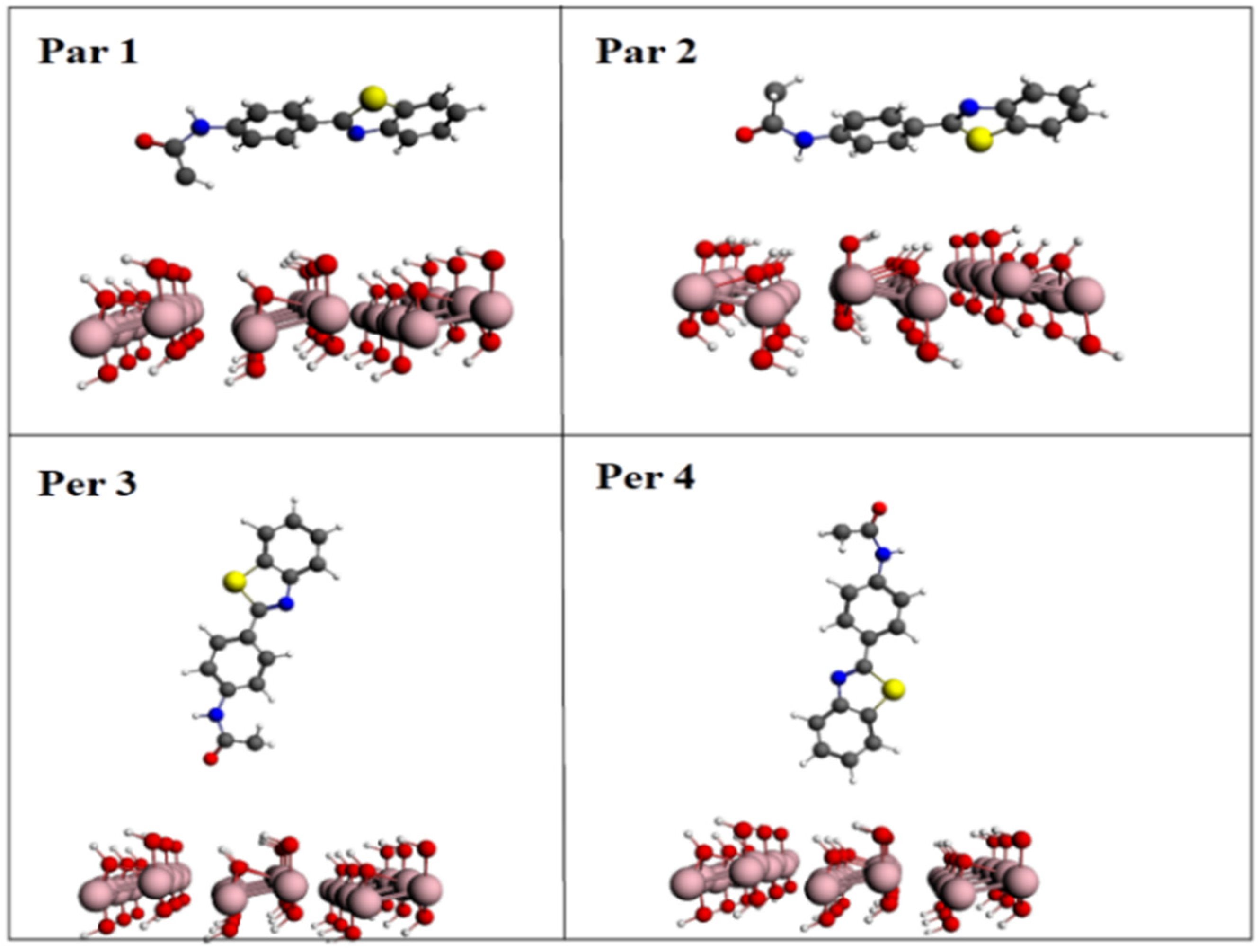
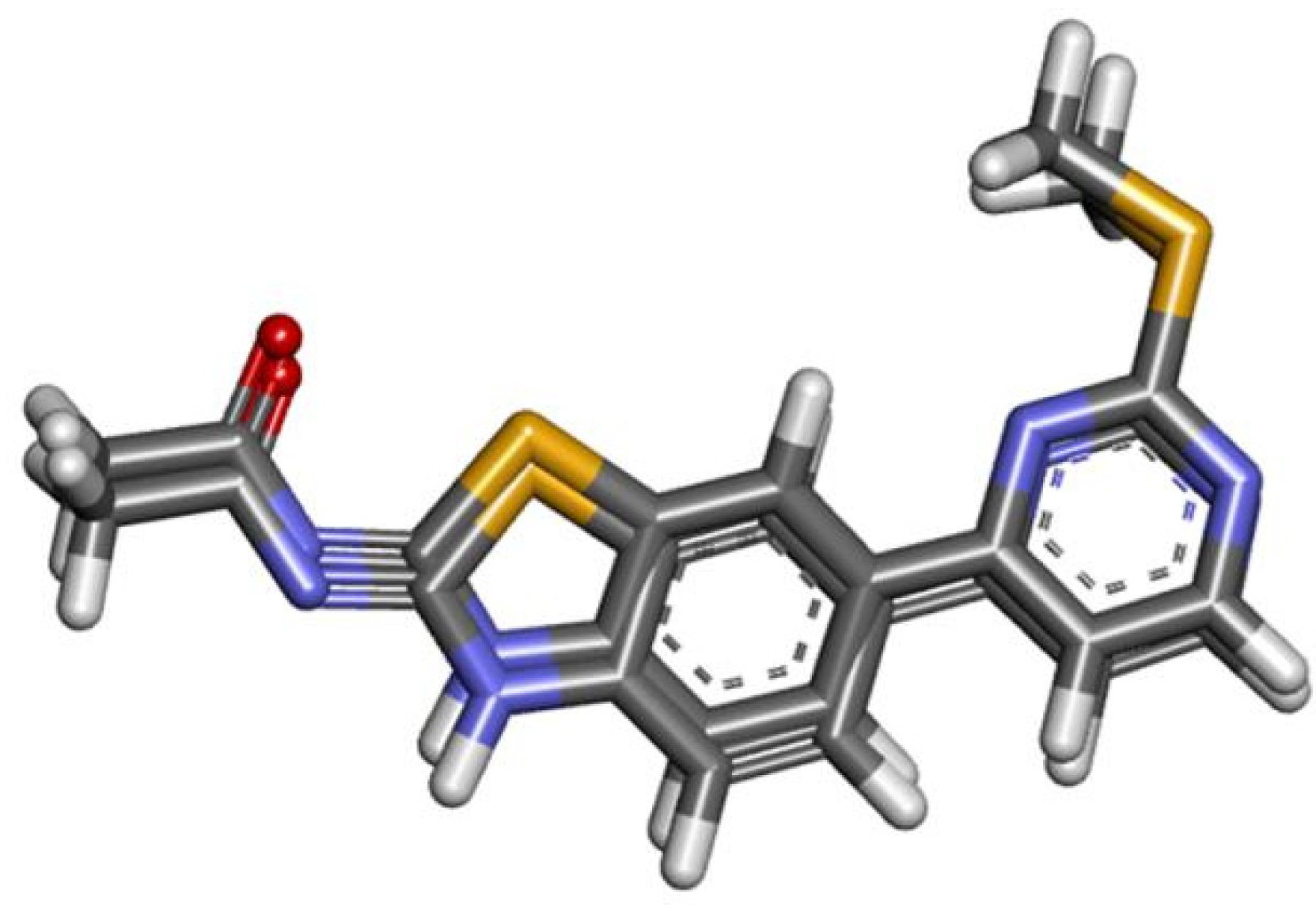
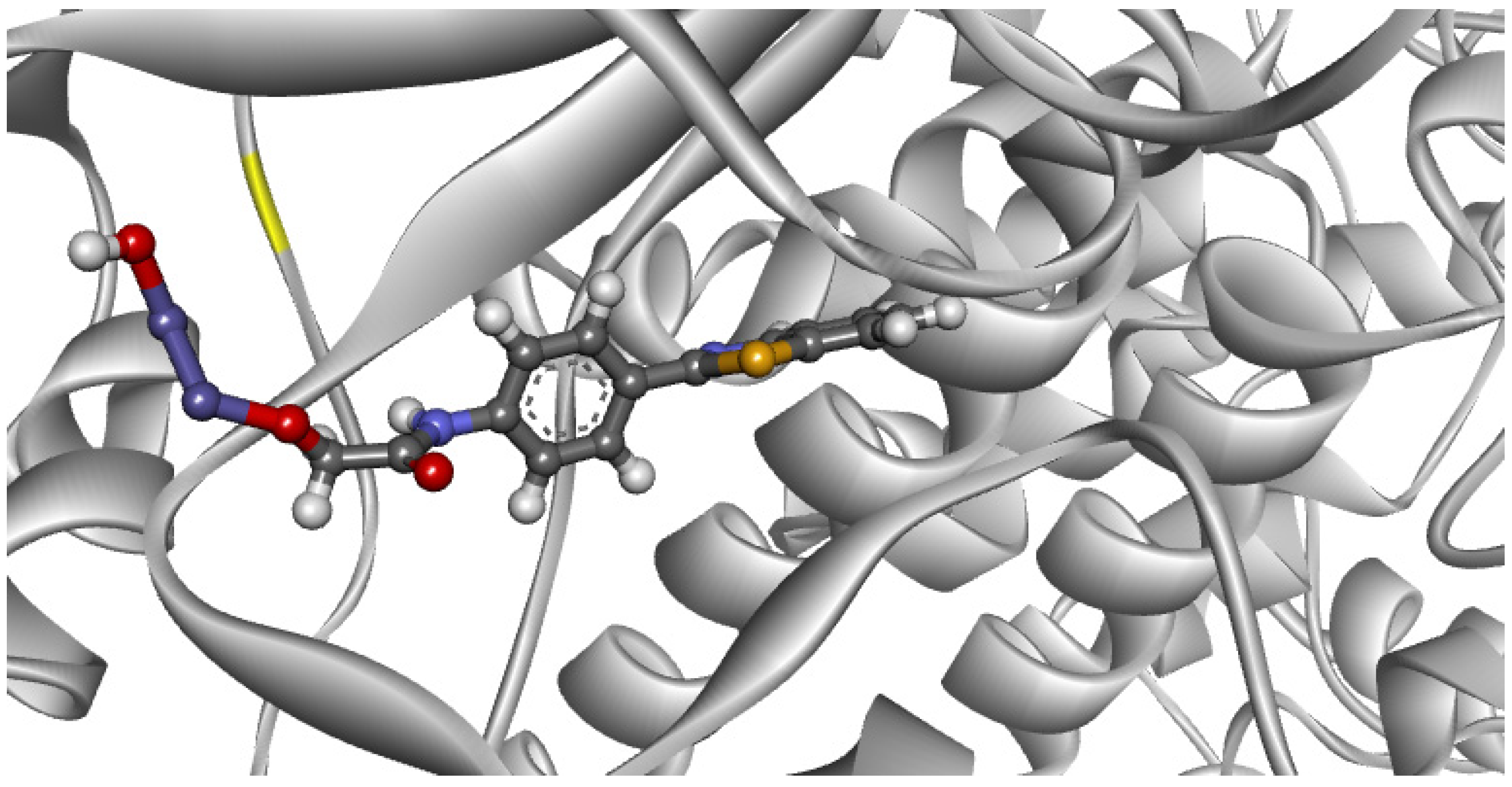
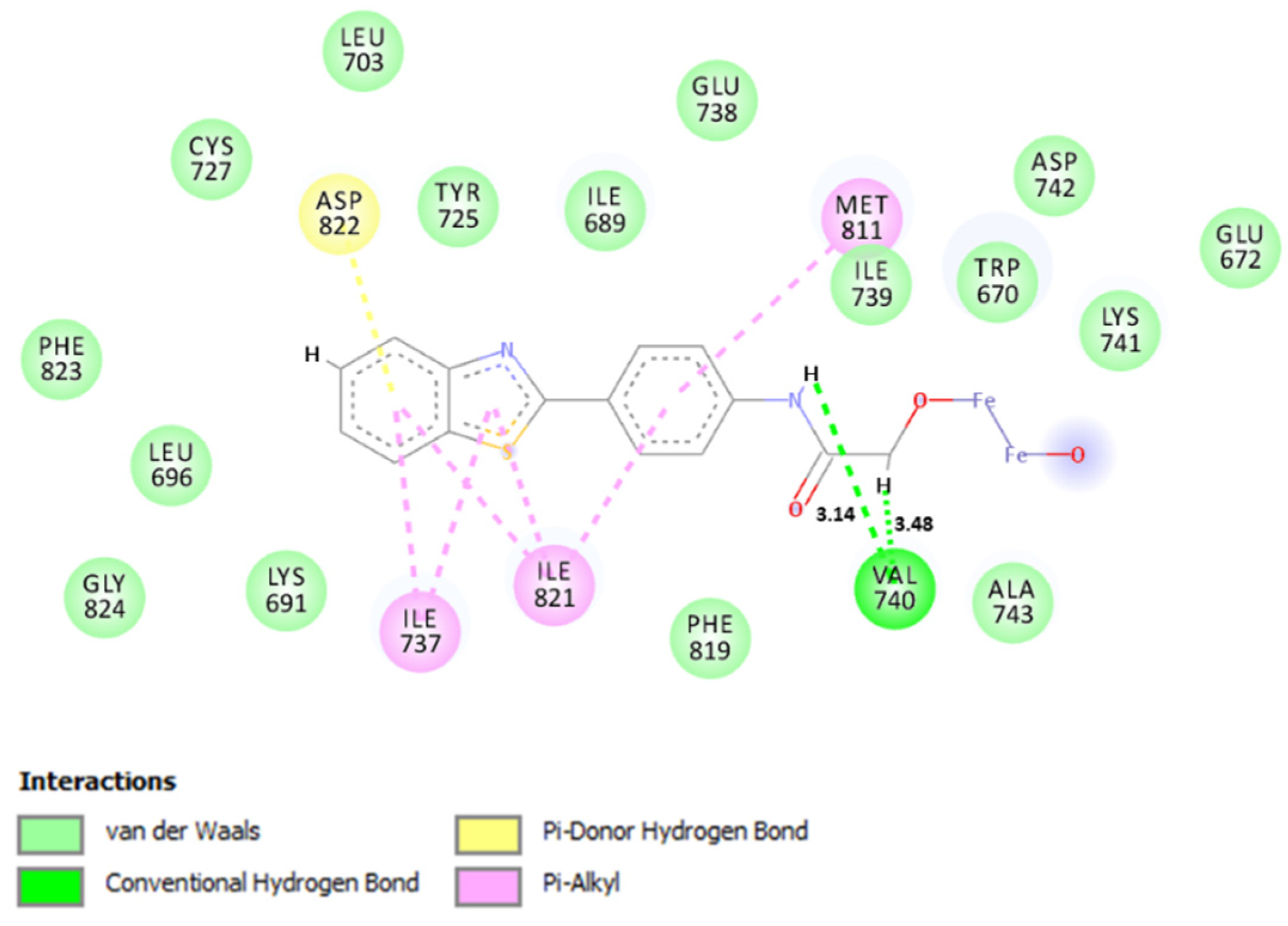
2. Results
Theoretical Studies
3. Materials and Methods
3.1. Synthesis of Nanoparticles
3.2. Characterizations of Materials
3.3. Computational Details
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wakap, S.N.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef]
- Ferreira, C.R. The burden of rare diseases. Am. J. Med. Genet. Part A 2019, 179, 885–892. [Google Scholar] [CrossRef]
- Oo, C.; Rusch, L.M. A personal perspective of orphan drug development for rare diseases: A golden opportunity or an unsustainable future? J. Clin. Pharmacol. 2015, 56, 257–259. [Google Scholar] [CrossRef]
- Taruscio, D.; Capozzoli, F.; Frank, C. Rare diseases and orphan drugs. Ann. Dell’istituto Super. Sanità 2011, 47, 83–93. [Google Scholar]
- Rosa, I.A.; Giacoppo, J.D.O.S.; Da Cunha, E.F.F.; Fernandes, Í.A.; Horvath, R.D.O.; Ionta, M.; Dos Santos, M.H.; Ramalho, T.C. Structure-Based Virtual Screening and Synthesis of Mn 2+ Complexes of Benzothiazole Derivatives for Designing New MRI Probes. ChemistrySelect 2019, 4, 3118–3122. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; Santos, L.S.; Peixoto, F.C.; Da Cunha, E.F.F.; Silva, T.C.; Ramalho, T.C. Comparing Structure and Dynamics of Solvation of Different Iron Oxide Phases for Enhanced Magnetic Resonance Imaging. ChemistrySelect 2017, 2, 10136–10142. [Google Scholar] [CrossRef]
- Mancini, D.T.; Souza, E.F.; Caetano, M.S.; Ramalho, T.C. 99Tc NMR as a promising technique for structural investigation of biomolecules: Theoretical studies on the solvent and thermal effects of phenylbenzothiazole complex. Magn. Reson. Chem. 2014, 52, 129–137. [Google Scholar] [CrossRef]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An overview on its versatile biological behavior. Eur. J. Med. Chem. 2015, 97, 612–648. [Google Scholar] [CrossRef]
- Wu, B.; Warnock, G.; Zaiss, M.; Lin, C.; Chen, M.; Zhou, Z.; Mu, L.; Nanz, D.; Tuura, R.; Delso, G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016, 3, 19. [Google Scholar] [CrossRef]
- Akram, M.W.; Fakhar-E-Alam, M.; Butt, A.R.; Munir, T.; Ali, A.; Alimgeer, K.S.; Mehmood-Ur-Rehman, K.; Iqbal, S.; Ali, S.; Ikram, M.; et al. Magnesium Oxide in Nanodimension: Model for MRI and Multimodal Therapy. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Kuo, P.H.; Kanal, E.; Abu-Alfa, A.K.; Cowper, S.E. Gadolinium-based MR Contrast. Radiology 2007, 242, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Holban, A.M.; Călugăreanu, A.; Orzan, O.A. Recent advances in diagnosis and therapy of skin cancers through nanotechnological approaches. Nanostruct. Cancer Ther. 2017, 285–306. [Google Scholar] [CrossRef]
- Ramalho, T.C.; de Castro, A.A.; Silva, D.R.; Silva, M.C.; Franca, T.C.C.; Bennion, B.J.; Kuca, K. Computational Enzymology and Organophosphorus Degrading Enzymes: Promising Approaches Toward Remediation Technologies of Warfare Agents and Pesticides. Curr. Med. Chem. 2016, 23, 1041. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Corrêa, S.; Lacerda, L.C.T.; Pires, M.S.; Rocha, M.V.J.; Nogueira, F.G.E.; da Silva, A.C.; Pereira, M.C.; de Brito, A.D.B.; da Cunha, E.F.F.; Ramalho, T.C. Synthesis, structural characterization and thermal properties of the Poly (methylmethacrylate)/δ -FeOOH hybrid material: An experimental and theoretical study. J. Nanomater. 2016. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Expanding the Limits of Computational Chemistry. Available online: https://gaussian.com/citation/ (accessed on 7 November 2020).
- D’Angelo, N.D.; Kim, T.-S.; Andrews, K.; Booker, S.K.; Caenepeel, S.; Chen, K.; D’Amico, D.; Freeman, D.; Jiang, J.; Liu, L.; et al. Discovery and Optimization of a Series of Benzothiazole Phosphoinositide 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Dual Inhibitors. J. Med. Chem. 2011, 54, 1789–1811. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Kemmish, H.; Fasnacht, M.; Yan, L. Fully automated antibody structure prediction using BIOVIA tools: Validation study. PLoS ONE 2017, 12, e0177923. [Google Scholar] [CrossRef]
- Giacoppo, J.O.S.; Franca, T.C.C.; Kuca, K.; da Cunha, E.F.F.; Abagyan, R.; Mancini, D.T.; Ramalho, T.C. Molecular modeling and in vitro reactivation study between the oxime BI-6 and acetylcholinesterase inhibited by different nerve agents. J. Biomol. Struct. Dyn. 2015, 33, 2048. [Google Scholar] [CrossRef]
- Velde, G.T.; Bickelhaupt, F.M.; Baerends, E.J.; Guerra, C.F.; Van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Leone, V.O.; Pereira, M.C.; Aquino, S.F.; Oliveira, L.C.A.; Correa, S.; Ramalho, T.C.; Gurgel, L.V.A.; Silva, A.C. Adsorption of diclofenac on a magnetic adsorbent based on maghemite: Experimental and theoretical studies. New J. Chem. 2017, 42, 437–449. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Identificação Espectrofotométrica de Compostos Orgânicos, 8th ed.; LTC: Rio de Janeiro, Brazil, 2019; p. 451. [Google Scholar]
- Benvidi, A.; Jahanbani, S.; Mirjalili, B.F.; Zare, R. Electrocatalytic oxidation of hydrazine on magnetic bar carbon paste electrode modified with benzothiazole and iron oxide nanoparticles: Simultaneous determination of hydrazine and phenol. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 549–560. [Google Scholar] [CrossRef]
- Thomas, R.C.; Chidester, C.G. Albocycline: Structure determination by X-ray crystallography. J. Antibiot. 1982, 35, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.L.; Ramalho, T.C.; Santiago, R.T.; Rocha, M.V. Orbital Signatures as a Descriptor of Regioselectivity and Chemical Reactivity: The Role of the Frontier Orbitals on 1,3-Dipolar Cycloadditions. J. Phys. Chem. 2011, 115, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Leach, A.R.; Shoichet, B.K.; Peishoff, C.E. Prediction of Protein−Ligand Interactions. Docking and Scoring: Successes and Gaps. J. Med. Chem. 2006, 49, 5851–5855. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Andrews, C.W.; Capelli, A.M.; Clarke, B.; LaLonde, J.; Lambert, M.H.; Lindvall, M.; Nevins, N.; Semus, S.F.; Senger, S.; et al. A Critical Assessment of Docking Programs and Scoring Functions. J. Med. Chem. 2006, 49, 5912–5931. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; da Cunha, E.F.F.; Peixoto, F.C.; Ramalho, T.C. Probing thermal and solvent effects on hyperfine interactions and spin relaxation rate of δ-FeOOH (1 0 0) and [MnH3buea (OH)] 2−: Toward new MRI probes. Comput. Theor. Chem. 2015, 1069, 96–104. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; Ramalho, T.C. Relaxation parameters of water molecules coordinated with Gd (III) complexes and hybrid materials based on δ-FeOOH (100) nanoparticles: A theoretical study of hyperfine inter-actions for CAs in MRI. Eclética Química J. 2020, 45, 12–20. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa, S.; Rosa, I.A.; Andolpho, G.A.; Assis, L.C.d.; Pires, M.d.S.; Lacerda, L.C.T.; Nogueira, F.G.E.; Cunha, E.F.F.d.; Nepovimova, E.; Kuca, K.; et al. Hybrid Materials Based on Magnetic Iron Oxides with Benzothiazole Derivatives: A Plausible Potential Spectroscopy Probe. Int. J. Mol. Sci. 2021, 22, 3980. https://doi.org/10.3390/ijms22083980
Corrêa S, Rosa IA, Andolpho GA, Assis LCd, Pires MdS, Lacerda LCT, Nogueira FGE, Cunha EFFd, Nepovimova E, Kuca K, et al. Hybrid Materials Based on Magnetic Iron Oxides with Benzothiazole Derivatives: A Plausible Potential Spectroscopy Probe. International Journal of Molecular Sciences. 2021; 22(8):3980. https://doi.org/10.3390/ijms22083980
Chicago/Turabian StyleCorrêa, Silviana, Isael Aparecido Rosa, Gustavo A. Andolpho, Letícia Cristina de Assis, Maíra dos S. Pires, Lívia C. T. Lacerda, Francisco G. E. Nogueira, Elaine F. F. da Cunha, Eugenie Nepovimova, Kamil Kuca, and et al. 2021. "Hybrid Materials Based on Magnetic Iron Oxides with Benzothiazole Derivatives: A Plausible Potential Spectroscopy Probe" International Journal of Molecular Sciences 22, no. 8: 3980. https://doi.org/10.3390/ijms22083980
APA StyleCorrêa, S., Rosa, I. A., Andolpho, G. A., Assis, L. C. d., Pires, M. d. S., Lacerda, L. C. T., Nogueira, F. G. E., Cunha, E. F. F. d., Nepovimova, E., Kuca, K., & Ramalho, T. C. (2021). Hybrid Materials Based on Magnetic Iron Oxides with Benzothiazole Derivatives: A Plausible Potential Spectroscopy Probe. International Journal of Molecular Sciences, 22(8), 3980. https://doi.org/10.3390/ijms22083980








