The MarR-Type Regulator PA3458 Is Involved in Osmoadaptation Control in Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results
2.1. The PA3458–PA3461 Gene Cluster of P. aeruginosa
2.2. Effect of PA3458 Lack or Excess on Bacterial Growth
2.3. Effect of Increased PA3458 Level on Gene Expression
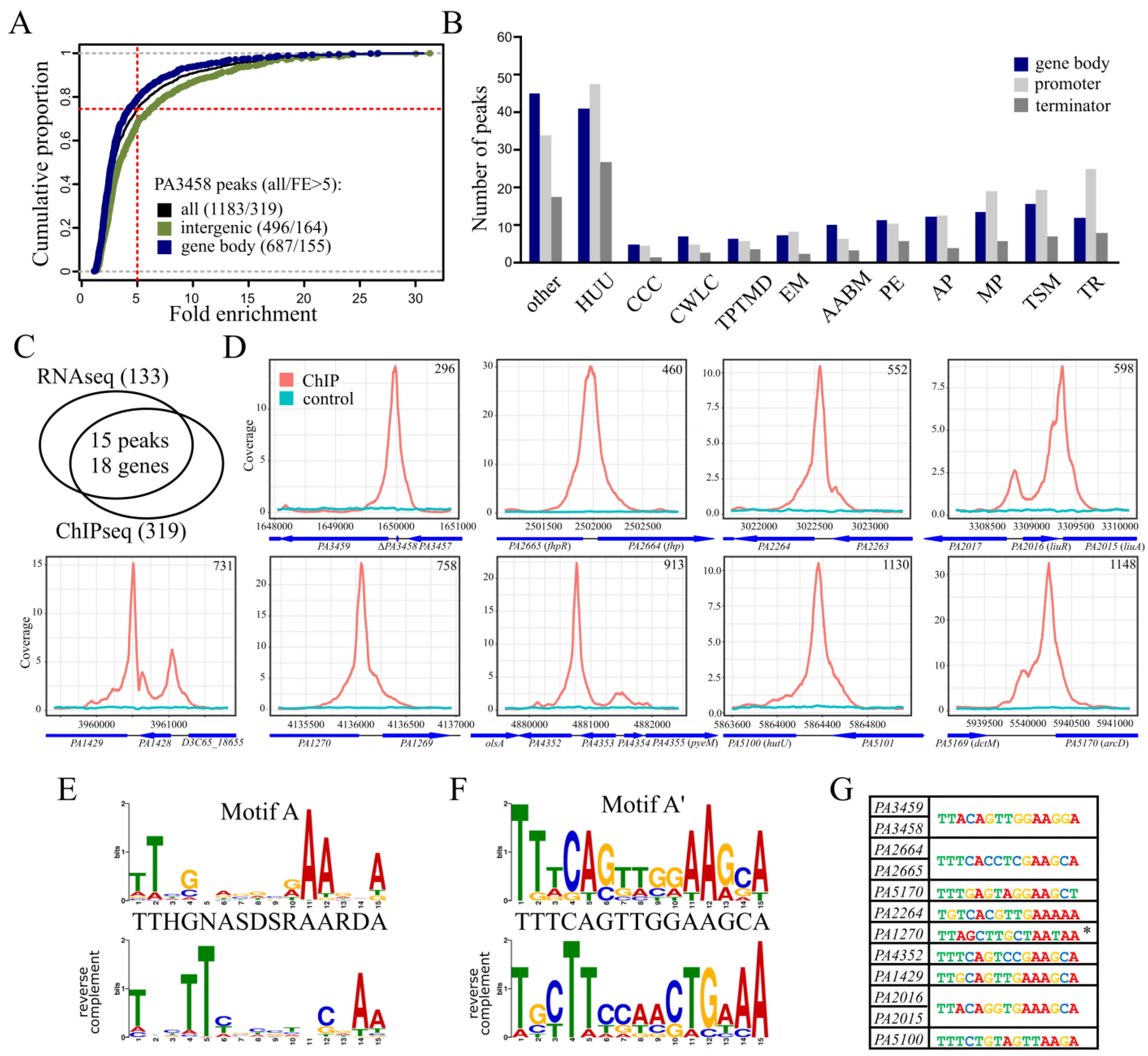
2.4. Identification of PA3458 Binding Sites in P. aeruginosa
2.5. Regulation of Gene Expression by PA3458
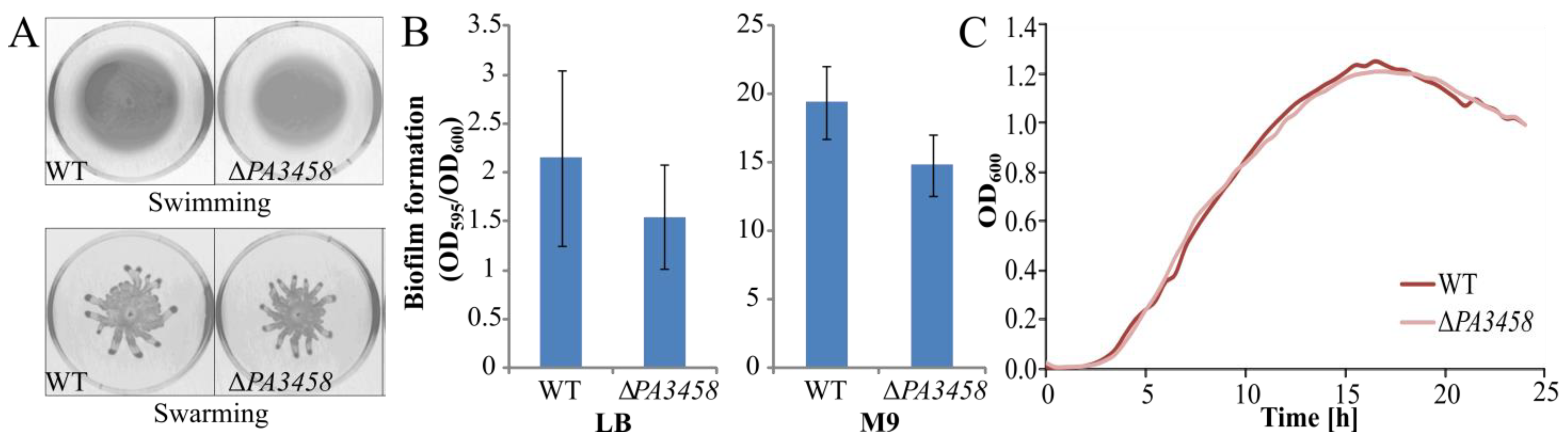
2.6. Phenotypic Characterization of PAO1161 ΔPA3458, ΔPA3459, and ΔPA3459–PA3461 Strains
2.7. Distribution and Evolutionary Conservation of PA3458–PA3461 Cluster in Bacteria
3. Discussion
4. Materials and Methods
4.1. Growth Conditions, Bacterial Strains, and Plasmids Manipulations
4.2. Construction of Expression Vectors and Protein Purification
4.3. Glutaraldehyde Crosslinking
4.4. SEC-MALS Analysis
4.5. RNA Isolation, RNA-seq, and RT-qPCR Analysis
4.6. Chromatin Immunoprecipitation with Sequencing (ChIP-seq)
4.7. Construction of Promotor-lacZ Transcriptional Fusions and Promoter Activity Testing
4.8. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| E. coli Strains | ||
|---|---|---|
| DH5α | F− (φ80dlacZΔM15) recA1 endA1 gyrA96 thi-1 hsdR17 (rk−mk+) supE44 relA1 deoR Δ(lacZYA–argF)U196 | [91] |
| BL21 | F− ompT hsdSB (rb−mb−)gal dcm (λ DE3) | [91] |
| S17-1 | pro hsdR hsdM recA TpR SmR ΩRP4-Tc∷Mu-Km∷Tn7 | [91] |
| DH5α Δlac | F- (80dlacZM15), recA1, en-dA1, gyrA96, thi-1, hsdR17 (rk-mk+), supE44, relA1, deoR Δ(lacZYA-argF), U196 | lab collection |
| Pseudomonas aeruginosaStrains | ||
| PAO1161 leu− | leu− r−m+ RifR | [38] |
| PAO1161 leu− ΔPA3458 | deletion of 462 bp fragment encompassing PA3458 gene | this work |
| PAO1161 leu− ΔPA3459 | deletion of 1758 bp fragment encompassing PA3459 gene | this work |
| PAO1161 leu− ΔPA3459–PA3461 | deletion of 4749 bp fragment encompassing PA3459–PA3461 putative operon | this work |
| PAO1161 leu+ | r−m+ RifR | [48] |
| PAO1161 leu+ ΔPA3458 | deletion of 462 bp fragment encompassing PA3458 gene | this work |
| PAO1161 leu+ ΔPA3459 | deletion of 1758 bp fragment encompassing PA3459 gene | this work |
| PAO1161 leu+ ΔPA3459–PA3461 | deletion of 4749 bp fragment encompassing PA3459–PA3461 putative operon | this work |
| Plasmids | ||
| pAKE600 | Apr; oriMB1oriTRK2, sacB | [77] |
| pKKB2.61 | Apr; pAKE600 derivative with 437 bp fragment encompassing up- and down-sequence of PA3458, amplified using 1#/2# and 3#/4# primers and cloned using BamHI, HindIII/HindIII, EcoRI | this work |
| pKKB2.62 | pAKE600 derivative with 530 bp fragment encompassing up- and down-sequence of PA3459, amplified using 5#/6# and 7#/8# primers and cloned using BamHI, HindIII/HindIII, EcoRI | this work |
| pKKB2.63 | pAKE600 derivative with 541 bp fragment encompassing up- and down-sequence of PA3459–PA3461, amplified using 5#/6# and 9#/10# primers and cloned using BamHI, HindIII/HindIII, EcoRI | this work |
| pET28a(+) | Kmr; ori pBR322; ori f1; expression vector | Novagen |
| pKKB2.21 | pET28a (+) derivative with 513 bp fragment, encoding His6-PA3458, amplified using 11#/12# primers and cloned using EcoRI, SacI | this work |
| pKKB2.22 | pET28a (+) derivative with 483 bp fragment, encoding PA3458-His6, amplified using 13#/14# primers and cloned using NcoI, HindIII | this work |
| pKGB8 | Cmr; IncA/C broad-host-range cloning vector, mob, araC-pBADp | [46] |
| pABB28.3 | pKGB8 derivative with flag | this work |
| pKKB2.11 | pKGB8 derivative with 513 bp fragment, encoding PA3458, amplified using 11#/12# primers and cloned using EcoRI, SacI | this work |
| pKAB20B | Apr; pUC19 derivative with His6-mcs (MunI, HindIII, NotI, XhoI, BamHI)-flag | this work |
| pKKB2.91 | pKAB20B derivative with 483 bp fragment, encoding PA3458–FLAG, amplified using 11#/15# primers and cloned using NcoI, BamHI | this work |
| pKKB2.12 | pKGB8 derivative with 618 bp fragment encoding PA3458-FLAG cloned using EcoRI, SalI cut from pKKB2.91 | this work |
| pCM132 | Kmr; oriVRK2; oriTRK2; promoter less lacZ reporter gene | [51] |
| pKKB2.31 | pCM132 derivative with 281 bp fragment amplified with primers 17#/18#, cloned using EcoRI, BamHI/BglII encompassing PA3458 promoter (PA3458p) | this work |
| pKKB2.32 | pCM132 derivative with 266 bp fragment amplified with primers 19#/20#, cloned using EcoRI, BamHI/BglII encompassing PA3459 promoter (PA3459p) | this work |
| Nr | Starter Name | Use | Seqence |
|---|---|---|---|
| #1 | 3458mLF | PA3458 gene deletion | gcggatccACGAAACTCTCCTGATATG (BamHI) |
| #2 | 3458mLR | gcaagcTTACTTTCAACCATAATAAATTCTTC (HindIII) | |
| #3 | 3458mPF | gcaagctttaatAGTGAGGGCGCCAGGTTCG (HindIII) | |
| #4 | 3458mPR | gcgaattCAACGCCTGTTCGCGCTGATGCTG (EcoRI) | |
| #5 | 3459–61mLF | PA3459 gene and PA3459–61 operon deletion | gcggatccAGACGCTTGGAGTGGATTTC (BamHI) |
| #6 | 3459–61mLR | gcaagcttGCCGCACATATTCCTTACC(HindIII) | |
| #7 | 3459mPF | PA3459 gene deletion | gcaagctttaatagTGAAAATGGGCCCCCAACGC(HindIII) |
| #8 | 3459mPR | gcgaattcACAGGTCGCGGGCCAATTCC (EcoRI) | |
| #9 | 3459–61mLF | PA3459–61 operon deletion | gcaagctttagtaaTGAGCCCGCGCCGGCCT (HindIII) |
| #10 | 3459–61mLR | gcgaattcGTACTCGGCGTATGCGCCCAGG (EcoRI) | |
| #11 | 3458eF | PA3458 expression | gcgaattcATGGTTGAAAGTAATAAGACCG (EcoRI) |
| #12 | 3458eR | gcgagctcCTGTCTTTCGTAGCGAAC (SacI) | |
| #13 | 3458eF3 | cgccatggATGGTTGAAAGTAATAAGACCGCTGC (NcoI) | |
| #14 | 3458eR3 | gcaagcttCTCTTCTTCGACGTCCTCTTCC (HindIII) | |
| #15 | 3458eR4 | gcggatccCTCTTCTTCGACGTCCTCTTCC (BamHI) | |
| #16 | 3458pF | PA3458 promoter cloning | cagaattcgcatgcCCAGGTCCATGATCTTCAGC (EcoRI/SphI) |
| #17 | 3458pR | gcggatccATAAATTCTTCGTTAAGGGTCCTGATAG (BamHI) | |
| #18 | 3459pF | PA3459 promoter cloning | cagaattcgcatgcCGTATCGGCTGAGACGCTTG (EcoRI/SphI) |
| #19 | 3459pR | gcggatccATTCCTTACCGGTTCTCCGTTG (BamHI) | |
| #20 | nadBqF | RT-qPCR | CTACCTGGACATCAGCCACA |
| #21 | nadBqR | GGTAATGTCGATGCCGAAGT | |
| #22 | proCqF | CAGGCCGGGCAGTTGCTGTC | |
| #23 | proCqR | GGTCAGGCGCGAGGCTGTCT | |
| #24 | 3458qF | TAATAAGACCGCTGCCGATACC | |
| #25 | 3458qR | TGAGACGCTTGGAGTGGATTTC | |
| #26 | 3459qF | CCCGGCTCAATGGGATGTTC | |
| #27 | 3459qR | AGCGGTCGAGGCTGTAGTAG | |
| #28 | 3973qF | GGATCCTGAAGTCGACGAGC | |
| #29 | 3973qR | GAAAGCTGGAATGCGCCAC | |
| #30 | 2204qF | CAGGTGGACTTCAGCATCCC | |
| #31 | 2204qR | CGCCACTTGTTGAGGACTTC | |
| #32 | 2252qF | GGGTGCCTACTTCACGATCC | |
| #33 | 2252qR | ATCAGGGCCTGGAAGGAACT | |
| #34 | 2264qF | CTACCAGCCTCGCTTCTTCA | |
| #35 | 2264qR | CTGTCGGTCGATGAACTCCG | |
| #36 | 2776qF | ACGGTATCCAATGCGACCTG | |
| #37 | 2776qR | GGCGTCCATGATCTCCAACT | |
| #38 | 3356qF | GGGCGAAACATCTTCAGCGA | |
| #39 | 3356qR | GCTGGAAGGAGTTCACATGC | |
| #40 | 3923qF | CTACTGCGAGAACGATGGCT | |
| #41 | 3923qR | ACACTGGGCTTGAGGTTCAC | |
| #42 | 4296qF | CTACCGTTGCGTAACCAGCA | |
| #43 | 4296qR | GCTCTCGATCAGGCGGATAC | |
| #44 | 4352qF | ATGAAACGAATCCTGGTGGC | |
| #45 | 4352qR | CACGTTCAGCACGGTCAGTT | |
| #46 | 5099qF | GACGTATAGCCGTAGTGGGC | |
| #47 | 5099qR | GTGCTATCCGGACGATCCAC | |
| #48 | 5100qF | GTGATCGTCTGCGACGGTAG | |
| #49 | 5100qR | CAATCGATGGCGACCTGGTA | |
| #50 | 5170qF | TCTTCTCCCTCCCGCAAAAC | |
| #51 | 5170qR | AAGACGAAAGCGAGGGTGAG | |
| #52 | 1270qF | CTATAGCGGCATGGTCCTGG | |
| #53 | 1270qR | GATGCCTGGCCATAGAGTGC | |
| #54 | 3461qF | CGGACCTCAACTACCTGCAA | |
| #55 | 3461qR | CGCACGATGGTATCGGTGAA |
References
- Gellatly, S.L.; Hancock, R.E.W. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palser, S.; Smith, S.; Nash, E.F.; Agarwal, A.; Smyth, A.R. Treatments for preventing recurrence of infection with Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst. Rev. 2019, 12, CD012300. [Google Scholar] [CrossRef]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Stress responses as determinants of antimicrobial resistance in Pseudomonas aeruginosa: Multidrug efflux and more. Can. J. Microbiol. 2014, 60, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Allan, B.; Linseman, M.; MacDonald, L.A.; Lam, J.S.; Kropinski, A.M. Heat shock response of Pseudomonas aeruginosa. J. Bacteriol. 1988, 170, 3668–3674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, Y.; Tomida, J.; Kawamura, Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front. Microbiol. 2014, 4, 422. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, P.; Pourhajibagher, M.; Chiniforush, N.; Hosseini, N.; Bahador, A. The effect of quorum-sensing and efflux pumps interactions in Pseudomonas aeruginosa against photooxidative stress. J. Lasers Med. Sci. 2018, 9, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Aspedon, A.; Palmer, K.; Whiteley, M. Microarray analysis of the osmotic stress response in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 2721–2725. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Ha, J.; Lee, H.; Lee, S.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y.; Choi, K.-H. Role of Pseudomonas aeruginosa DesB in adaptation to osmotic stress. J. Food Prot. 2019, 82, 1278–1282. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef]
- Perez-Rueda, E.; Hernandez-Guerrero, R.; Martinez-Nuñez, M.A.; Armenta-Medina, D.; Sanchez, I.; Ibarra, J.A. Abundance, diversity and domain architecture variability in prokaryotic DNA-binding transcription factors. PLoS ONE 2018, 13, e0195332. [Google Scholar] [CrossRef] [Green Version]
- Grove, A. MarR family transcription factors. Curr. Biol. 2013, 23, R142–R143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alekshun, M.N.; Levy, S.B.; Mealy, T.R.; Seaton, B.A.; Head, J.F. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 2001, 8, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Regulation of chromosomally mediated multiple antibiotic resistance: The Mar regulon. Antimicrob. Agents Chemother. 1997, 41, 2067–2075. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.G.; Rosner, J.L. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to Mar operator sequences. Proc. Natl. Acad. Sci. USA 1995, 92, 5456–5460. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, T.M.; Levy, S.B. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J. Bacteriol. 2000, 182, 3467–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.G.; Jair, K.W.; Wolf, R.E.; Rosner, J.L. Autoactivation of the MarRAB multiple antibiotic resistance operon by the MarA transcriptional activator in Escherichia coli. J. Bacteriol. 1996, 178, 2216–2223. [Google Scholar] [CrossRef] [Green Version]
- Deochand, D.K.; Grove, A. MarR family transcription factors: Dynamic variations on a common scaffold. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 595–613. [Google Scholar] [CrossRef]
- Wei, K.; Tang, D.-J.; He, Y.-Q.; Feng, J.-X.; Jiang, B.-L.; Lu, G.-T.; Chen, B.; Tang, J.-L. HpaR, a putative MarR family transcriptional regulator, is positively controlled by HrpG and HrpX and involved in the pathogenesis, hypersensitive response, and extracellular protease production of Xanthomonas cmpestris pathovar campestris. J. Bacteriol. 2007, 189, 2055–2062. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Luo, F.; Wu, D.; Hikichi, Y.; Kiba, A.; Igarashi, Y.; Ding, W.; Ohnishi, K. PrhN, a putative MarR family transcriptional regulator, is involved in positive regulation of Type III Secretion System and full virulence of Ralstonia solanacearum. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Haque, M.M.; Kabir, M.S.; Aini, L.Q.; Hirata, H.; Tsuyumu, S. SlyA, a MarR family transcriptional regulator, is essential for virulence in Dickeya dadantii 3937. J. Bacteriol. 2009, 191, 5409–5418. [Google Scholar] [CrossRef] [Green Version]
- Michaux, C.; Sanguinetti, M.; Reffuveille, F.; Auffray, Y.; Posteraro, B.; Gilmore, M.S.; Hartke, A.; Giard, J.-C. SlyA is a transcriptional regulator involved in the virulence of Enterococcus faecalis. Infect. Immun. 2011, 79, 2638–2645. [Google Scholar] [CrossRef] [Green Version]
- Michaux, C.; Martini, C.; Hanin, A.; Auffray, Y.; Hartke, A.; Giard, J.-C. SlyA Regulator is involved in bile salts stress response of Enterococcus faecalis. FEMS Microbiol. Lett. 2011, 324, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Buchmeier, N.; Bossie, S.; Chen, C.Y.; Fang, F.C.; Guiney, D.G.; Libby, S.J. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect. Immun. 1997, 65, 3725–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leelakriangsak, M.; Huyen, N.T.T.; Töwe, S.; Duy, N.V.; Becher, D.; Hecker, M.; Antelmann, H.; Zuber, P. Regulation of quinone detoxification by the thiol stress sensing DUF24/MarR-like repressor, YodB in Bacillus subtilis. Mol. Microbiol. 2008, 67, 1108–1124. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Chen, C.; Su, T.; Che, C.; Yao, S.; Liang, G.; Li, G.; Yang, G. CosR is an oxidative stress sensing a MarR-Type transcriptional repressor in Corynebacterium glutamicum. Biochem. J. 2018, 475, 3979–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.L.; Silva, D.N.; Pauer, H.; Ferreira, L.Q.; Ferreira, E.; Domingues, R.M.; Lobo, L.A. The role of BmoR, a MarR family regulator, in the survival of Bacteroides fragilis during oxidative stress. Int. J. Med. Microbiol. 2013, 303, 443–448. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [Green Version]
- Winsor, G.L.; Lo, R.; Sui, S.J.H.; Ung, K.S.E.; Huang, S.; Cheng, D.; Ching, W.-K.H.; Hancock, R.E.W.; Brinkman, F.S.L. Pseudomonas aeruginosa genome database and PseudoCAP: Facilitating community-based, continually updated, genome annotation. Nucleic Acids Res. 2005, 33, D338–D343. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, P.; Rojo, F.; Martínez, J.L. Transcriptional regulation of MexR, the repressor of Pseudomonas aeruginosa MexAB-OprM multidrug efflux pump. FEMS Microbiol. Lett. 2002, 207, 63–68. [Google Scholar] [CrossRef]
- Ziha-Zarifi, I.; Llanes, C.; Köhler, T.; Pechere, J.-C.; Plesiat, P. In Vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob. Agents Chemother. 1999, 43, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hu, J.; Chen, P.R.; Lan, L.; Li, Z.; Hicks, L.M.; Dinner, A.R.; He, C. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc. Natl. Acad. Sci. USA 2008, 105, 13586–13591. [Google Scholar] [CrossRef] [Green Version]
- Atichartpongkul, S.; Vattanaviboon, P.; Wisitkamol, R.; Jaroensuk, J.; Mongkolsuk, S.; Fuangthong, M. Regulation of organic hydroperoxide stress response by two OhrR homologs in Pseudomonas aeruginosa. PLoS ONE 2016, 11, e0161982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Park, S.-Y.; Heo, Y.-J.; Cho, Y.-H. Drosophila melanogaster-based screening for multihost virulence factors of Pseudomonas aeruginosa PA14 and identification of a virulence-attenuating factor, HudA. Infect. Immun. 2008, 76, 4152–4162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosik, A.A.; Glabski, K.; Jecz, P.; Mikulska, S.; Fogtman, A.; Koblowska, M.; Jagura-Burdzy, G. Transcriptional profiling of parA and parB mutants in actively dividing cells of an opportunistic human pathogen Pseudomonas aeruginosa. PLoS ONE 2014, 9, e87276. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, A.A.; Mierzejewska, J.; Thomas, C.M.; Jagura-Burdzy, G. ParB deficiency in Pseudomonas aeruginosa destabilizes the partner protein ParA and affects a variety of physiological parameters. Microbiology 2009, 155, 1080–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasocki, K.; Bartosik, A.A.; Mierzejewska, J.; Thomas, C.M.; Jagura-Burdzy, G. Deletion of the ParA (Soj) homologue in Pseudomonas aeruginosa causes ParB instability and affects growth rate, chromosome segregation, and motility. J. Bacteriol. 2007, 189, 5762–5772. [Google Scholar] [CrossRef] [Green Version]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK Server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Yaron, S.; White, D.G.; Matthews, K.R. Characterization of an Escherichia coli O157:H7 MarR mutant. Int. J. Food Microbiol. 2003, 85, 281–291. [Google Scholar] [CrossRef]
- Will, W.R.; Brzovic, P.; Le Trong, I.; Stenkamp, R.E.; Lawrenz, M.B.; Karlinsey, J.E.; Navarre, W.W.; Main-Hester, K.; Miller, V.L.; Libby, S.J.; et al. The evolution of SlyA/RovA transcription factors from repressors to countersilencers in Enterobacteriaceae. MBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.R.; Brown, B.L.; Page, R.; Sello, J.K. Study of PcaV from Streptomyces coelicolor yields new insights into ligand-responsive MarR family transcription factors. Nucleic Acids Res. 2013, 41, 3888–3900. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-R.; Li, D.-F.; Fleming, J.; Zhou, Y.-F.; Liu, Y.; Deng, J.-Y.; Zhou, L.; Zhou, J.; Zhu, G.-F.; Zhang, X.-E.; et al. Structural analysis of the regulatory mechanism of MarR protein Rv2887 in M. tuberculosis. Sci. Rep. 2017, 7, 6471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawalek, A.; Glabski, K.; Bartosik, A.A.; Fogtman, A.; Jagura-Burdzy, G. Increased ParB level affects expression of stress response, adaptation and virulence operons and potentiates repression of promoters adjacent to the high affinity binding sites ParS3 and ParS4 in Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0181726. [Google Scholar] [CrossRef] [Green Version]
- Kovach, M.E.; Phillips, R.W.; Elzer, P.H.; Roop, R.M.; Peterson, K.M. PBBR1MCS: A broad-host-range cloning vector. Biotechniques 1994, 16, 800–802. [Google Scholar] [PubMed]
- Kawalek, A.; Kotecka, K.; Modrzejewska, M.; Gawor, J.; Jagura-Burdzy, G.; Bartosik, A.A. Genome sequence of Pseudomonas aeruginosa PAO1161, a PAO1 derivative with the ICEPae1161 integrative and conjugative element. BMC Genom. 2020, 21, 14. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Machanick, P.; Bailey, T.L. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 2011, 27, 1696–1697. [Google Scholar] [CrossRef] [Green Version]
- Marx, C.J.; Lidstrom, M.E. Development of improved versatile broad-host-range vectors for use in methylotrophs and other gram-negative bacteria. Microbiology 2001, 147, 2065–2075. [Google Scholar] [CrossRef] [Green Version]
- Sagot, B.; Gaysinski, M.; Mehiri, M.; Guigonis, J.-M.; Rudulier, D.L.; Alloing, G. Osmotically induced synthesis of the dipeptide N-Acetylglutaminylglutamine amide is mediated by a new pathway conserved among bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 12652–12657. [Google Scholar] [CrossRef] [Green Version]
- Medema, M.H.; Takano, E.; Breitling, R. Detecting sequence homology at the gene cluster level with MultiGeneBlast. Mol. Biol. Evol. 2013, 30, 1218–1223. [Google Scholar] [CrossRef]
- Tümmler, B.; Wiehlmann, L.; Klockgether, J.; Cramer, N. Advances in understanding Pseudomonas. F1000Prime Rep. 2014, 6, 9. [Google Scholar] [CrossRef]
- Will, W.R.; Fang, F.C. The evolution of MarR family transcription factors as counter-silencers in regulatory networks. Curr. Opin. Microbiol. 2020, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- D’Souza-Ault, M.R.; Smith, L.T.; Smith, G.M. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl. Environ. Microbiol. 1993, 59, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, S.J.; Goebel, W.; Ludwig, A.; Buchmeier, N.; Bowe, F.; Fang, F.C.; Guiney, D.G.; Songer, J.G.; Heffron, F. A Cytolysin encoded by Salmonella is required for survival within macrophages. Proc. Natl. Acad. Sci. USA 1994, 91, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, I.R.; Higgins, C.F. Enteric bacteria and osmotic stress: Intracellular potassium glutamate as a secondary signal of osmotic stress? FEMS Microbiol. Rev. 1990, 6, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Dorman, C.J.; Dorman, M.J. DNA Supercoiling is a fundamental regulatory principle in the control of bacterial gene expression. Biophys. Rev. 2016, 8, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Dillon, S.C.; Dorman, C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010, 8, 185–195. [Google Scholar] [CrossRef]
- Hünnefeld, M.; Persicke, M.; Kalinowski, J.; Frunzke, J. The MarR-Type Regulator MalR is involved in stress-responsive cell envelope remodeling in Corynebacterium glutamicum. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Liang, F.; Li, R.-J.; Qian, W. MarR-family transcription factor HpaR controls expression of the VgrR-VgrS operon of Xanthomonas campestris pv. campestris. Mol. Plant Microbe Interact. 2017, 31, 299–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, G.J.; Morreale, D.P.; Boyd, E.F. CosR is a global regulator of the osmotic stress response with widespread distribution among bacteria. Appl. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Kallio, P.T. Induction of Pseudomonas aeruginosa Fhp and FhpR by reactive oxygen Species. Can. J. Microbiol. 2009, 55, 657–663. [Google Scholar] [CrossRef]
- Forrester, M.T.; Foster, M.W. Protection from nitrosative stress: A central role for microbial flavohemoglobin. Free Radic. Biol. Med. 2012, 52, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Firoved, A.M.; Wood, S.R.; Ornatowski, W.; Deretic, V.; Timmins, G.S. Microarray analysis and functional characterization of the nitrosative stress response in nonmucoid and mucoid Pseudomonas aeruginosa. J. Bacteriol. 2004, 186, 4046–4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Qin, J.; Tan, B.; Kong, W.; Chen, G.; Zhang, C.; Liang, H. The P-type ATPase PA1429 regulates quorum-sensing systems and bacterial virulence. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Borriello, G.; Richards, L.; Ehrlich, G.D.; Stewart, P.S. Arginine or nitrate enhances antibiotic susceptibility of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 2006, 50, 382–384. [Google Scholar] [CrossRef] [Green Version]
- Lüthi, E.; Baur, H.; Gamper, M.; Brunner, F.; Villeval, D.; Mercenier, A.; Haas, D. The Arc operon for anaerobic arginine catabolism in Pseudomonas aeruginosa contains an additional gene, arcD, encoding a membrane protein. Gene 1990, 87, 37–43. [Google Scholar] [CrossRef]
- Benkert, B.; Quäck, N.; Schreiber, K.; Jaensch, L.; Jahn, D.; Schobert, M. Nitrate-responsive NarX-NarL represses arginine-mediated induction of the Pseudomonas aeruginosa arginine fermentation arcDABC operon. Microbiology 2008, 154, 3053–3060. [Google Scholar] [CrossRef] [Green Version]
- Chou, H.T.; Li, J.-Y.; Peng, Y.-C.; Lu, C.-D. Molecular characterization of PauR and its role in control of putrescine and cadaverine catabolism through the γ-glutamylation pathway in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2013, 195, 3906–3913. [Google Scholar] [CrossRef] [Green Version]
- Koskenkorva, T.; Aro-Kärkkäinen, N.; Bachmann, D.; Arai, H.; Frey, A.D.; Kallio, P.T. Transcriptional activity of Pseudomonas aeruginosa fhp promoter is dependent on two regulators in addition to FhpR. Arch. Microbiol. 2008, 189, 385–396. [Google Scholar] [CrossRef]
- Díaz-Pérez, A.L.; Núñez, C.; Meza Carmen, V.; Campos-García, J. The expression of the genes involved in leucine catabolism of Pseudomonas aeruginosa is controlled by the transcriptional regulator LiuR and by the CbrAB/Crc system. Res. Microbiol. 2018, 169, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Boes, N.; Schreiber, K.; Härtig, E.; Jaensch, L.; Schobert, M. The Pseudomonas aeruginosa universal stress protein PA4352 is essential for surviving anaerobic energy stress. J. Bacteriol. 2006, 188, 6529–6538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, M.; Kolter, R.; Thomas, C.; Figurski, D.; Meyer, R.; Remaut, E.; Helinski, D.R. Plasmid Cloning Vehicles Derived from Plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979, 68, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Maniatis, T.; Fritsch, E.F.; Laboratory, C.S.H. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1987; ISBN 978-0-87969-309-1. [Google Scholar]
- El-Sayed, A.K.; Hothersall, J.; Thomas, C.M. Quorum-sensing-dependent regulation of biosynthesis of the polyketide antibiotic mupirocin in Pseudomonas fluorescens NCIMB 10586. Microbiology 2001, 147, 2127–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Häussler, S. Chromatin immunoprecipitation for ChIP-chip and ChIP-seq. In Pseudomonas Methods and Protocols; Filloux, A., Ramos, J.-L., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; pp. 591–605. ISBN 978-1-4939-0473-0. [Google Scholar]
- Kawalek, A.; Bartosik, A.A.; Glabski, K.; Jagura-Burdzy, G. Pseudomonas aeruginosa partitioning protein ParB acts as a nucleoid-associated protein binding to multiple copies of a ParS-related motif. Nucleic Acids Res. 2018, 46, 4592–4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome project data processing subgroup the sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, F.; Ryan, D.P.; Grüning, B.; Bhardwaj, V.; Kilpert, F.; Richter, A.S.; Heyne, S.; Dündar, F.; Manke, T. DeepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016, 44, W160–W165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Salamov, V.S.A.; Solovyevand, A. Automatic annotation of microbial genomes and metagenomic sequences. In Metagenomics and Its Applications in Agriculture, Biomedicine and Environmental Studies; Li, R.W., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 61–78. [Google Scholar]
- Goudenège, D.; Avner, S.; Lucchetti-Miganeh, C.; Barloy-Hubler, F. CoBaltDB: Complete bacterial and archaeal orfeomes subcellular localization database and associated resources. BMC Microbiol. 2010, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Simon, R.; O’Connell, M.; Labes, M.; Pühler, A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986, 118, 640–659. [Google Scholar] [PubMed]





| Peak nr | Peak Summit | Fold Enrichment | Fold Change | Feature * | PAO1161 [D3C65_] ID | PAO1 ID | Gene Name | Gene Product |
|---|---|---|---|---|---|---|---|---|
| 296 | 1650070 | 27.17 | −3.62 | promoter − | 07825 | PA3459 | N-acetylglutaminylglutamine amidotransferase | |
| 49.67 | promoter + | 07830 | PA3458 | MarR family transcriptional regulator | ||||
| 460 | 2502004 | 26.49 | −42.63 | promoter + | 12095 | PA2664 | fhp | NO-inducible flavohemoprotein |
| −2.05 | promoter - | 12090 | PA2665 | fhpR (norR) | nitric oxide reductase transcriptional regulator FhpR (NorR) | |||
| 1148 | 5940238 | 23.15 | −9.38 | promoter + | 28110 | PA5170 | arcD | arginine:ornithine antiporter |
| 552 | 3022546 | 11.73 | 2.27 | promoter − | 14200 | PA2264 | gluconate 2-dehydrogenase subunit 3 family protein | |
| 758 | 4136054 | 18.41 | 5.03 | promoter − | 19465 | PA1270 | FUSC family protein | |
| 905 | 4819815 | 17.66 | 2.08 | gene body | 22785 | PA4295 | fppA | type 4b pilus Flp prepilin peptidase |
| 913 | 4880773 | 16.55 | −11.11 | promoter − | 23080 | PA4352 | universal stress protein | |
| 731 | 3960512 | 13.75 | −15.23 | promoter − | 18645 | PA1429 | cation-transporting P-type ATPase | |
| 686 | 3777799 | 12.53 | −3.87 | gene body | 17765 | PA1596 | htpG | molecular chaperone HtpG |
| 598 | 3309346 | 11.61 | −3.39 | terminator + | 15560 | PA2016 | liuR | MerR family DNA-binding transcriptional regulator |
| −3.09 | promoter + | 15565 | PA2015 | liuA | isovaleryl-CoA dehydrogenase | |||
| 770 | 4215905 | 8.68 | −2.01 | terminator − | 19850 | PA1197 | hypothetical protein | |
| 1130 | 5864366 | 7.23 | −6.09 | promoter − | 27730 | PA5100 | hutU | urocanate hydratase |
| 195 | 1065438 | 6.33 | −2.47 | terminator + | 05130 | PA3971 | PaaI family thioesterase | |
| 555 | 3042187 | 6.20 | −3.60 | gene body | 14290 | PA2247 | bkdA1 | 3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring subunit alpha |
| 860 | 4567471 | 6.11 | −2.76 | gene body | 21640 | PA0866 | aroP2 | amino acid permease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotecka, K.; Kawalek, A.; Kobylecki, K.; Bartosik, A.A. The MarR-Type Regulator PA3458 Is Involved in Osmoadaptation Control in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 3982. https://doi.org/10.3390/ijms22083982
Kotecka K, Kawalek A, Kobylecki K, Bartosik AA. The MarR-Type Regulator PA3458 Is Involved in Osmoadaptation Control in Pseudomonas aeruginosa. International Journal of Molecular Sciences. 2021; 22(8):3982. https://doi.org/10.3390/ijms22083982
Chicago/Turabian StyleKotecka, Karolina, Adam Kawalek, Kamil Kobylecki, and Aneta Agnieszka Bartosik. 2021. "The MarR-Type Regulator PA3458 Is Involved in Osmoadaptation Control in Pseudomonas aeruginosa" International Journal of Molecular Sciences 22, no. 8: 3982. https://doi.org/10.3390/ijms22083982
APA StyleKotecka, K., Kawalek, A., Kobylecki, K., & Bartosik, A. A. (2021). The MarR-Type Regulator PA3458 Is Involved in Osmoadaptation Control in Pseudomonas aeruginosa. International Journal of Molecular Sciences, 22(8), 3982. https://doi.org/10.3390/ijms22083982






