Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Ethylene–norbornene Copolymer (Topas) and Polylactide (PLA) Composites with Cannabidiol (CBD) Extract
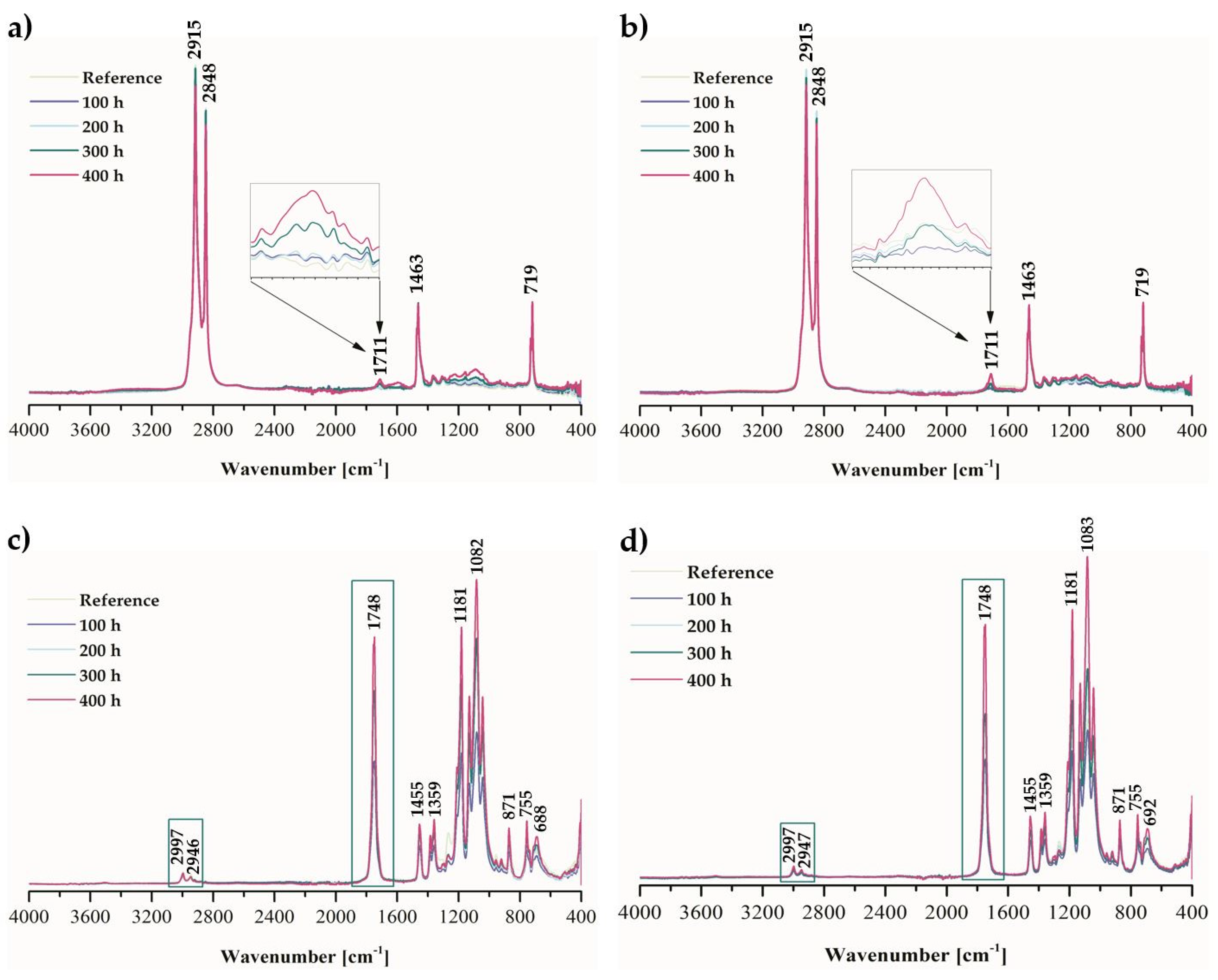
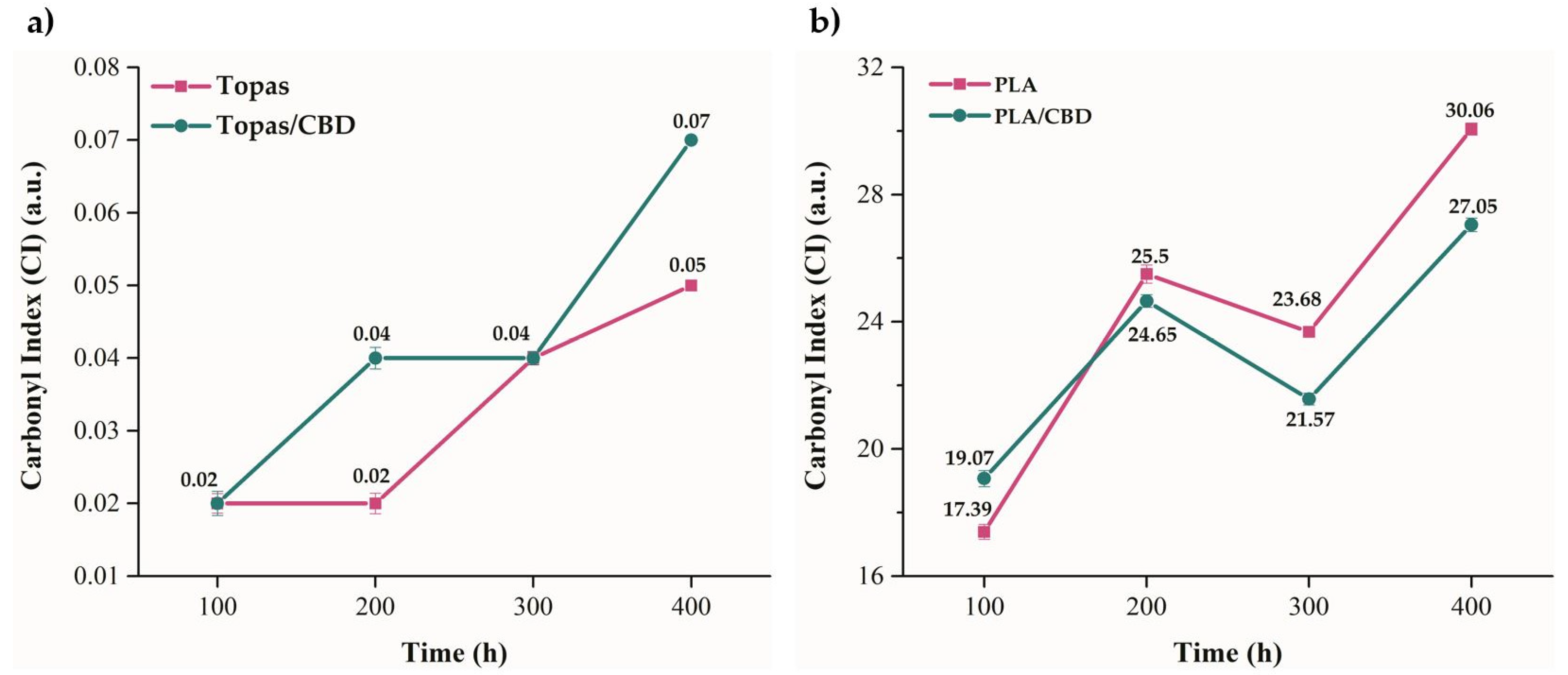
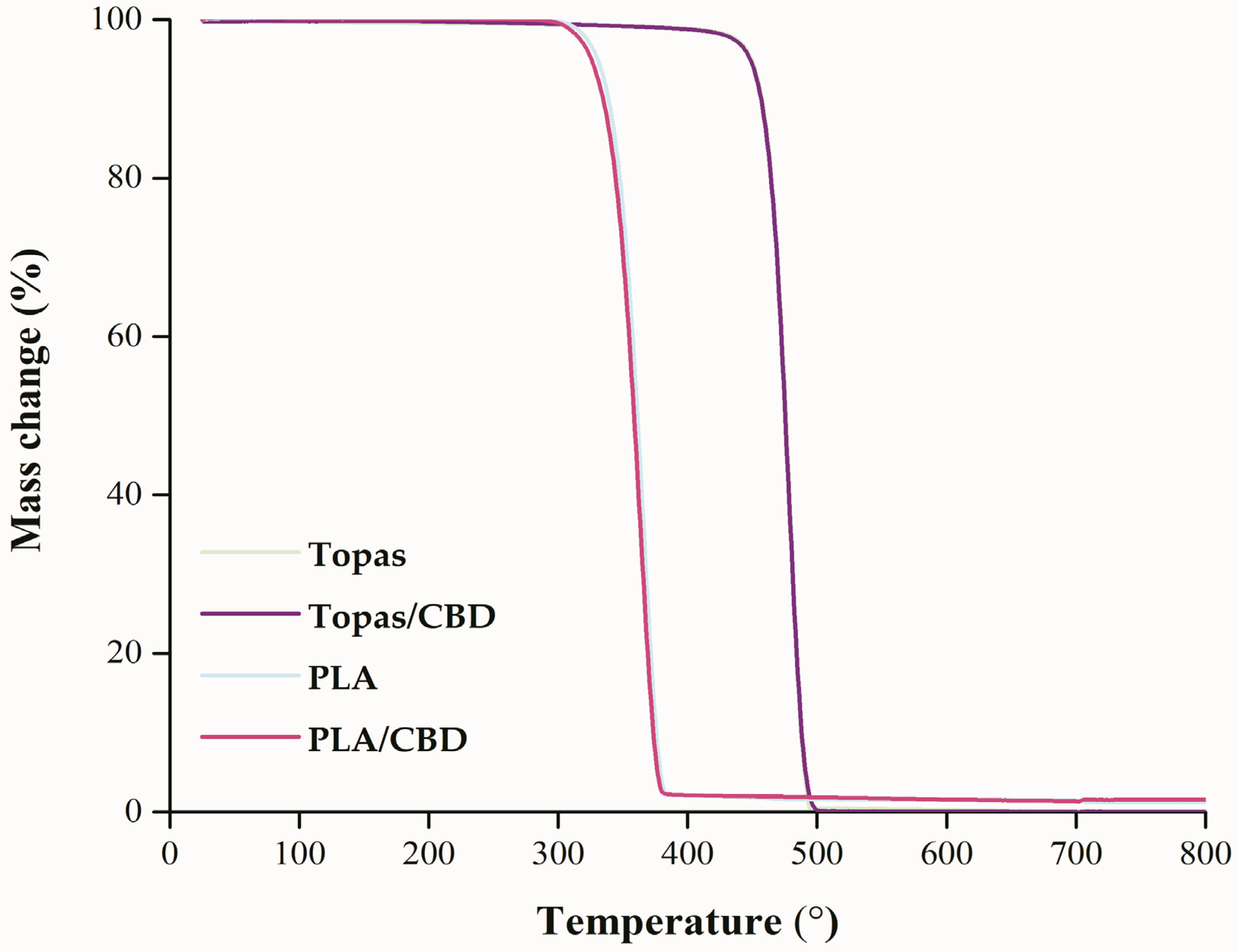
2.2. Thermal Analysis of Ethylene-norbornene Copolymer (Topas) and Polylactide (PLA) Composites with Cannabidiol (CBD) Extract
3. Materials and Methods
3.1. Reagents
3.2. Methods of Samples Preparation
3.3. Weather-Aging
3.4. Measurement Methods
3.4.1. Surface Free Energy (SFE)
3.4.2. Surface Morphology Analysis
3.4.3. Fourier-Transform Infrared Spectroscopy
3.4.4. Change in Color Measurements
3.4.5. Thermogravimetric Analysis (TGA)
3.4.6. Oxidative-Induction Time (OIT)
- Heating from 0 to 200 °C for 15 min under an argon atmosphere at a flow rate of 50 mL/min;
- Heating in the investigation temperature, 200 °C for 10 min under an argon atmosphere at a flow rate of 50 mL/min;
- Cooling from 200 to 0 °C for 20 min under an argon atmosphere at a flow rate of 50 mL/min;
- Heating from 0 to 350 °C for 30 min under an air atmosphere at a flow rate of 50 mL/min.
4. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol extract obtained from Cannabis Plants |
| PLA | Polylactide |
| COCs | Cyclic Olefin Copolymers |
| Topas | Ethylene–Norbornene Copolymer |
| PS | Polystyrene |
| PP | Polypropylene |
| PE | Polyethylene |
| PHAs | Polyhydroxyalkanoates from aliphatic polyesters group |
| BHA | Butylated Hydroxyanisole |
| BHT | Butylated Hydroxytoluene |
| THC | Tetrahydrocannabinol |
| FTIR | Fourier-transform Infrared Spectroscopy |
| OIT | Oxidation Induction Time/Temperature |
| SFE | Surface Free Energy [mJ/m2] |
| CI | Carbonyl Index [–] |
| TGA | Thermogravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
References
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangroniz, A.; Zhu, J.B.; Tang, X.; Etxeberria, A.; Chen, E.Y.X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kurtela, A.; Antolović, N. The problem of plastic waste and microplastics in the seas and oceans: Impact on marine organisms. Ribar. Croat. J. Fish. 2019, 77, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Rajmohan, K.V.S.; Ramya, C.; Raja Viswanathan, M.; Varjani, S. Plastic pollutants: Effective waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Khare, A.; Deshmukh, S. Studies toward producing eco-friendly plastics. J. Plast. Film Sheeting 2006, 22, 193–211. [Google Scholar] [CrossRef]
- Sudesh, K.; Iwata, T. Sustainability of biobased and biodegradable plastics. CLEAN—Soil Air Water 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [Green Version]
- Nkwachukwu, O.; Chima, C.; Ikenna, A.; Albert, L. Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. Int. J. Ind. Chem. 2013, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of Bio-Based Products on Waste Management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Haider, T.P.; Vçlker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and fossil-based polymeric blends and nanocomposites for packaging: Structure-property relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Wu, H.; Liu, R.; Wu, C. Preparation of Long Sisal Fiber-Reinforced Polylactic Acid Biocomposites with Highly Improved Mechanical Performance. Polymers 2021, 13, 1124. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, M.; Jawaid, M.; Parveez, B. Bamboo Fiber Based Cellulose Nanocrystals/Poly(Lactic Acid)/Poly(Butylene Succinate) Nanocomposites: Morphological, Mechanical and Thermal Properties. Polymers 2021, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Brounstein, Z.; Yeager, C.M.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerín, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltrán, J.A.; Roncalés, P. Stabilization of beef meat by a new active packaging containing natural antioxidants. J. Agric. Food Chem. 2006, 54, 7840–7846. [Google Scholar] [CrossRef]
- Marcos, B.; Sárraga, C.; Castellari, M.; Kappen, F.; Schennink, G.; Arnau, J. Development of biodegradable films with antioxidant properties based on polyesters containing α-tocopherol and olive leaf extract for food packaging applications. Food Packag. Shelf Life 2014, 1, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Masek, A.; Latos, M.; Piotrowska, M.; Zaborski, M. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packag. Shelf Life 2018, 16, 51–58. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic profile and antioxidant activity of Juglans regia L. leaves and husk extracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef] [Green Version]
- Paradiso, V.M.; Flamminii, F.; Pittia, P.; Caponio, F.; Di Mattia, C. Radical Scavenging Activity of Olive Oil Phenolic Antioxidants in Oil or Water Phase during the Oxidation of O/W Emulsions: An Oxidomics Approach. Antioxidants 2020, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Emad, S., Ed.; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Giordano, M.E.; Caricato, R.; Lionetto, M.G. Concentration dependence of the antioxidant and prooxidant activity of trolox in hela cells: Involvement in the induction of apoptotic volume decrease. Antioxidants 2020, 9, 1058. [Google Scholar] [CrossRef]
- Lukanina, Y.K.; Kolesnikova, N.N.; Popov, A.A.; Khvatov, A.V. Oxo-degradation of LDPE with pro-oxidant additive. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 22–23 August 2019; Volume 525, p. 012104. [Google Scholar]
- Dintcheva, N.T.; Gennaro, D.; Teresi, R.; Baiamonte, M. Pro-degradant activity of naturally occurring compounds on polyethylene in accelerate weathering conditions. Materials 2019, 12, 195. [Google Scholar] [CrossRef] [Green Version]
- Lukanina, Y.K.; Popov, A.A.; Khvatov, A.V. Biodegradation of polymer compositions with pro-oxidants. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Karnataka, India, 17–18 July 2020; Volume 921, p. 012016. [Google Scholar]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A.; Zawadziłło, J. Processability and Mechanical Properties of Thermoplastic Polylactide/Polyhydroxybutyrate (PLA/PHB) Bioblends. Materials 2021, 14, 898. [Google Scholar] [CrossRef]
- Szadkowski, B.; Kuśmierek, M.; Rybiński, P.; Zukowski, W.; Marzec, A. Application of earth pigments in cycloolefin copolymer: Protection against combustion and accelerated aging in the full sunlight spectrum. Materials 2020, 13, 3381. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Toschi, T.G. Preliminary study: Comparison of antioxidant activity of cannabidiol (CBD) and α-tocopherol added to refined olive and sunflower oils. Molecules 2019, 24, 3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.V.; Shah, S.; Albornoz, C.A.; Saedi, N. Consumer interest in topical cannabidiol (CBD): An examination of online search trends from 2015–2019. Clin. Dermatol. 2020. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Singh Chauhan, B.P.; Sharma, V. Challenges of cannabinoid delivery: How can nanomedicine help? Nanomedicine 2020, 15, 2023–2028. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Cannabinoid Pharmacology; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 80, pp. 73–115. [Google Scholar]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion and friction properties of micro-and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970–4980. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Schmid, M.; Hauser, C. Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food. Foods 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.M.B.; Tomé, L.C.; Garcia, H.; Brandão, L.; Mendes, A.M.; Marrucho, I.M. Effect of natural and synthetic antioxidants incorporation on the gas permeation properties of poly(lactic acid) films. J. Food Eng. 2013, 116, 562–571. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Kloziński, A. Accelerated weathering of polylactide-based composites filled with linseed cake: The influence of time and oil content within the filler. Polymers 2019, 11, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Islam, M.R.; Beg, M.D.H.; Pickering, K.L. Effect of accelerated weathering on physico-mechanical properties of polylactide bio-composites. J. Polym. Environ. 2019, 27, 942–955. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Zaborski, M. Morin hydrate as pro-ecological antioxidant and pigment for polyolefin polymers. C. R. Chim. 2013, 16, 990–996. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Anthocyanin—A Natural Dye for Smart Food Packaging Systems. Korean J. Packag. Sci. Technol. 2018, 24, 167–180. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Effect of impregnation of biodegradable polyesters with polyphenols from cistus linnaeus and Juglans regia Linnaeus walnut green husk. Polymers 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The potential of flavonoids as natural antioxidants and UV light stabilizers for polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The application of (+)-catechin and polydatin as functional additives for biodegradable polyesters. Int. J. Mol. Sci. 2020, 21, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masek, A.; Latos-Brozio, M. The effect of substances of plant origin on the thermal and thermo-oxidative ageing of aliphatic polyesters (PLA, PHA). Polymers 2018, 10, 1252. [Google Scholar] [CrossRef] [Green Version]
- Krehula, L.K.; Katancǐć, Z.; Siročić, A.P.; Hrnjak-Murgić, Z. Weathering of high-density polyethylene-wood plastic composites. J. Wood Chem. Technol. 2014, 34, 39–54. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Wolski, K. Innovative cellulose fibres reinforced ethylene-norbornene copolymer composites of an increased degradation potential. Polym. Degrad. Stab. 2019, 159, 174–183. [Google Scholar] [CrossRef]
- Tomoda, B.T.; Yassue-Cordeiro, P.H.; Ernesto, J.V.; Lopes, P.S.; Péres, L.O.; da Silva, C.F.; de Moraes, M.A. Chapter 3—Characterization of biopolymer membranes and films: Physicochemical, mechanical, barrier, and biological properties. In Biopolymer Membranes and Films; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 67–95. ISBN 9780128181348. [Google Scholar]

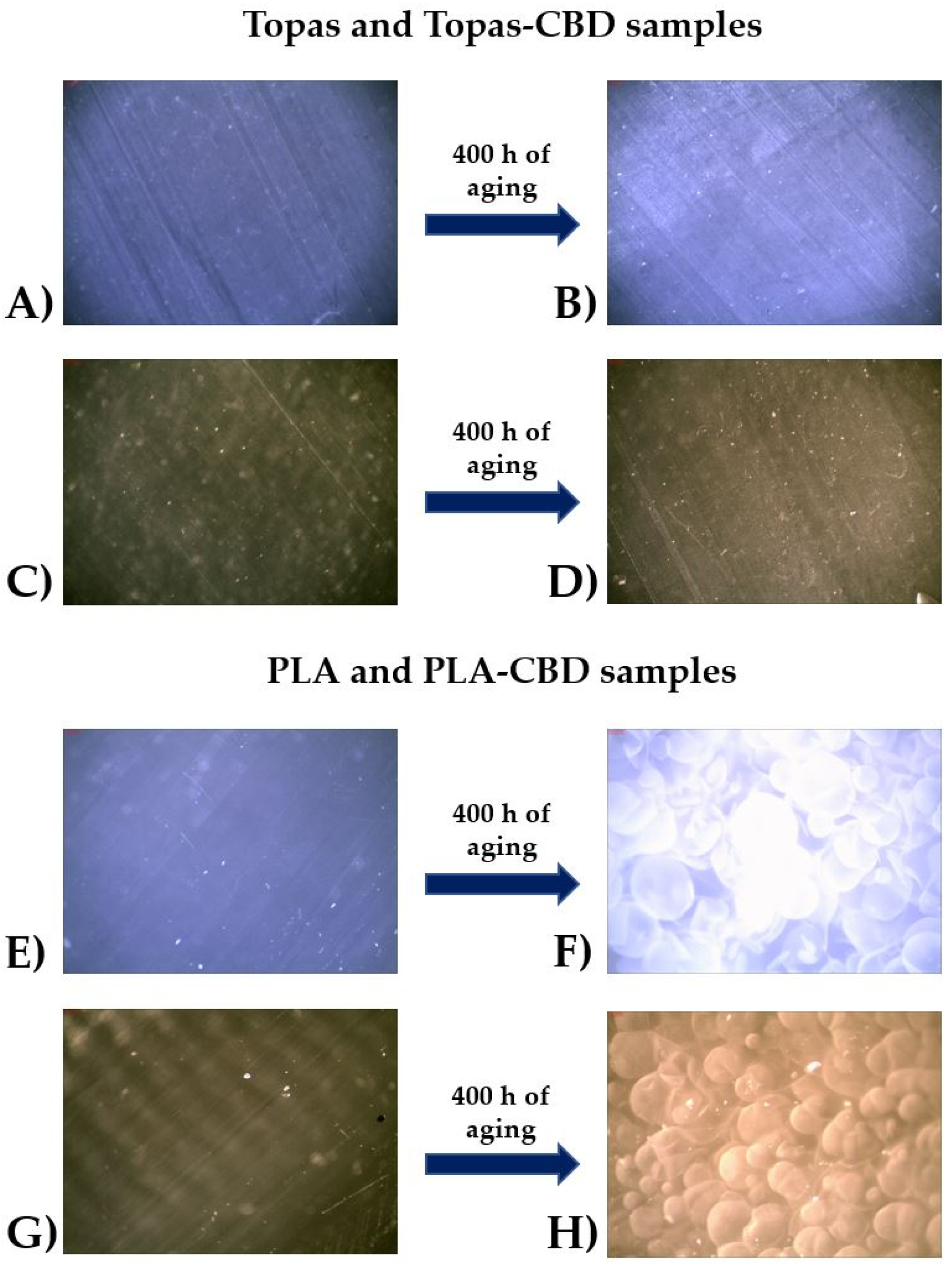

| Liquid | Contact Angle after Weathering Aging (°) | ||||
|---|---|---|---|---|---|
| Reference | 100 h | 200 h | 300 h | 400 h | |
| Topas | |||||
| Water | 87.6 ± 0.79 | 90.6 ± 6.61 | 98.2 ± 1.01 | 97.8 ± 1.73 | 97.6 ± 0.97 |
| Diiodomethane | 61.6 ± 1.43 | 59.9 ± 0.74 | 55.4 ± 1.89 | 62.5 ± 1.73 | 53.8 ± 1.68 |
| Ethylene glycol | 63.3 ± 1.72 | 65.4 ± 1.79 | 74.9 ± 1.04 | 75.0 ± 0.66 | 69.5 ± 2.05 |
| Topas/CBD | |||||
| Water | 71.2 ± 0.92 | 97.3 ± 1.08 | 85.9 ± 1.08 | 92.9 ± 1.04 | 97.1 ± 0.99 |
| Diiodomethane | 55.4 ± 0.95 | 51.1 ± 0.74 | 47.0 ± 0.56 | 48.6 ± 0.96 | 49.5 ± 1.28 |
| Ethylene glycol | 48.1 ± 2.29 | 71.9 ± 1.88 | 58.0 ± 1.32 | 65.9 ± 1.17 | 69.4 ± 1.09 |
| PLA | |||||
| Water | 82.1 ± 0.84 | 86.1 ± 0.54 | 76.0 ± 0.95 | 75.0 ± 1.25 | 65.1 ± 0.73 |
| Diiodomethane | 54.2 ± 1.17 | 51.6 ± 1.74 | 44.0 ± 1.32 | 46.9 ± 2.50 | 41.3 ± 3.50 |
| Ethylene glycol | 57.8 ± 1.47 | 61.2 ± 1.45 | 49.5 ± 0.85 | 48.0 ± 1.10 | 42.7 ± 1.65 |
| PLA/CBD | |||||
| Water | 87.8 ± 1.44 | 78.9 ± 1.62 | 76.8 ± 1.76 | 71.6 ± 1.01 | 67.9 ± 2.04 |
| Diiodomethane | 47.6 ± 2.23 | 46.8 ± 1.46 | 51.4 ± 2.68 | 40.3 ± 1.23 | 33.8 ± 2.43 |
| Ethylene glycol | 58.9 ± 1.34 | 51.1 ± 1.28 | 48.2 ± 0.84 | 50.3 ± 1.84 | 40.7 ± 3.32 |
| Mixture | Temperatures of Mass Change (°C) | ||||||
|---|---|---|---|---|---|---|---|
| T2% | T5% | T10% | T20% | T50% | T70% | T90% | |
| Topas | 432.5 | 449.2 | 456.7 | 464.2 | 475.8 | 480.8 | 487.5 |
| Topas/CBD | 429.2 | 448.3 | 456.7 | 464.2 | 475.0 | 480.8 | 487.5 |
| PLA | 320.0 | 330.0 | 338.3 | 347.5 | 360.8 | 367.5 | 375.0 |
| PLA/CBD | 314.2 | 325.0 | 334.2 | 344.2 | 358.3 | 365.0 | 373.3 |
| Mixture | Oxidative-Induction Time (min) | Energy of Oxidation (J/g) |
|---|---|---|
| Topas | 5.09 | 170 |
| Topas/CBD | 8.49 | 223 |
| Mixture | Oxidative-Induction Temperatures (°C) | Energy of Oxidation (J/g) | |
|---|---|---|---|
| Onset (°C) | Endset (°C) | ||
| PLA | 222.0 | 261.3 | 11 |
| PLA/CBD | 276.1 | 305.9 | 8 |
| Mixture | Weight Composition (phr) | ||
|---|---|---|---|
| Topas | PLA | CBD Extract | |
| 1 | 100 | - | - |
| 2 | 100 | - | 1 |
| 3 | - | 100 | - |
| 4 | - | 100 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plota, A.; Masek, A. Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. Int. J. Mol. Sci. 2021, 22, 4012. https://doi.org/10.3390/ijms22084012
Plota A, Masek A. Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. International Journal of Molecular Sciences. 2021; 22(8):4012. https://doi.org/10.3390/ijms22084012
Chicago/Turabian StylePlota, Angelika, and Anna Masek. 2021. "Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials" International Journal of Molecular Sciences 22, no. 8: 4012. https://doi.org/10.3390/ijms22084012
APA StylePlota, A., & Masek, A. (2021). Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. International Journal of Molecular Sciences, 22(8), 4012. https://doi.org/10.3390/ijms22084012







