Large-Pore Platelet-Rich Fibrin with a Mg Ring to Allow MC3T3-E1 Preosteoblast Migration and to Improve Osteogenic Ability for Bone Defect Repair
Abstract
1. Introduction
2. Results
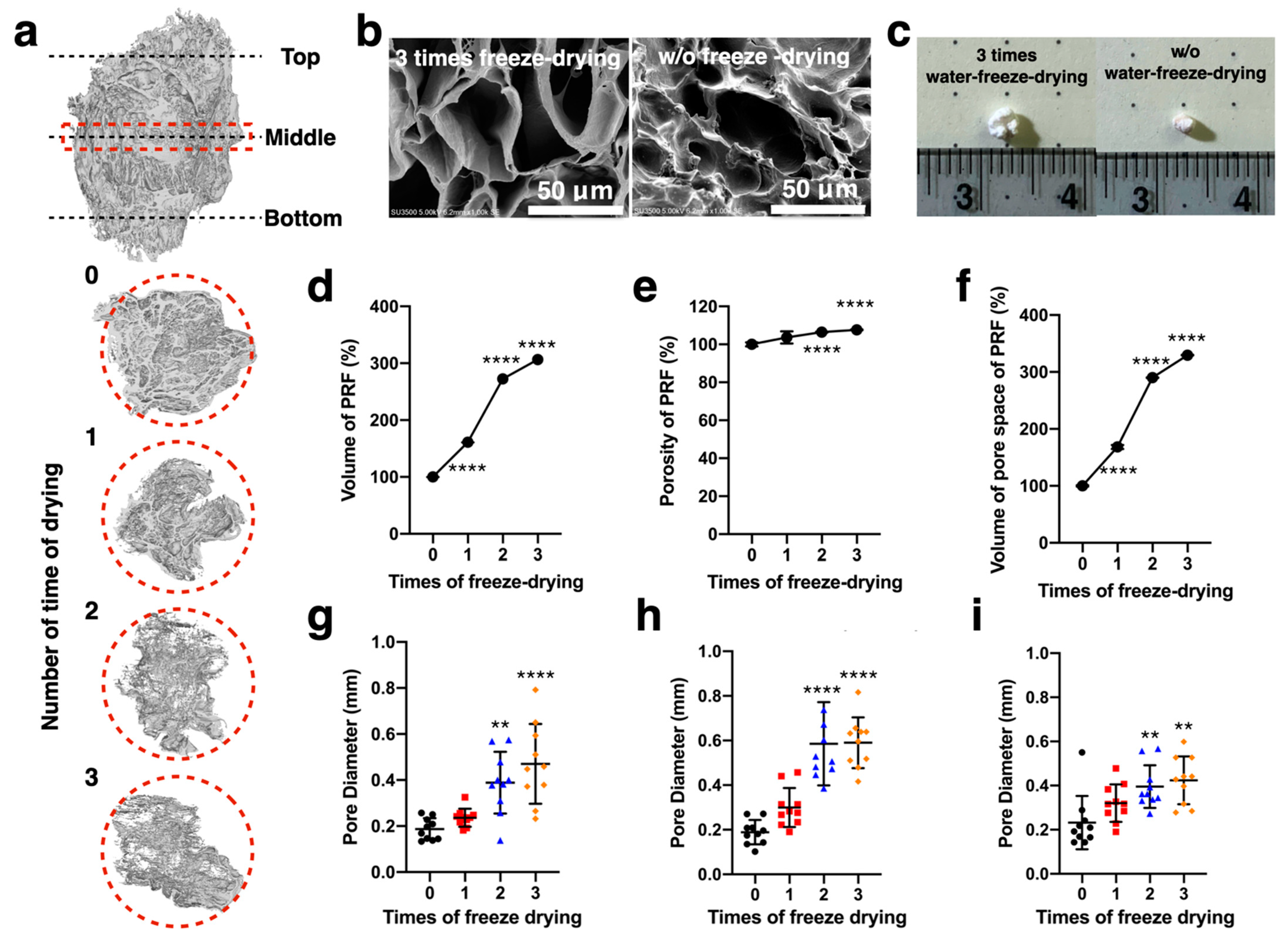
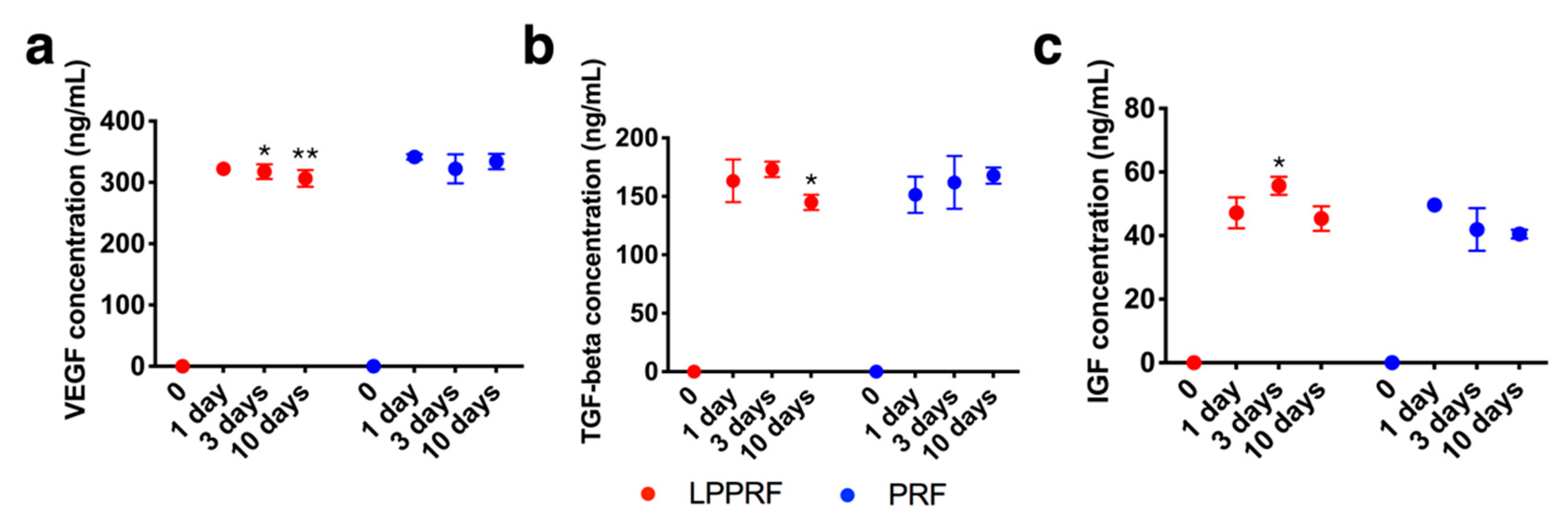
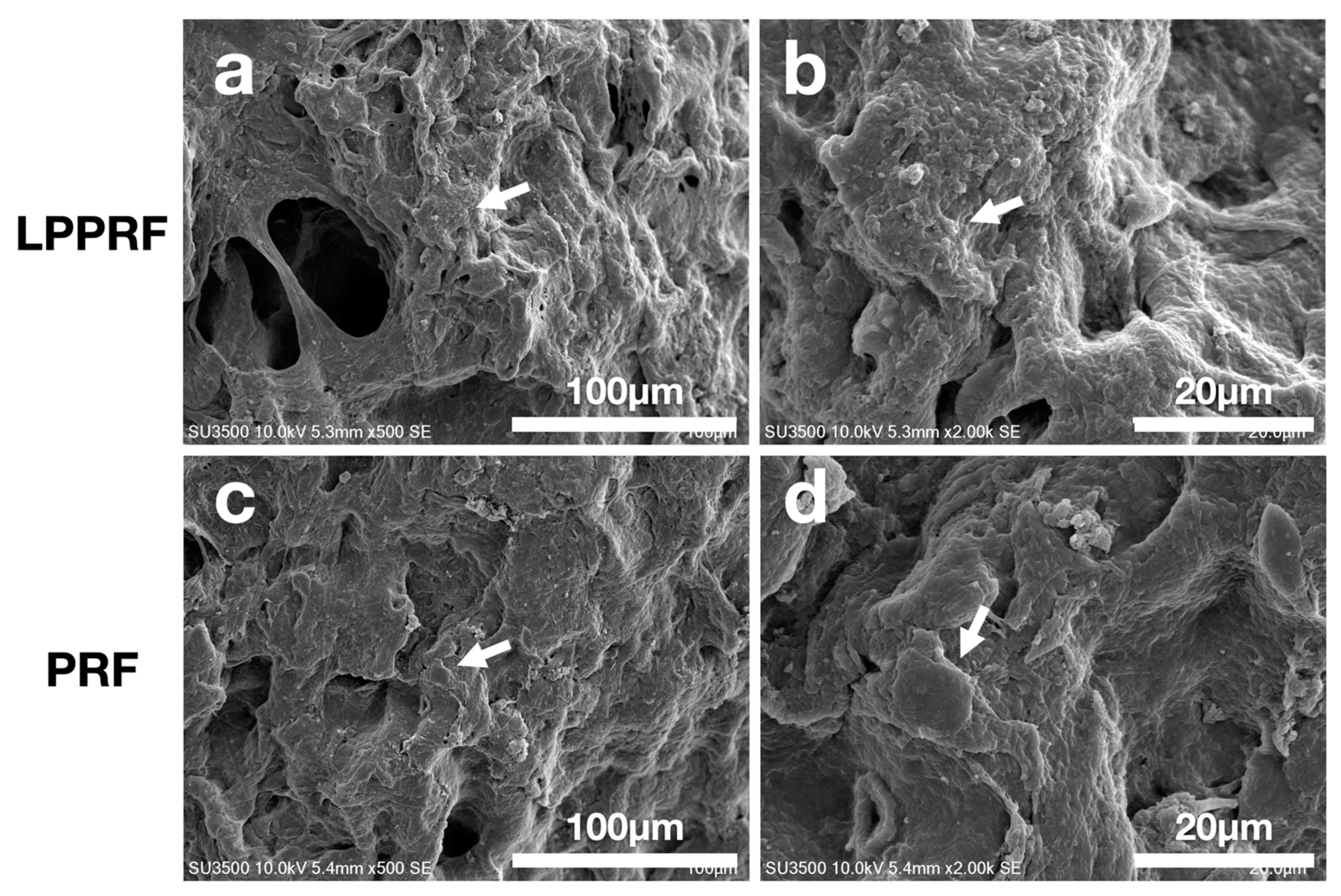
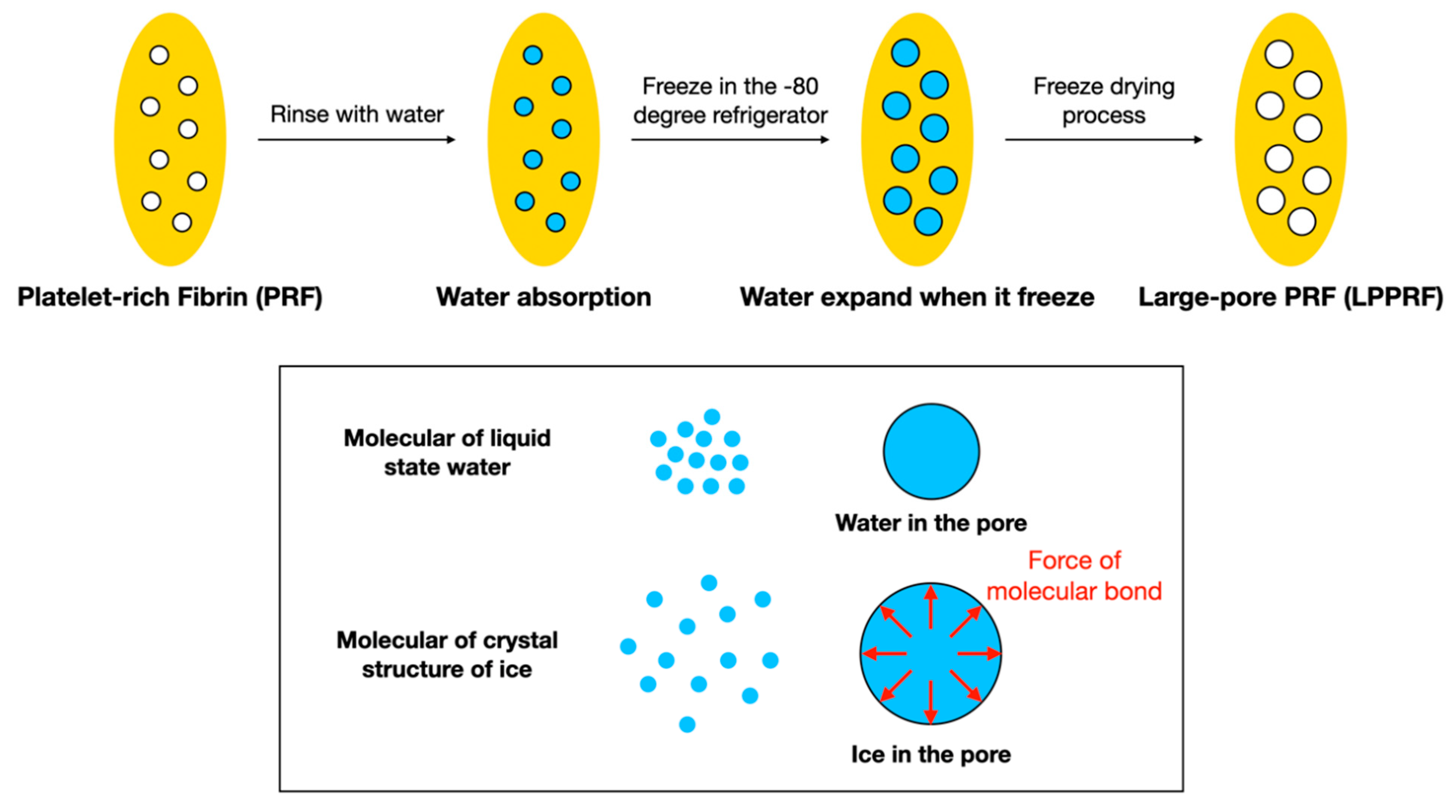
2.1. Fundamental Properties of Modified PRF
2.2. Degradation Behavior of Metallic Rings
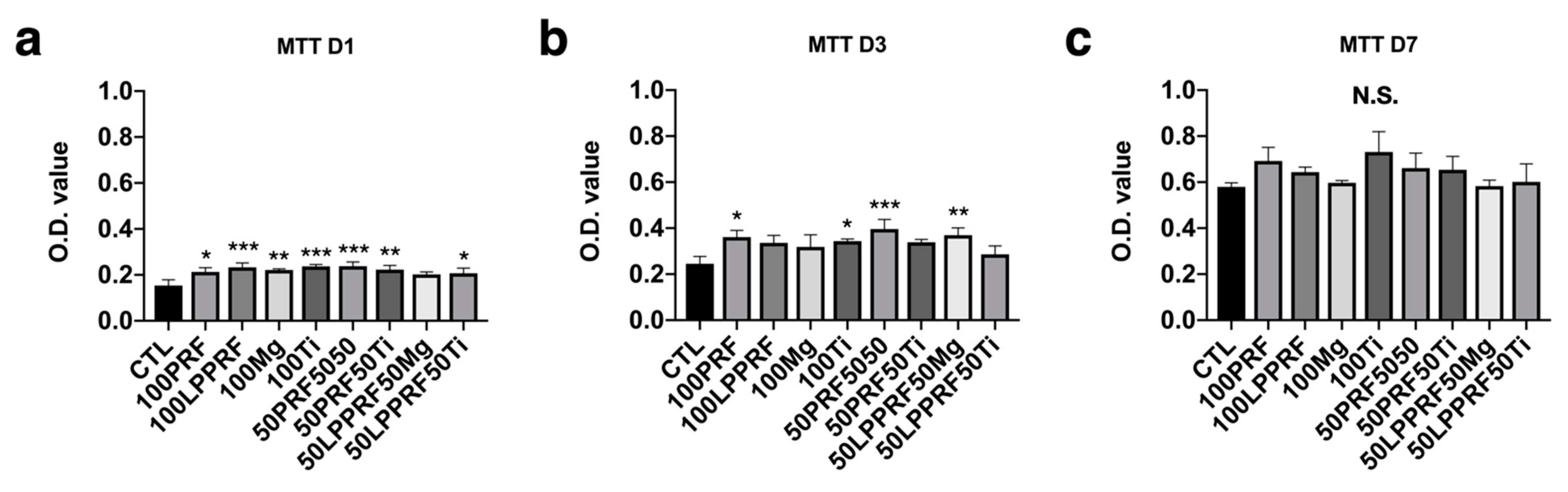
2.3. Cell Viability of MC3T3-E1 Preosteoblasts Treated with PRF, LPPRF, Mg Ring and Ti Ring Precipitate Media and Corresponding Mixtures
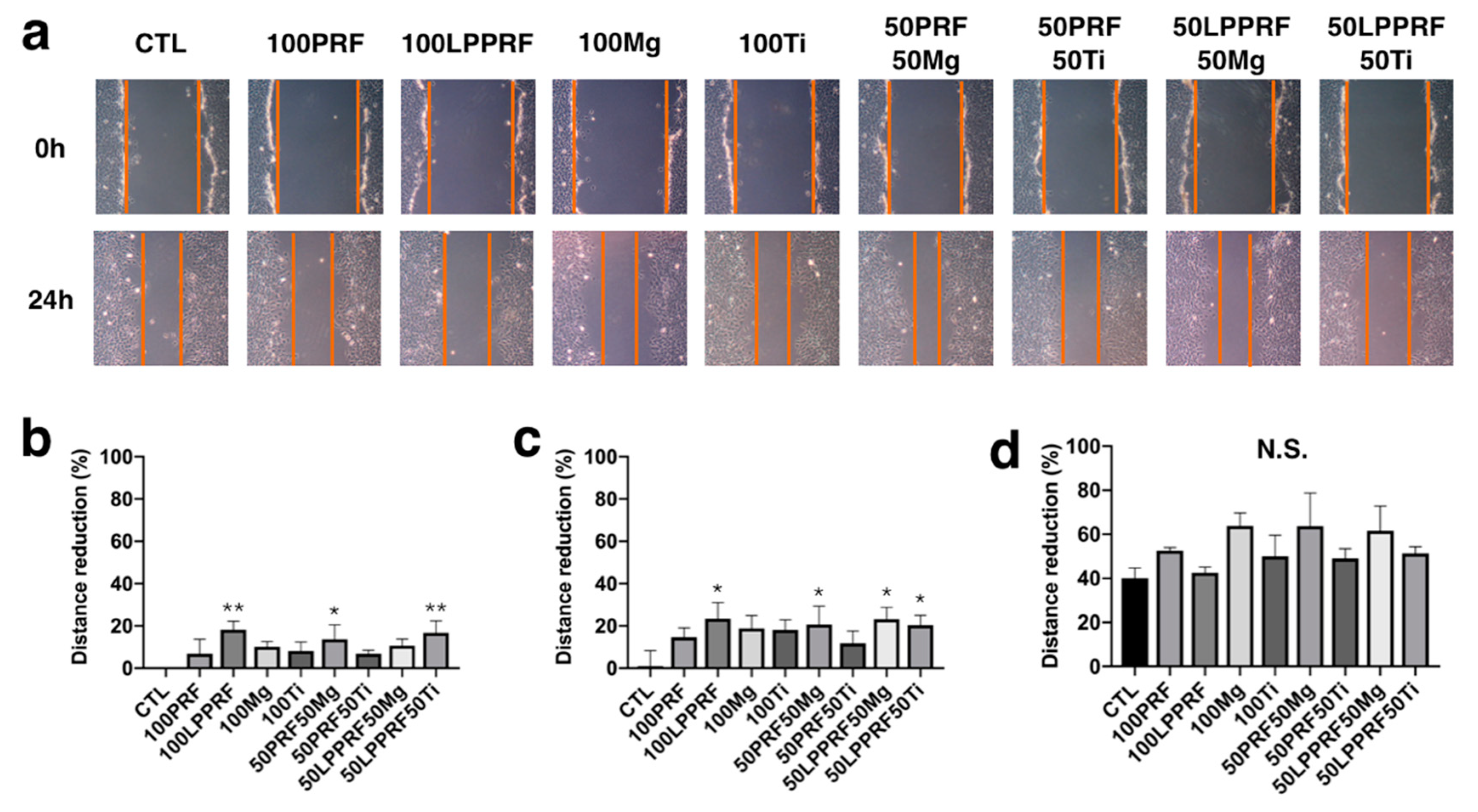
2.4. Migration Capacity of MC3T3-E1 Preosteoblasts Treated with PRF, LPPRF, Mg Ring and Ti Ring Precipitate Media and Their Mixtures
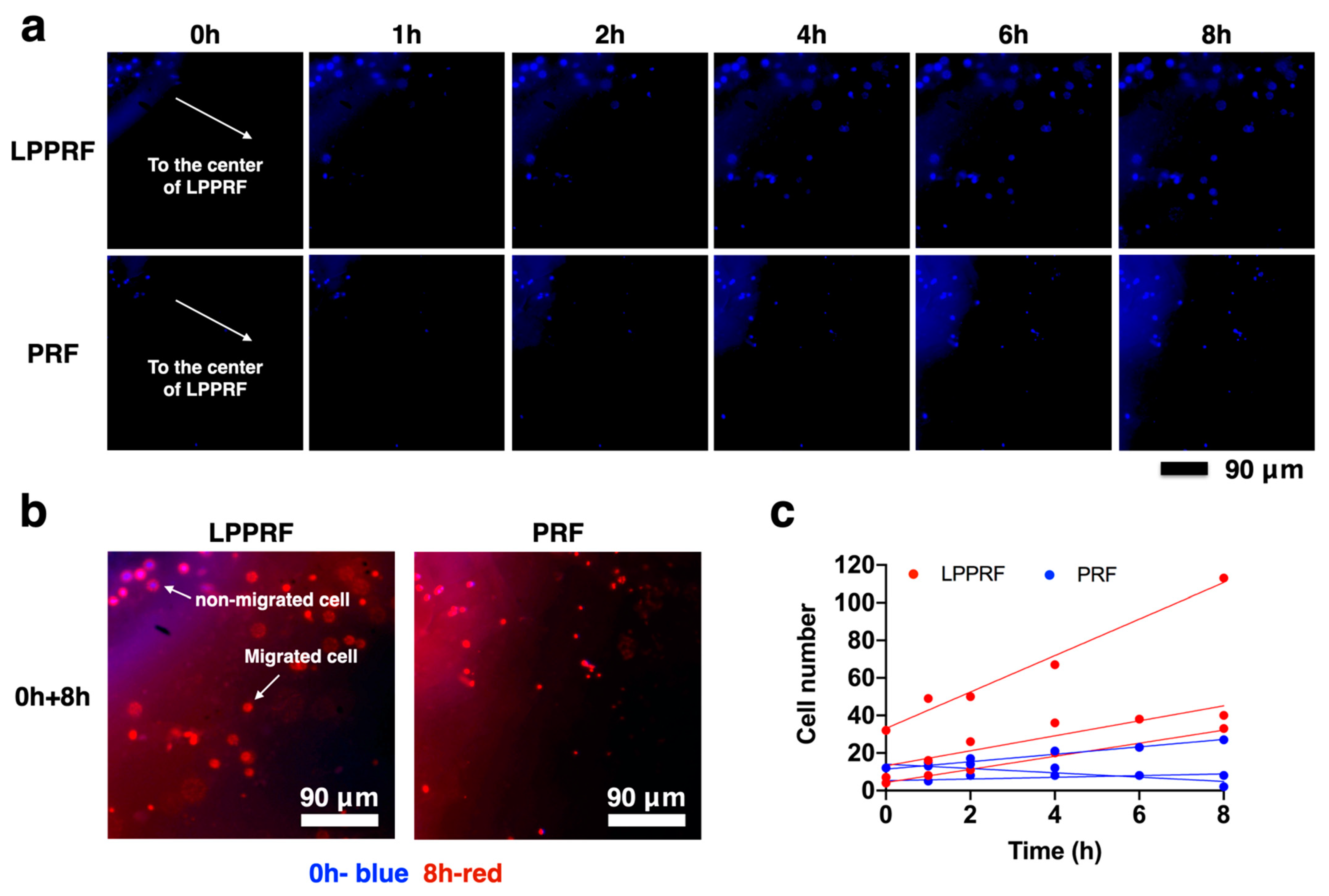
2.5. Migration Capacity of MC3T3-E1 Preosteoblasts on PRF and LPPRF Sheets
2.6. Cell Morphology of MC3T3-E1 Preosteoblasts on PRF and LPPRF
2.7. Calcium Deposition of MC3T3-E1 Preosteoblasts Treated with PRF, LPPRF, Mg Ring and Ti Ring Precipitate Media and Their Mixtures
2.8. Calcium Deposition of MC3T3-E1 Preosteoblasts on PRF and LPPRF
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. PRF Collection and Analysis
4.3. Analysis of PRF and LPPRF Structures
4.4. Growth Factor Tests
4.5. Preparation of Mg and Ti scaffolds
4.6. Degradation Behavior of the Metallic Ring
4.7. Surface Morphology Observation of Metallic Ring and MC3T3-E1 Preosteoblasts When Attached to PRF and LPPRF
4.8. Precipitate Medium Preparation
4.9. Cell Viability of MC3T3-E1 Preosteoblasts
4.10. Analysis of MC3T3-E1 Cell Migration Capacity through a Scratch Assay
4.11. Migration Capacity of MC3T3-E1 Cells in PRF and LPPRF Sheets
4.12. Extracellular Matrix Calcium Deposition of MC3T3-E1 Cells with Precipitate Medium Culture
4.13. Extracellular Matrix Calcium Deposition of MC3T3-E1 Cells on PRF and LPPRF Scaffolds with Mg and Ti Rings
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kheirallah, M.; Almeshaly, H. Present Strategies for Critical Bone Defects Regeneration. J. Soc. 2016, 2, 3–8. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. A review of recent developments in the molecular mechanisms of bone healing. Int. J. Mol. Sci. 2021, 22, 767. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, O.J. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Kleinschmidt, J.C. The Critical Size Defect as an Experimental Model to Test Bone Repair Materials. J. Craniofacial Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef]
- Cowan, C.M.; Shi, Y.-Y.; Aalami, O.O.; Chou, Y.-F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef]
- Christou, C.O.; Pelletier, R.A.; Pelletier, M.H.; Walsh, W.R. Ovine model for critical-size tibial segmental defects. Comp. Med. 2014, 64, 377–385. [Google Scholar]
- Gálvez-Sirvent, E.; Ibarzabal-Gil, A.; Rodríguez-Merchán, E.C. Treatment options for aseptic tibial diaphyseal nonunion: A review of selected studies. EFORT Open Rev. 2020, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- De Long, W.G.E.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: A critical analysis. J. Bone Jt. Surg. Am. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice: The evidence for their use. Bone Jt. J. 2013, 95, 583–597. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Lauritano, D.; Avantaggiato, A.; Candotto, V.; Zollino, I.; Carinci, F. Is platelet-rich fibrin really useful in oral and maxillofacial surgery? Lights and shadows of this new technique. Ann. Oral Maxillofac. Surg. 2013, 1, 25. [Google Scholar] [CrossRef]
- Kumar, K.R.; Genmorgan, K.; Rahman, S.M.A.; Rajan, M.A.; Kumar, T.A.; Prasad, V.S. Role of plasma-rich fibrin in oral surgery. J. Pharm. Bioallied Sci. 2016, 8, S36–S38. [Google Scholar]
- Lucarelli, E.B.; Dozza, R.B.; Tazzari, P.L.; O’Connel, S.M.; Ricci, F.; Pierini, M.; Squarzoni, S.; Pagliaro, P.P.; Oprita, E.I.; Donati, D. A recently developed bifacial platelet-rich fibrin matrix. Eur. Cell Mater. 2010, 20, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.T.; Daren, L.F.; Moran, C.J.; Mullett, H. The Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2019, 47, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Antuña, S.B.; Martínez Diez, R.; Sánchez Márquez, J.M. Platelet-rich fibrin in arthroscopic repair of massive rotator cuff tears: A prospective randomized pilot clinical trial. Acta Orthop. Belg. 2013, 79, 25–30. [Google Scholar]
- Jee, Y.-J. Use of platelet-rich fibrin and natural bone regeneration in regenerative surgery. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 121–122. [Google Scholar] [CrossRef]
- Barbon, S.S.; Macchi, E.V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P.p.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. [Google Scholar] [CrossRef]
- Bennardo, F.B.; Del Duca, L.E.; Patruno, C.; Fortunato, L.; Giudice, A.; Nisticò, S.P. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 2020, 33, e13334. [Google Scholar] [CrossRef]
- Friedenberg, Z.B.; Lawrence, R.R. The regeneration of bone in defects of varying size. Surg. Gynecol. Obstet. 1962, 114, 721–726. [Google Scholar]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Hakim, S.G. Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci. Rep. 2019, 9, 8310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, L.; Sun, T.; Wang, W.; Li, X.; Wu, B. Preparation and effect of lyophilized platelet-rich fibrin on the osteogenic potential of bone marrow mesenchymal stem cells in vitro and in vivo. Heliyon 2019, 5, e02739. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Chai, J.K.; Han, Y.F.; Sun, T.J.; Deng, H.P.; Zhao, J.Y.; Liu, L.Y. Growth and migration of umbilical cord mesenchymal stem cells on polycarbonate membrane with different pore sizes. Zhonghua Yi Xue Za Zhi 2011, 91, 699–702. [Google Scholar]
- Peyton, S.R.; Kalcioglu, Z.I.; Cohen, J.C.; Runkle, A.P.; Van Vliet, K.J.; Lauffenburger, D.A.; Griffith, L.G. Marrow-Derived stem cell motility in 3D synthetic scaffold is governed by geometry along with adhesivity and stiffness. Biotechnol. Bioeng. 2010, 108, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.H.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D.L. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Wong, C.-C.; Wong, P.-C.; Tsai, P.-H.; Jang, J.S.-C.; Cheng, C.-K.; Chen, H.-H.; Chen, C.-H. Biocompatibility and Osteogenic Capacity of Mg-Zn-Ca Bulk Metallic Glass for Rabbit Tendon-Bone Interference Fixation. Int. J. Mol. Sci. 2019, 20, 2191. [Google Scholar] [CrossRef]
- Wong, P.-C.; Tsai, P.-H.; Li, T.-H.; Cheng, C.-K.; Jang, J.; Huang, J. Degradation behavior and mechanical strength of Mg-Zn-Ca bulk metallic glass composites with Ti particles as biodegradable materials. J. Alloys Compd. 2017, 699, 914–920. [Google Scholar] [CrossRef]
- Wong, P.-C.; Song, S.-M.; Tsai, P.-H.; Nien, Y.-Y.; Jang, J.S.-C.; Cheng, C.-K.; Chen, C.-H. Relationship between the Surface Roughness of Biodegradable Mg-Based Bulk Metallic Glass and the Osteogenetic Ability of MG63 Osteoblast-Like Cells. Materials 2020, 13, 1188. [Google Scholar] [CrossRef]
- Song, S.-M.; Wong, P.-C.; Chiang, C.-W.; Tsai, P.-H.; Jang, J.; Chen, C.-H. A bi-phase core–shell structure of Mg-based bulk metallic glass for application in orthopedic fixation implants. Mater. Sci. Eng. C 2020, 111, 110783. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium–Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-kappaB and NFATc1 signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials 2015, 64, 57–69. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Song, B.; Chow, D.H.; Yung, P.S.-H.; Qin, L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017, 63, 393–410. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, P.-C.; Wang, C.-Y.; Jang, J.S.-C.; Lee, C.-H.; Wu, J.-L. Large-Pore Platelet-Rich Fibrin with a Mg Ring to Allow MC3T3-E1 Preosteoblast Migration and to Improve Osteogenic Ability for Bone Defect Repair. Int. J. Mol. Sci. 2021, 22, 4022. https://doi.org/10.3390/ijms22084022
Wong P-C, Wang C-Y, Jang JS-C, Lee C-H, Wu J-L. Large-Pore Platelet-Rich Fibrin with a Mg Ring to Allow MC3T3-E1 Preosteoblast Migration and to Improve Osteogenic Ability for Bone Defect Repair. International Journal of Molecular Sciences. 2021; 22(8):4022. https://doi.org/10.3390/ijms22084022
Chicago/Turabian StyleWong, Pei-Chun, Chen-Yun Wang, Jason Shian-Ching Jang, Chian-Her Lee, and Jia-Lin Wu. 2021. "Large-Pore Platelet-Rich Fibrin with a Mg Ring to Allow MC3T3-E1 Preosteoblast Migration and to Improve Osteogenic Ability for Bone Defect Repair" International Journal of Molecular Sciences 22, no. 8: 4022. https://doi.org/10.3390/ijms22084022
APA StyleWong, P.-C., Wang, C.-Y., Jang, J. S.-C., Lee, C.-H., & Wu, J.-L. (2021). Large-Pore Platelet-Rich Fibrin with a Mg Ring to Allow MC3T3-E1 Preosteoblast Migration and to Improve Osteogenic Ability for Bone Defect Repair. International Journal of Molecular Sciences, 22(8), 4022. https://doi.org/10.3390/ijms22084022








