Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders
Abstract
:1. Introduction
2. Protective Role of Exercise in Preventing Mood Disorders and Neurodegenerative Diseases
3. BDNF: A Key Trophic Signalling Molecule in the Brain
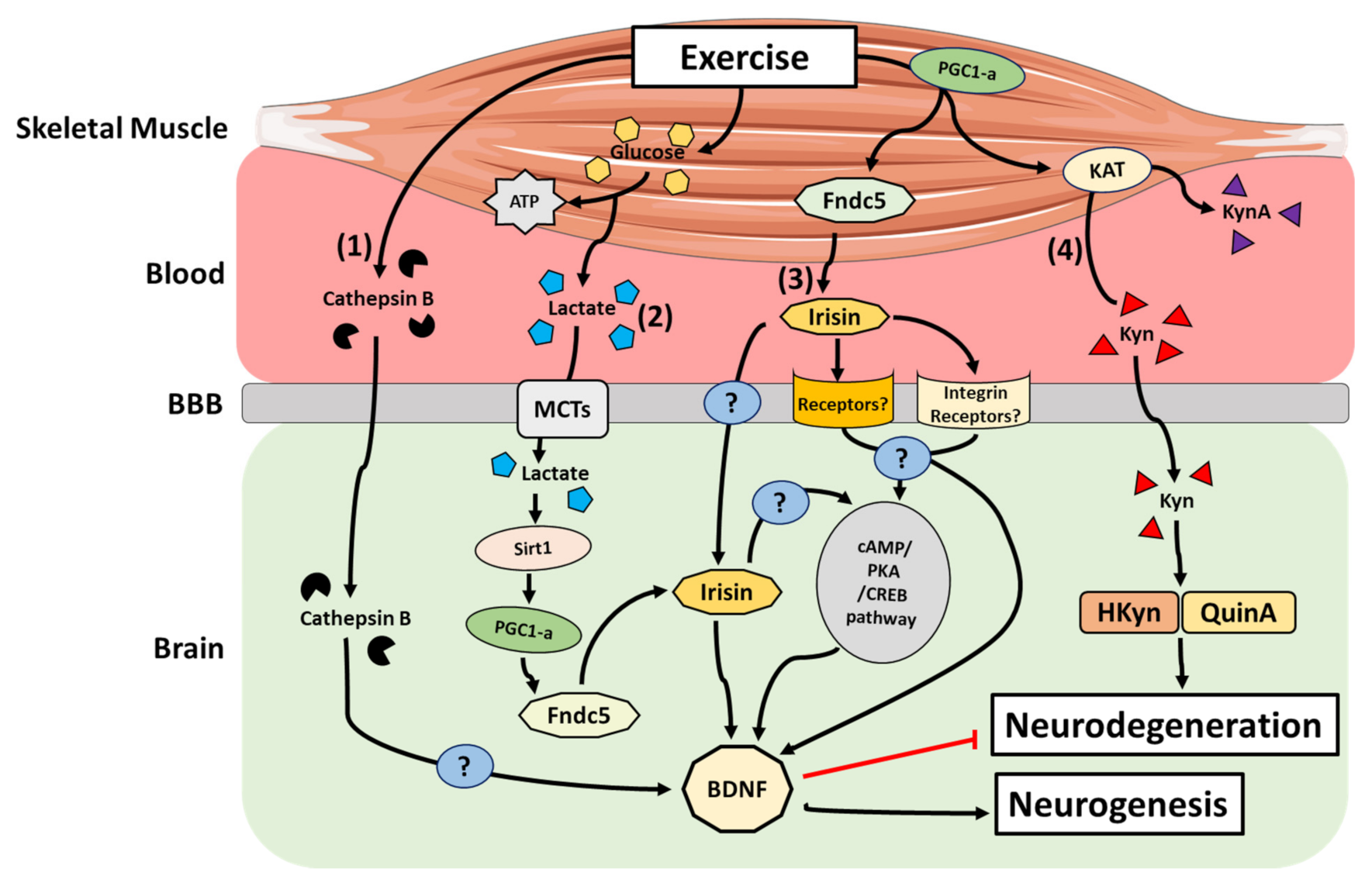
4. Hormones and Metabolites of the Muscle–Brain Axis
- Irisin
- Lactate
- Cathepsin B
- Kynurenine
5. Exercise and Liver–Brain Axis
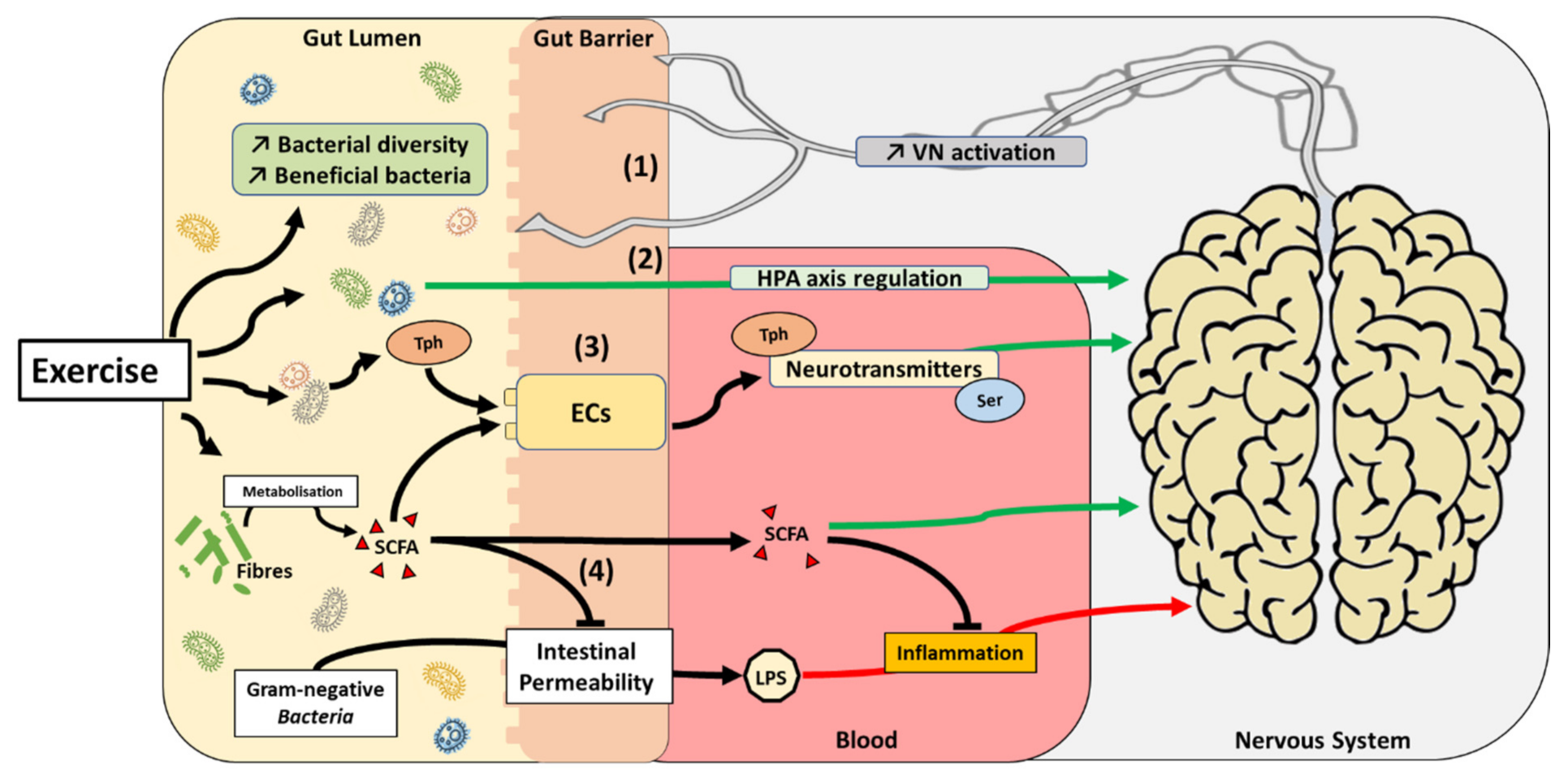
6. Exercise and the Microbiome–Gut–Brain Axis
7. Iron: An Emerging Factor in the Muscle–Brain Axis
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BBB | Blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| cAMP | cyclic adenosine monophosphate |
| CNS | central nervous system |
| CREB | cAMP-response element-binding protein |
| CTSB | cathepsin B |
| EPO | erythropoietin |
| FGF21 | fibroblast growth factor 21 |
| FNDC5 | fibronectin type III domain-containing Protein 5 |
| HKyn | hydroxykynurenine |
| HPA | hypothalamic-pituitary-adrenal |
| IGF-1 | insulin-like growth factor 1 |
| KATs | kynurenine aminotransferases |
| KPM | kynurenine pathway metabolism |
| Kyn | kynurenine |
| LPS | lipopolysaccharide |
| MCTs | monocarboxylate transporters |
| PD | Parkinson’s disease |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PKA | protein kinase A |
| QuinA | quinolinic acid |
| SCFAs | short chain fatty acids |
| Ser | serotonin |
| SIRT1 | sirtuin 1 |
| Tph | Tryptophane |
| TrkB | tropomyosin receptor kinase B |
| VEGF | vascular endothelial growth factor |
| VN | vagus nerve |
| β-HB | β-hydroxybutyrate |
References
- Steel, Z.; Marnane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The Global Prevalence of Common Mental Disorders: A Systematic Review and Meta-Analysis 1980–2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [Green Version]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Canady, V.A. Mental Illness Will Cost the World $16 Trillion (USD) by 2030. Ment. Health Wkly. 2018, 28, 7–8. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Qiu, C.; Cheng, F. Global and Regional Economic Costs of Dementia: A Systematic Review. Lancet 2017, 390, S47. [Google Scholar] [CrossRef] [Green Version]
- Ahlskog, J.E.; Geda, Y.E.; Graff-Radford, N.R.; Petersen, R.C. Physical Exercise as a Preventive or Disease-Modifying Treatment of Dementia and Brain Aging. Mayo Clin. Proc. 2011, 86, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the Treatment of Depression and Anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, T.; Chu, J.M.-T.; Chen, Y.; Dunnett, S.; Ho, Y.-S.; Wong, G.T.-C.; Chang, R.C.-C. The Beneficial Effects of Physical Exercise in the Brain and Related Pathophysiological Mechanisms in Neurodegenerative Diseases. Lab. Invest. 2019, 99, 943–957. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and Mental Health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- Farmer, M.E.; Locke, B.Z.; Mościcki, E.K.; Dannenberg, A.L.; Larson, D.B.; Radloff, L.S. Physical Activity and Depressive Symptoms: The NHANES I Epidemiologic Follow-up Study. Am. J. Epidemiol. 1988, 128, 1340–1351. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a Treatment for Depression: A Meta-Analysis Adjusting for Publication Bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a Treatment for Depression: A Meta-Analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, L.A.; Asmundson, G.J. Is There a Negative Association between Anxiety Sensitivity and Arousal-Increasing Substances and Activities? J. Anxiety Disord. 2001, 15, 161–170. [Google Scholar] [CrossRef]
- Anderson, E.; Shivakumar, G. Effects of Exercise and Physical Activity on Anxiety. Front. Psychiatry 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vancampfort, D.; De Hert, M.; Knapen, J.; Wampers, M.; Demunter, H.; Deckx, S.; Maurissen, K.; Probst, M. State Anxiety, Psychological Stress and Positive Well-Being Responses to Yoga and Aerobic Exercise in People with Schizophrenia: A Pilot Study. Disabil. Rehabil. 2011, 33, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-W.; Lin, H.-C.; Su, C.-Y.; Chen, M.-D.; Lin, K.C.; Ko, C.-H.; Yen, C.-F. Effect of Aerobic Exercise on Improving Symptoms of Individuals with Schizophrenia: A Single Blinded Randomized Control Study. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Petrus, C.; Adamson, S.R.; Block, L.; Einarson, S.J.; Sharifnejad, M.; Harris, S.R. Effects of Exercise Interventions on Stereotypic Behaviours in Children with Autism Spectrum Disorder. Physiother. Can. 2008, 60, 134–145. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Ghiarone, T.; Cabral Júnior, C.R.; Furtado, G.E.; Moreira Carvalho, H.; Machado-Rodrigues, A.M.; Andrade Toscano, C.V. Effects of Physical Exercise on the Stereotyped Behavior of Children with Autism Spectrum Disorders. Medicina 2019, 55, 685. [Google Scholar] [CrossRef] [Green Version]
- Thomson, D.; Turner, A.; Lauder, S.; Gigler, M.E.; Berk, L.; Singh, A.B.; Pasco, J.A.; Berk, M.; Sylvia, L. A Brief Review of Exercise, Bipolar Disorder, and Mechanistic Pathways. Front. Psychol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Biddle, S.J.H.; Asare, M. Physical Activity and Mental Health in Children and Adolescents: A Review of Reviews. Br. J. Sports Med. 2011, 45, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Frazzitta, G.; Balbi, P.; Maestri, R.; Bertotti, G.; Boveri, N.; Pezzoli, G. The Beneficial Role of Intensive Exercise on Parkinson Disease Progression. Am. J. Phys. Med. Rehabil. 2013, 92, 523–532. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Al-Jarrah, M.D.; Erekat, N.S. Parkinson Disease-Induced Upregulation of Apoptotic Mediators Could Be Attenuated in the Skeletal Muscle Following Chronic Exercise Training. NeuroRehabilitation 2017, 41, 823–830. [Google Scholar] [CrossRef]
- Lau, Y.-S.; Patki, G.; Das-Panja, K.; Le, W.-D.; Ahmad, S.O. Neuroprotective Effects and Mechanisms of Exercise in a Chronic Mouse Model of Parkinson’s Disease with Moderate Neurodegeneration. Eur. J. Neurosci. 2011, 33, 1264–1274. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.-S.; Kim, T.-W.; Lee, J.-M.; Ji, E.-S.; Lim, B.-V. Treadmill Exercise Alleviates Nigrostriatal Dopaminergic Loss of Neurons and Fibers in Rotenone-Induced Parkinson Rats. J. Exerc. Rehabil. 2017, 13, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.; Ekavali. A Review on Alzheimer’s Disease Pathophysiology and Its Management: An Update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.-J.; Laurent, C.; Lestavel, S.; Figeac, M.; Sultan, A.; Troquier, L.; Leboucher, A.; Caillierez, R.; et al. Beneficial Effects of Exercise in a Transgenic Mouse Model of Alzheimer’s Disease-like Tau Pathology. Neurobiol. Dis. 2011, 43, 486–494. [Google Scholar] [CrossRef]
- Frederiksen, K.S.; Gjerum, L.; Waldemar, G.; Hasselbalch, S.G. Effects of Physical Exercise on Alzheimer’s Disease Biomarkers: A Systematic Review of Intervention Studies. J. Alzheimers Dis. JAD 2018, 61, 359–372. [Google Scholar] [CrossRef]
- Law, L.L.; Rol, R.N.; Schultz, S.A.; Dougherty, R.J.; Edwards, D.F.; Koscik, R.L.; Gallagher, C.L.; Carlsson, C.M.; Bendlin, B.B.; Zetterberg, H.; et al. Moderate Intensity Physical Activity Associates with CSF Biomarkers in a Cohort at Risk for Alzheimer’s Disease. Alzheimers Dement. Amst. Neth. 2018, 10, 188–195. [Google Scholar] [CrossRef]
- Lima, M.G.P.; Schimidt, H.L.; Garcia, A.; Daré, L.R.; Carpes, F.P.; Izquierdo, I.; Mello-Carpes, P.B. Environmental Enrichment and Exercise Are Better than Social Enrichment to Reduce Memory Deficits in Amyloid Beta Neurotoxicity. Proc. Natl. Acad. Sci. USA 2018, 115, E2403–E2409. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Lin, M.-S.; Tzeng, I.-S. Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.Y.; Mintun, M.A.; Fagan, A.M.; Goate, A.M.; Bugg, J.M.; Holtzman, D.M.; Morris, J.C.; Head, D. Exercise and Alzheimer’s Disease Biomarkers in Cognitively Normal Older Adults. Ann. Neurol. 2010, 68, 311–318. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Miller, A.C.; Hayes, T.; Shaw, E. Cognitive and Behavioral Changes in Huntington Disease before Diagnosis. Handb. Clin. Neurol. 2017, 144, 69–91. [Google Scholar] [CrossRef]
- Mueller, S.M.; Petersen, J.A.; Jung, H.H. Exercise in Huntington’s Disease: Current State and Clinical Significance. Tremor Other Hyperkinet. Mov. 2019, 9. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. Yakhteh 2017, 19, 1–10. [Google Scholar]
- Reynolds, E.R.; Ashbaugh, A.D.; Hockenberry, B.J.; McGrew, C.A. Multiple Sclerosis and Exercise: A Literature Review. Curr. Sports Med. Rep. 2018, 17, 31–35. [Google Scholar] [CrossRef]
- Devine, J.M.; Wong, B.; Gervino, E.; Pascual-Leone, A.; Alexander, M.P. Independent, Community-Based Aerobic Exercise Training for People with Moderate-to-Severe Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2016, 97, 1392–1397. [Google Scholar] [CrossRef]
- Esquenazi, A.; Lee, S.; Wikoff, A.; Packel, A.; Toczylowski, T.; Feeley, J. A Randomized Comparison of Locomotor Therapy Interventions: Partial Body Weight Supported Treadmill, Lokomat and G-Eo Training in Traumatic Brain Injury. PM&R 2016, 8, S154. [Google Scholar] [CrossRef]
- Pearn, M.L.; Niesman, I.R.; Egawa, J.; Sawada, A.; Almenar-Queralt, A.; Shah, S.B.; Duckworth, J.L.; Head, B.P. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell. Mol. Neurobiol. 2017, 37, 571–585. [Google Scholar] [CrossRef]
- Wogensen, E.; Malá, H.; Mogensen, J. The Effects of Exercise on Cognitive Recovery after Acquired Brain Injury in Animal Models: A Systematic Review. Neural Plast. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Seo, T.-B.; Kim, B.-K.; Ko, I.-G.; Kim, D.-H.; Shin, M.-S.; Kim, C.-J.; Yoon, J.-H.; Kim, H. Effect of Treadmill Exercise on Purkinje Cell Loss and Astrocytic Reaction in the Cerebellum after Traumatic Brain Injury. Neurosci. Lett. 2010, 481, 178–182. [Google Scholar] [CrossRef]
- Taylor, J.M.; Montgomery, M.H.; Gregory, E.J.; Berman, N.E.J. Exercise Preconditioning Improves Traumatic Brain Injury Outcomes. Brain Res. 2015, 1622, 414–429. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Drudis, L.; Amorós-Aguilar, L.; Torras-Garcia, M.; Serra-Elias, B.; Costa-Miserachs, D.; Portell-Cortés, I.; Coll-Andreu, M. Delayed Voluntary Physical Exercise Restores “When” and “Where” Object Recognition Memory after Traumatic Brain Injury. Behav. Brain Res. 2021, 400, 113048. [Google Scholar] [CrossRef]
- Khaku, A.S.; Tadi, P. Cerebrovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Schmidt, W.; Endres, M.; Dimeo, F.; Jungehulsing, G.J. Train the Vessel, Gain the Brain: Physical Activity and Vessel Function and the Impact on Stroke Prevention and Outcome in Cerebrovascular Disease. Cerebrovasc. Dis. 2013, 35, 303–312. [Google Scholar] [CrossRef]
- Kramer, S.F.; Hung, S.H.; Brodtmann, A. The Impact of Physical Activity Before and After Stroke on Stroke Risk and Recovery: A Narrative Review. Curr. Neurol. Neurosci. Rep. 2019, 19, 28. [Google Scholar] [CrossRef]
- Endres, M.; Gertz, K.; Lindauer, U.; Katchanov, J.; Schultze, J.; Schröck, H.; Nickenig, G.; Kuschinsky, W.; Dirnagl, U.; Laufs, U. Mechanisms of Stroke Protection by Physical Activity. Ann. Neurol. 2003, 54, 582–590. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.S.; Dhikav, V. Hippocampus in Health and Disease: An Overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-Based Synaptic Repair as a Disease-Modifying Strategy for Neurodegenerative Diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuccato, C.; Cattaneo, E. Brain-Derived Neurotrophic Factor in Neurodegenerative Diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Kim, K.; Sung, Y.-H.; Seo, J.-H.; Lee, S.-W.; Lim, B.-V.; Lee, C.-Y.; Chung, Y.-R. Effects of Treadmill Exercise-Intensity on Short-Term Memory in the Rats Born of the Lipopolysaccharide-Exposed Maternal Rats. J. Exerc. Rehabil. 2015, 11, 296–302. [Google Scholar] [CrossRef]
- Russo-Neustadt, A.; Beard, R.C.; Cotman, C.W. Exercise, Antidepressant Medications, and Enhanced Brain Derived Neurotrophic Factor Expression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1999, 21, 679–682. [Google Scholar] [CrossRef] [Green Version]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF Mediates the Efficacy of Exercise on Synaptic Plasticity and Cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A Meta-Analytic Review of the Effects of Exercise on Brain-Derived Neurotrophic Factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a Release of Brain-Derived Neurotrophic Factor from the Brain during Exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Seifert, T.; Brassard, P.; Wissenberg, M.; Rasmussen, P.; Nordby, P.; Stallknecht, B.; Adser, H.; Jakobsen, A.H.; Pilegaard, H.; Nielsen, H.B.; et al. Endurance Training Enhances BDNF Release from the Human Brain. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 298, R372–R377. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Ye, M.; Li, Z.; Bu, S.; Zhang, Y. Long-Term Supplementation of Dehydroepiandrosterone Improved Depressive-like Behaviors by Increasing BDNF Expression in the Hippocampus in Ovariectomized Rats. Heliyon 2020, 6, e05180. [Google Scholar] [CrossRef]
- Gross, K.S.; Alf, R.L.; Polzin, T.R.; Frick, K.M. 17β-Estradiol Activation of Dorsal Hippocampal TrkB Is Independent of Increased Mature BDNF Expression and Is Required for Enhanced Memory Consolidation in Female Mice. Psychoneuroendocrinology 2021, 125, 105110. [Google Scholar] [CrossRef]
- Matthews, V.B.; Åström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-Derived Neurotrophic Factor Is Produced by Skeletal Muscle Cells in Response to Contraction and Enhances Fat Oxidation via Activation of AMP-Activated Protein Kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. MRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Lecker, S.H.; Zavin, A.; Cao, P.; Arena, R.; Allsup, K.; Daniels, K.M.; Joseph, J.; Schulze, P.C.; Forman, D.E. Expression of the Irisin Precursor FNDC5 in Skeletal Muscle Correlates with Aerobic Exercise Performance in Patients with Heart Failure. Circ. Heart Fail. 2012, 5, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Arias-Loste, M.T.; Ranchal, I.; Romero-Gómez, M.; Crespo, J. Irisin, a Link among Fatty Liver Disease, Physical Inactivity and Insulin Resistance. Int. J. Mol. Sci. 2014, 15, 23163–23178. [Google Scholar] [CrossRef] [Green Version]
- Teufel, A.; Malik, N.; Mukhopadhyay, M.; Westphal, H. Frcp1 and Frcp2, Two Novel Fibronectin Type III Repeat Containing Genes. Gene 2002, 297, 79–83. [Google Scholar] [CrossRef]
- Hashemi, M.-S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.H.; Baharvand, H. Fndc5 Knockdown Significantly Decreased Neural Differentiation Rate of Mouse Embryonic Stem Cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Duman, R.S.; Nestler, E.J. The Many Faces of CREB. Trends Neurosci. 2005, 28, 436–445. [Google Scholar] [CrossRef]
- Wrann, C.D. FNDC5/Irisin—Their Role in the Nervous System and as a Mediator for Beneficial Effects of Exercise on the Brain. Brain Plast. 2015, 1, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [Green Version]
- Jackson, T.C.; Gorse, K.; Herrmann, J.R.; Kochanek, P.M. Hippocampal and Prefrontal Cortical Brain Tissue Levels of Irisin and GDF15 Receptor Subunits in Children. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive Review on Lactate Metabolism in Human Health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef]
- Brooks, G.A. Anaerobic Threshold: Review of the Concept and Directions for Future Research. Med. Sci. Sports Exerc. 1985, 17, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Ruchti, E.; Petit, J.-M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate Promotes Plasticity Gene Expression by Potentiating NMDA Signaling in Neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundquist, A.J.; Gallagher, T.J.; Petzinger, G.M.; Jakowec, M.W. Exogenous L-Lactate Promotes Astrocyte Plasticity but Is Not Sufficient for Enhancing Striatal Synaptogenesis or Motor Behavior in Mice. J. Neurosci. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, T.; Schulte, S.; Sperlich, B.; Achtzehn, S.; Fricke, H.; Strüder, H.K. Lactate Infusion at Rest Increases BDNF Blood Concentration in Humans. Neurosci. Lett. 2011, 488, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef]
- Hayek, L.E.; Khalifeh, M.; Zibara, V.; Assaad, R.A.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [Green Version]
- Mort, J.S.; Buttle, D.J. Cathepsin B. Int. J. Biochem. Cell Biol. 1997, 29, 715–720. [Google Scholar] [CrossRef]
- Norheim, F.; Raastad, T.; Thiede, B.; Rustan, A.C.; Drevon, C.A.; Haugen, F. Proteomic Identification of Secreted Proteins from Human Skeletal Muscle Cells and Expression in Response to Strength Training. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1013–E1021. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression—Preclinical Approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117–138. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The Kynurenine Connection: How Exercise Shifts Muscle Tryptophan Metabolism and Affects Energy Homeostasis, the Immune System, and the Brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef]
- Bryleva, E.Y.; Brundin, L. Kynurenine Pathway Metabolites and Suicidality. Neuropharmacology 2017, 112, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Réus, G.Z.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine Pathway Dysfunction in the Pathophysiology and Treatment of Depression: Evidences from Animal and Human Studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the Mammalian Brain: When Physiology meets Pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Stone, T.W.; Forrest, C.M.; Darlington, L.G. Kynurenine Pathway Inhibition as a Therapeutic Strategy for Neuroprotection. FEBS J. 2012, 279, 1386–1397. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal Muscle PGC-1α1 Modulates Kynurenine Metabolism and Mediates Resilience to Stress-Induced Depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance Exercise Increases Skeletal Muscle Kynurenine Aminotransferases and Plasma Kynurenic Acid in Humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef] [Green Version]
- Koeslag, J.H.; Noakes, T.D.; Sloan, A.W. Post-Exercise Ketosis. J. Physiol. 1980, 301, 79–90. [Google Scholar] [CrossRef]
- Mitchell, G.A.; Kassovska-Bratinova, S.; Boukaftane, Y.; Robert, M.F.; Wang, S.P.; Ashmarina, L.; Lambert, M.; Lapierre, P.; Potier, E. Medical Aspects of Ketone Body Metabolism. Clin. Investig. Med. Med. Clin. Exp. 1995, 18, 193–216. [Google Scholar]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of β-Hydroxybutyrate on Cognition in Memory-Impaired Adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Lim, S.; Chesser, A.S.; Grima, J.C.; Rappold, P.M.; Blum, D.; Przedborski, S.; Tieu, K. D-β-Hydroxybutyrate Is Protective in Mouse Models of Huntington’s Disease. PLoS ONE 2011, 6, e24620. [Google Scholar] [CrossRef] [Green Version]
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The Mechanisms by Which the Ketone Body D-β-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson’s Disease. Front. Nutr. 2019, 6, 63. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. eLife 2016, 5. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate Regulates Energy Metabolism and Induces BDNF Expression in Cerebral Cortical Neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef]
- Hu, E.; Du, H.; Zhu, X.; Wang, L.; Shang, S.; Wu, X.; Lu, H.; Lu, X. Beta-Hydroxybutyrate Promotes the Expression of BDNF in Hippocampal Neurons under Adequate Glucose Supply. Neuroscience 2018, 386, 315–325. [Google Scholar] [CrossRef]
- Hansen, J.S.; Pedersen, B.K.; Xu, G.; Lehmann, R.; Weigert, C.; Plomgaard, P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2816–2825. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Kim, S.H.; Min, Y.-K.; Yang, H.-M.; Lee, J.-B.; Lee, M.-S. Acute Exercise Induces FGF21 Expression in Mice and in Healthy Humans. PLoS ONE 2013, 8, e63517. [Google Scholar] [CrossRef]
- Sa-nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Satjaritanun, P.; Wang, X.; Liang, G.; Li, X.; Jiang, C.; Pratchayasakul, W.; Chattipakorn, N.; et al. FGF21 Improves Cognition by Restored Synaptic Plasticity, Dendritic Spine Density, Brain Mitochondrial Function and Cell Apoptosis in Obese-Insulin Resistant Male Rats. Horm. Behav. 2016, 85, 86–95. [Google Scholar] [CrossRef]
- Sjögren, K.; Liu, J.-L.; Blad, K.; Skrtic, S.; Vidal, O.; Wallenius, V.; LeRoith, D.; Törnell, J.; Isaksson, O.G.P.; Jansson, J.-O.; et al. Liver-Derived Insulin-like Growth Factor I (IGF-I) Is the Principal Source of IGF-I in Blood but Is Not Required for Postnatal Body Growth in Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 7088–7092. [Google Scholar] [CrossRef] [Green Version]
- Shavlakadze, T.; Chai, J.; Maley, K.; Cozens, G.; Grounds, G.; Winn, N.; Rosenthal, N.; Grounds, M.D. A Growth Stimulus Is Needed for IGF-1 to Induce Skeletal Muscle Hypertrophy in Vivo. J. Cell Sci. 2010, 123, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Zanconato, S.; Moromisato, D.Y.; Moromisato, M.Y.; Woods, J.; Brasel, J.A.; Leroith, D.; Roberts, C.T.; Cooper, D.M. Effect of Training and Growth Hormone Suppression on Insulin-like Growth Factor I MRNA in Young Rats. J. Appl. Physiol. 1994, 76, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Ha, C.H. The Effect of Exercise Intensity on Brain Derived Neurotrophic Factor and Memory in Adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, S.E.; De Hoyos, D.V.; Garzarella, L.; Vincent, K.; Pollock, B.H.; Lowenthal, D.T.; Pollock, M.L. Effects of Resistance Training on Insulin-like Growth Factor-I and IGF Binding Proteins. Med. Sci. Sports Exerc. 2001, 33, 648–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trejo, J.L.; Carro, E.; Torres-Aleman, I. Circulating Insulin-like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef] [Green Version]
- Carro, E.; Nuñez, A.; Busiguina, S.; Torres-Aleman, I. Circulating Insulin-Like Growth Factor I Mediates Effects of Exercise on the Brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef]
- Chen, M.J.; Russo-Neustadt, A.A. Running Exercise- and Antidepressant-Induced Increases in Growth and Survival-Associated Signaling Molecules Are IGF-Dependent. Growth Factors 2007, 25, 118–131. [Google Scholar] [CrossRef]
- Cani, P.D.; Knauf, C. How Gut Microbes Talk to Organs: The Role of Endocrine and Nervous Routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut Bacteria Are Critical for Optimal Muscle Function:A Potential Link with Glucose Homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef]
- Hawley, J.A. Microbiota and Muscle Highway—Two Way Traffic. Nat. Rev. Endocrinol. 2020, 16, 71–72. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and Exercise Orthogonally Alter the Gut Microbiome and Reveal Independent Associations with Anxiety and Cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- McFadzean, R. Exercise Can Help Modulate Human Gut Microbiota. Undergraduate Honors Thesis, University of Colorado, Boulder, CO, USA, July 2014. [Google Scholar]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [Green Version]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and Probiotics Attenuate the Development of Alzheimer’s Disease in Transgenic Mice: Role of Microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Feng, X.; Uchida, Y.; Koch, L.; Britton, S.; Hu, J.; Lutrin, D.; Maze, M. Exercise Prevents Enhanced Postoperative Neuroinflammation and Cognitive Decline and Rectifies the Gut Microbiome in a Rat Model of Metabolic Syndrome. Front. Immunol. 2017, 8, 1768. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise Influence on the Microbiome–Gut–Brain Axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef]
- Li, S.; Zhai, X.; Rong, P.; McCabe, M.F.; Wang, X.; Zhao, J.; Ben, H.; Wang, S. Therapeutic Effect of Vagus Nerve Stimulation on Depressive-Like Behavior, Hyperglycemia and Insulin Receptor Expression in Zucker Fatty Rats. PLoS ONE 2014, 9, e112066. [Google Scholar] [CrossRef] [Green Version]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The Anxiolytic Effect of Bifidobacterium Longum NCC3001 Involves Vagal Pathways for Gut-Brain Communication. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Malick, M.; Gilbert, K.; Daniel, J.; Arseneault-Breard, J.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Vagotomy Prevents the Effect of Probiotics on Caspase Activity in a Model of Postmyocardial Infarction Depression. Neurogastroenterol. Motil. 2015, 27, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Heaney, J. Hypothalamic-Pituitary-Adrenal Axis. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 1017–1018. ISBN 978-1-4419-1005-9. [Google Scholar]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Eutamene, H.; Lamine, F.; Chabo, C.; Theodorou, V.; Rochat, F.; Bergonzelli, G.E.; Corthésy-Theulaz, I.; Fioramonti, J.; Bueno, L. Synergy between Lactobacillus Paracasei and Its Bacterial Products to Counteract Stress-Induced Gut Permeability and Sensitivity Increase in Rats. J. Nutr. 2007, 137, 1901–1907. [Google Scholar] [CrossRef]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic Treatment of Rat Pups Normalises Corticosterone Release and Ameliorates Colonic Dysfunction Induced by Maternal Separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Sports Endocrinol. 2016, 47, 12–26. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central Mechanisms of HPA Axis Regulation by Voluntary Exercise. Neuromol. Med. 2008, 10, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The Microbiome of Professional Athletes Differs from That of More Sedentary Subjects in Composition and Particularly at the Functional Metabolic Level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-Like Effects of the Histone Deacetylase Inhibitor, Sodium Butyrate, in the Mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- Kim, H.J.; Leeds, P.; Chuang, D.-M. The HDAC Inhibitor, Sodium Butyrate, Stimulates Neurogenesis in the Ischemic Brain. J. Neurochem. 2009, 110, 1226–1240. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, D.W.; Chung, J.Y.; Choi, S.Y.; Yoon, Y.S.; Won, M.-H.; Hwang, I.K. Synergistic Effects of Sodium Butyrate, a Histone Deacetylase Inhibitor, on Increase of Neurogenesis Induced by Pyridoxine and Increase of Neural Proliferation in the Mouse Dentate Gyrus. Neurochem. Res. 2011, 36, 1850–1857. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C. The Gut-Brain Barrier in Major Depression: Intestinal Mucosal Dysfunction with an Increased Translocation of LPS from Gram Negative Enterobacteria (Leaky Gut) Plays a Role in the Inflammatory Pathophysiology of Depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Brown, G.C. The Endotoxin Hypothesis of Neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef] [Green Version]
- Keirns, B.H.; Koemel, N.A.; Sciarrillo, C.M.; Anderson, K.L.; Emerson, S.R. Exercise and Intestinal Permeability: Another Form of Exercise-Induced Hormesis? Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G512–G518. [Google Scholar] [CrossRef]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G.; Wall, R.; Rangel, I.; Marques, T.M.; Repsilber, D. Qualitative Modelling of the Interplay of Inflammatory Status and Butyrate in the Human Gut: A Hypotheses about Robust Bi-Stability. BMC Syst. Biol. 2018, 12, 144. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. The Microbiota Modulates Gut Physiology and Behavioral Abnormalities Associated with Autism. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Terry, N.; Margolis, K.G. Serotonergic Mechanisms Regulating the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Beattie, D.T.; Smith, J.A.M. Serotonin Pharmacology in the Gastrointestinal Tract: A Review. Naunyn. Schmiedebergs Arch. Pharmacol. 2008, 377, 181–203. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [Green Version]
- Reigstad, C.S.; Salmonson, C.E.; Iii, J.F.R.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef]
- Zhang, A.-S. Control of Systemic Iron Homeostasis by the Hemojuvelin-Hepcidin Axis12. Adv. Nutr. 2010, 1, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The Role of Iron in Brain Ageing and Neurodegenerative Disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.; Arosio, P.; Finazzi, D.; Koziorowski, D.; Galazka-Friedman, J. Ferritin as an Important Player in Neurodegeneration. Parkinsonism Relat. Disord. 2011, 17, 423–430. [Google Scholar] [CrossRef]
- Kim, J.; Wessling-Resnick, M. Iron and Mechanisms of Emotional Behavior. J. Nutr. Biochem. 2014, 25, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Sun, W.; Li, Y.; Ling, S.; Zhao, C.; Zhong, G.; Zhao, D.; Song, J.; Song, H.; Li, J.; et al. The Regulation of Iron Metabolism by Hepcidin Contributes to Unloading-Induced Bone Loss. Bone 2017, 94, 152–161. [Google Scholar] [CrossRef]
- Cavey, T.; Pierre, N.; Nay, K.; Allain, C.; Ropert, M.; Loréal, O.; Derbré, F. Simulated Microgravity Decreases Circulating Iron in Rats: Role of Inflammation-Induced Hepcidin Upregulation. Exp. Physiol. 2017, 102, 291–298. [Google Scholar] [CrossRef]
- Nay, K.; Martin, D.; Orfila, L.; Saligaut, D.; Martin, B.; Horeau, M.; Cavey, T.; Kenawi, M.; Island, M.-L.; Ropert, M.; et al. Intermittent Reloading Does Not Prevent Reduction in Iron Availability and Hepcidin Upregulation Caused by Hindlimb Unloading. Exp. Physiol. 2021, 106, 28–36. [Google Scholar] [CrossRef]
- Nay, K.; Koechlin-Ramonatxo, C.; Rochdi, S.; Island, M.-L.; Orfila, L.; Treffel, L.; Bareille, M.-P.; Beck, A.; Gauquelin-Koch, G.; Ropert, M.; et al. Simulated Microgravity Disturbs Iron Metabolism and Distribution in Humans: Lessons from Dry Immersion, an Innovative Ground-Based Human Model. FASEB J. 2020, 34, 14920–14929. [Google Scholar] [CrossRef]
- Nay, K.; Horeau, M.; Loréal, O.; Derbré, F. Métabolisme du fer: Impact de l’hypoactivité et mécanismes sous-jacents. Cah. Nutr. Diététique 2021. [Google Scholar] [CrossRef]
- Domínguez, R.; Sánchez-Oliver, A.J.; Mata-Ordoñez, F.; Feria-Madueño, A.; Grimaldi-Puyana, M.; López-Samanes, Á.; Pérez-López, A. Effects of an Acute Exercise Bout on Serum Hepcidin Levels. Nutrients 2018, 10, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, M.P. Effect of Exercise on Inflammation, Hepcidin Regulation and Iron Metabolism. Ph.D. Thesis, University of Western Australia, Perth, WA, Australia, 2014. [Google Scholar]
- Choi, D.-H.; Kwon, K.-C.; Hwang, D.-J.; Koo, J.-H.; Um, H.-S.; Song, H.-S.; Kim, J.-S.; Jang, Y.; Cho, J.-Y. Treadmill Exercise Alleviates Brain Iron Dyshomeostasis Accelerating Neuronal Amyloid-β Production, Neuronal Cell Death, and Cognitive Impairment in Transgenic Mice Model of Alzheimer’s Disease. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nay, K.; Smiles, W.J.; Kaiser, J.; McAloon, L.M.; Loh, K.; Galic, S.; Oakhill, J.S.; Gundlach, A.L.; Scott, J.W. Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4052. https://doi.org/10.3390/ijms22084052
Nay K, Smiles WJ, Kaiser J, McAloon LM, Loh K, Galic S, Oakhill JS, Gundlach AL, Scott JW. Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. International Journal of Molecular Sciences. 2021; 22(8):4052. https://doi.org/10.3390/ijms22084052
Chicago/Turabian StyleNay, Kévin, William J. Smiles, Jacqueline Kaiser, Luke M. McAloon, Kim Loh, Sandra Galic, Jonathan S. Oakhill, Andrew L. Gundlach, and John W. Scott. 2021. "Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders" International Journal of Molecular Sciences 22, no. 8: 4052. https://doi.org/10.3390/ijms22084052
APA StyleNay, K., Smiles, W. J., Kaiser, J., McAloon, L. M., Loh, K., Galic, S., Oakhill, J. S., Gundlach, A. L., & Scott, J. W. (2021). Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. International Journal of Molecular Sciences, 22(8), 4052. https://doi.org/10.3390/ijms22084052







