Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration
Abstract
1. Introduction
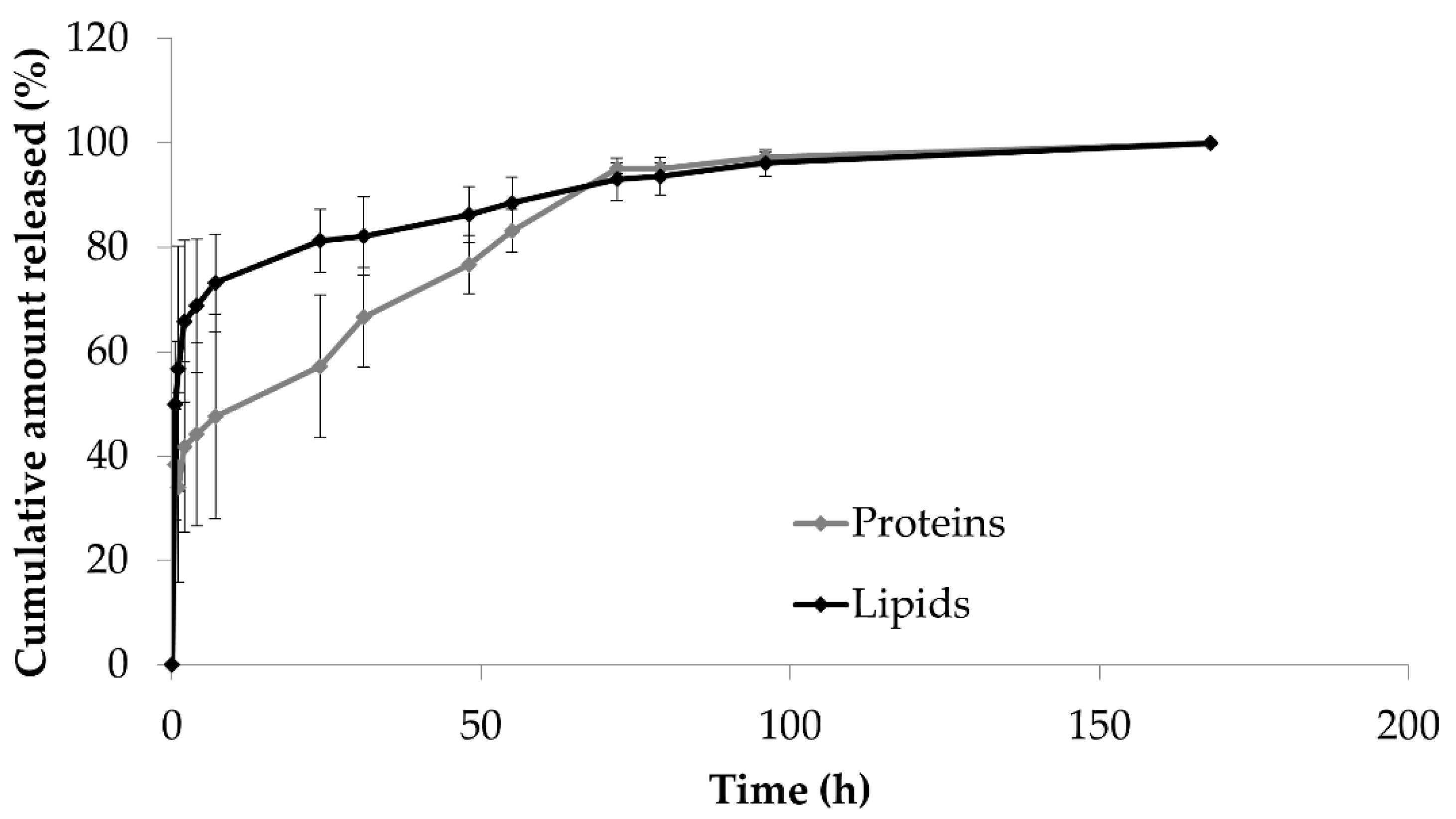
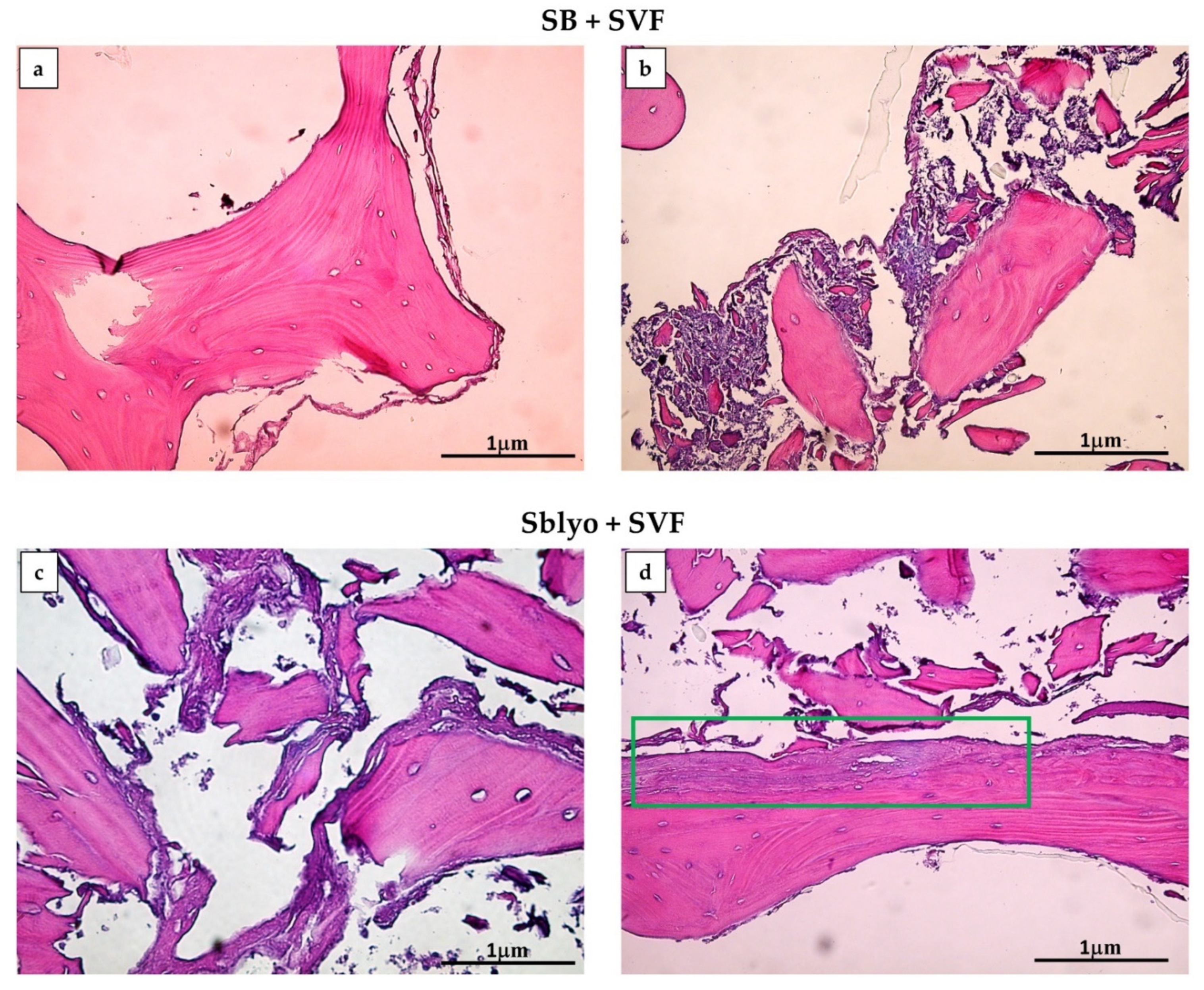
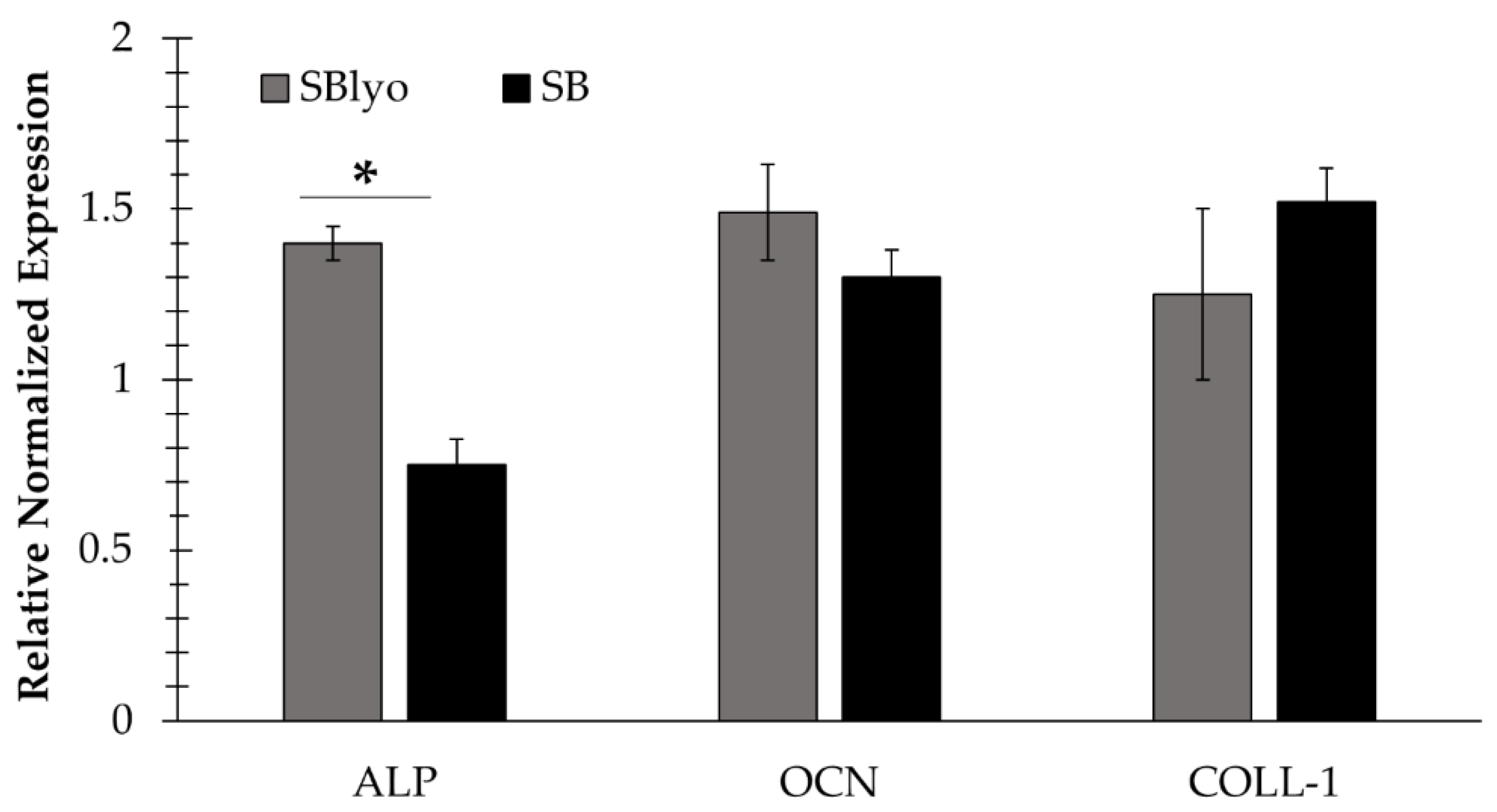
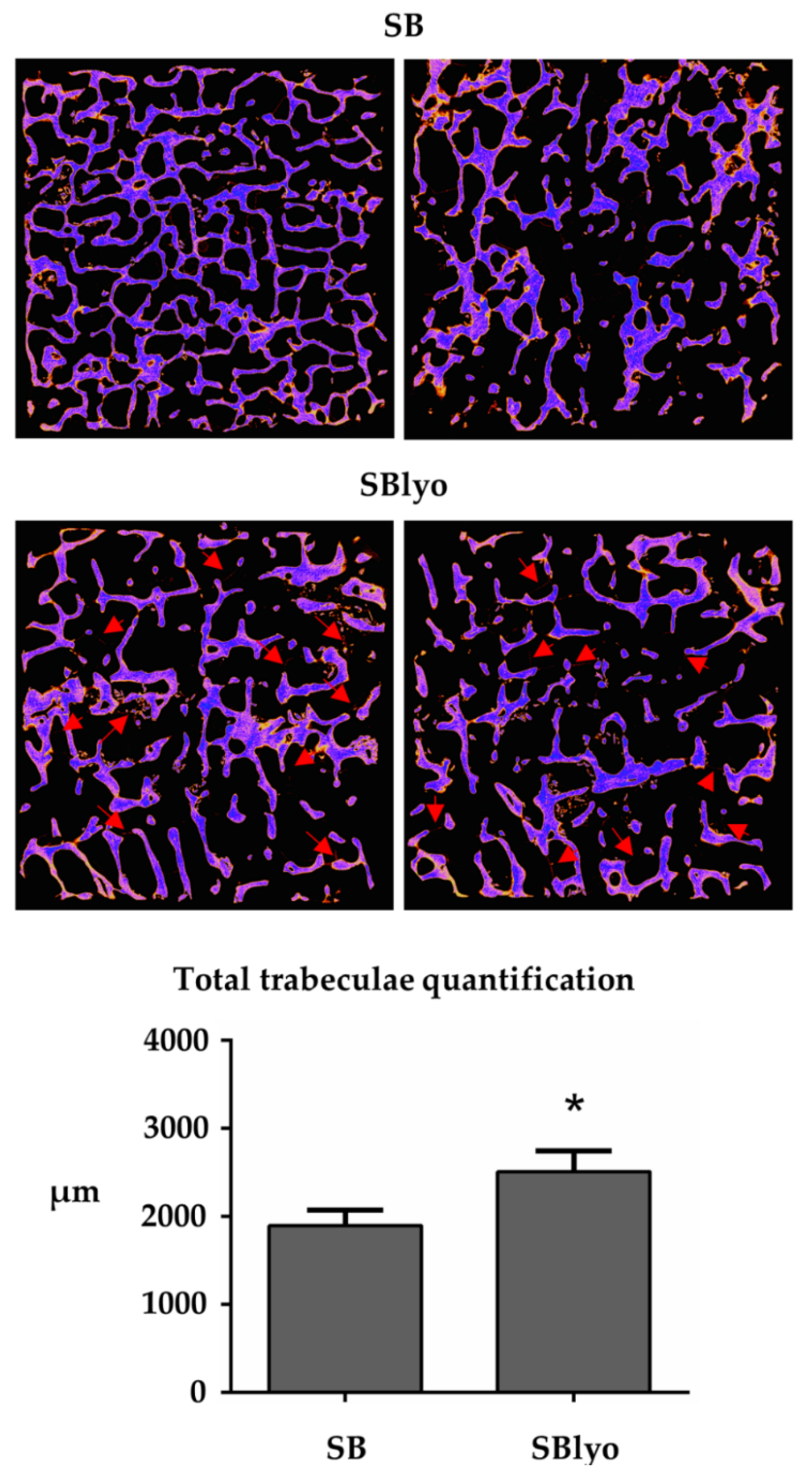
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Lyosecretome Preparation and Characterization
3.2.1. Lyosecretome Preparation
3.2.2. Lyosecretome Characterization
Total Proteins and Lipids
EV Particle Size and Concentration
3.3. Lyosecretome-Loaded SmartBone® (SBlyo) Preparation and Characterization
3.3.1. SBlyo Preparation
3.3.2. Drug Loading
3.3.3. Morphological Investigation by Environmental Scanning Electron Microscopy (ESEM)
3.3.4. Drug Release Studies
3.3.5. Drug Release Kinetic Study
3.4. In Vitro Evaluation of Lyosecretome Function
3.4.1. Isolation of the Stromal Vascular Fraction (SVF)
3.4.2. Cell Cultures
3.4.3. Evaluation of the Gene Expression
3.4.4. Micro-CT Analysis
3.4.5. Histochemical Analyses
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kon, E.; Filardo, G.; Roffi, A.; Di Martino, A.; Hamdan, M.; De Pasqual, L.; Merli, M.L.; Marcacci, M. Bone regeneration with mesenchymal stem cells. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2012, 9, 24–27. [Google Scholar]
- Garcia-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.; Perale, G.; Milazzo, M.; Moscato, S.; Stefanini, C.; Pertici, G.; Danti, S. Bovine bone matrix/poly(L-lactic-co-epsilon-caprolactone)/gelatin hybrid scaffold (SmartBone (R)) for maxillary sinus augmentation: A histologic study on bone regeneration. Int. J. Pharm. 2017, 523, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Pertici, G.; Rossi, F.; Casalini, T.; Perale, G. Composite polymer-coated mineral grafts for bone regeneration: Material characterisation and model study. Ann. Oral Maxillofac. Surg. 2014, 2, 4. [Google Scholar]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts-What they can offer and what they cannot. J. Bone Jt. Surg. Br. Vol. 2007, 89B, 574–579. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic bone tissue engineering graft substitutes: What is the future? Injury 2020. [Google Scholar] [CrossRef]
- Miramini, S.; Fegan, K.L.; Green, N.C.; Espino, D.M.; Zhang, L.H.; Thomas-Seale, L.E.J. The status and challenges of replicating the mechanical properties of connective tissues using additive manufacturing. J. Mech. Behav. Biomed. Mater. 2020, 103. [Google Scholar] [CrossRef]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.L.; Conti, M. 3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmaceutics 2021, 13, 515. [Google Scholar] [CrossRef]
- Cingolani, A.; Grottoli, C.F.; Esposito, R.; Villa, T.; Rossi, F.; Perale, G. Improving Bovine Bone Mechanical Characteristics for the Development of Xenohybrid Bone Grafts. Curr. Pharm. Biotechnol. 2018, 19, 1005–1013. [Google Scholar] [CrossRef]
- Grecchi, F.; Perale, G.; Candotto, V.; Busato, A.; Pascali, M.; Carinci, F. Reconstruction Of The Zygomatic Bone With Smartbone (R): Case Report. J. Biol. Regul. Homeost. Agents 2015, 29, 42–47. [Google Scholar] [PubMed]
- Mandelli, F.; Perale, G.; Danti, S.; D’Alessandro, D.; Ghensi, P. Clinical and histological evaluation of socket preservation using Smartbone®, a novel heterologous bone substitute: A case series study. Oral Implantol. 2018, 2, 87–94. [Google Scholar]
- Ferracini, R.; Bistolfi, A.; Garibaldi, R.; Furfaro, V.; Battista, A.; Perale, G. Composite Xenohybrid Bovine Bone-Derived Scaffold as Bone Substitute for the Treatment of Tibial Plateau Fractures. Appl. Sci. 2019, 9, 2675. [Google Scholar] [CrossRef]
- Boffano, M.; Ratto, N.; Conti, A.; Pellegrino, P.; Rossi, L.; Perale, G.; Piana, R. A Preliminary Study on the Mechanical Reliability and Regeneration Capability of Artificial Bone Grafts in Oncologic Cases, With and Without Osteosynthesis. J. Clin. Med. 2020, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Sallent, I.; Capella-Monsonis, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Muraglia, A.; Corsi, A.; Bianco, P.; Marcacci, M.; Martin, I.; Boyde, A.; Ruspantini, I.; Chistolini, P.; Rocca, M.; et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J. Biomed. Mater. Res. 2000, 49, 328–337. [Google Scholar] [CrossRef]
- Dallari, D.; Fini, M.; Stagni, C.; Torricelli, P.; Aldini, N.N.; Giavaresi, G.; Cenni, E.; Baldini, N.; Cenacchi, A.; Bassi, A.; et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J. Orthop. Res. 2006, 24, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Agacayak, S.; Gulsun, B.; Ucan, M.C.; Karaoz, E.; Nergiz, Y. Effects of mesenchymal stem cells in critical size bone defect. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 679–686. [Google Scholar]
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Boenig, H.; Henrich, D.; Marzi, I. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. 2018, 44, 649–665. [Google Scholar] [CrossRef]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control. Release 2019, 309, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, B.; Chlapanidas, T.; Perteghella, S.; Lucarelli, E.; Pascucci, L.; Brini, A.T.; Ferrero, I.; Marazzi, M.; Pessina, A.; Torre, M.L.; et al. Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J. Control. Release 2017, 262, 104–117. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Sorrenti, M.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, P.; et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells 2018, 7, 190. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Di Silvestre, D.; Mastracci, L.; Grillo, F.; Grisoli, P.; Marrubini, G.; Nardini, M.; Mastrogiacomo, M.; Sorlini, M.; Rossi, R.; et al. GMP-compliant sponge-like dressing containing MSC lyo-secretome: Proteomic network of healing in a murine wound model. Eur. J. Pharm. Biopharm. 2020, 155, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Ferrarotti, I.; Di Silvestre, D.; Grisoli, P.; Barzon, V.; Balderacchi, A.; Torre, M.L.; Rossi, R.; Mauri, P.; Corsico, A.G.; et al. Adipose Mesenchymal Extracellular Vesicles as Alpha-1-Antitrypsin Physiological Delivery Systems for Lung Regeneration. Cells 2019, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Belisario, D.C.; Compagno, M.; Verderio, L.; Sighinolfi, A.; Mussano, F.; Genova, T.; Veneziano, F.; Pertici, G.; Perale, G.; et al. Adipose-Derived Stromal Vascular Fraction/Xenohybrid Bone Scaffold: An Alternative Source for Bone Regeneration. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Costa, P.F. Bone Tissue Engineering Drug Delivery. Curr. Mol. Biol. Rep. 2015, 1, 87–93. [Google Scholar] [CrossRef]
- Wang, P.L.; Johnston, T.P. Enhanced Stability of 2 Model Proteins in an Agitated Solution Environment Using Poloxamer-407. J. Parenter. Sci. Technol. 1993, 47, 183–189. [Google Scholar] [PubMed]
- Katakam, M.; Banga, A.K. Use of poloxamer polymers to stabilize recombinant human growth hormone against various processing stresses. Pharm. Dev. Technol. 1997, 2, 143–149. [Google Scholar] [CrossRef]
- Telang, C.; Yu, L.; Suryanarayanan, R. Effective inhibition of mannitol crystallization in frozen solutions by sodium chloride. Pharm. Res. 2003, 20, 660–667. [Google Scholar] [CrossRef]
- Caccavo, D. An overview on the mathematical modeling of hydrogels’ behavior for drug delivery systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef]
- Pertici, G.; Carinci, F.; Carusi, G.; Epistatus, D.; Villa, T.; Crivelli, F.; Rossi, F.; Perale, G. Composite Polymer-Coated Mineral Scaffolds For Bone Regeneration: From Material Characterization To Human Studies. J. Biol. Regul. Homeost. Agents 2015, 29, 136–148. [Google Scholar] [PubMed]
- Liang, B.; Liang, J.-M.; Ding, J.-N.; Xu, J.; Xu, J.-G.; Chai, Y.-M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. Acs Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.X.; Li, S.H.; Gong, Y.S.; Zhou, M.W.; Zhang, J.H.; Zhu, G.Y. Effects of human umbilical cord mesenchymal stem cells-derived exosomes on fracture healing in rats through the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4954–4960. [Google Scholar]
- Narayanan, R.; Huang, C.-C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef]
- Wang, K.-X.; Xu, L.-L.; Rui, Y.-F.; Huang, S.; Lin, S.-E.; Xiong, J.-H.; Li, Y.-H.; Lee, W.Y.-W.; Li, G. The Effects of Secretion Factors from Umbilical Cord Derived Mesenchymal Stem Cells on Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0120593. [Google Scholar] [CrossRef] [PubMed]
- Moursi, A.M.; Damsky, C.H.; Lull, J.; Zimmerman, D.; Doty, S.B.; Aota, S.; Globus, R.K. Fibronectin regulates calvarial osteoblast differentiation. J. Cell Sci. 1996, 106, 1369–1380. [Google Scholar]
- Kang, Y.Y.; Georgiou, A.I.; MacFarlane, R.J.; Klontzas, M.E.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Fibronectin stimulates the osteogenic differentiation of murine embryonic stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Gavish, H.; Bab, I.; Tartakovsky, A.; Chorev, M.; Mansur, N.; Greenberg, Z.; NamdarAttar, H.; Muhlrad, A. Human alpha(2)-macroglobulin is an osteogenic growth peptide-binding protein. Biochemistry 1997, 36, 14883–14888. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Kalyvioti, E.; Papachristou, N.I.; Tourkova, I.L.; Syggelos, S.A.; Deligianni, D.; Orkoula, M.G.; Kontoyannis, C.G.; Karavia, E.A.; Kypreos, K.E.; et al. Apolipoprotein A-1 regulates osteoblast and lipoblast precursor cells in mice. Lab. Investig. 2016, 96, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Mundy, G.R. Role of Transforming Growth Factor-Beta in Bone Remodeling. Clin. Orthop. Relat. Res. 1990, 261–276. [Google Scholar] [CrossRef]
- Zhang, L.H.; Miramini, S.; Richardson, M.; Ebeling, P.; Little, D.; Yang, Y.; Huang, Z.Y. Computational modelling of bone fracture healing under partial weight-bearing exercise. Med. Eng. Phys. 2017, 42, 65–72. [Google Scholar] [CrossRef]
- Ganadhiepan, G.; Miramini, S.; Patel, M.; Mendis, P.; Zhang, L.H. Bone fracture healing under Ilizarov fixator: Influence of fixator configuration, fracture geometry, and loading. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3199. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Bucco, M.; Chlapanidas, T.; Lucconi, G.; Marazzi, M.; Tosca, M.C.; Gaetani, P.; Klinger, M.; Villani, S.; Ferretti, V.V.; et al. Nonexpanded Mesenchymal Stem Cells for Regenerative Medicine: Yield in Stromal Vascular Fraction from Adipose Tissues. Tissue Eng. Part C Methods 2010, 16, 1515–1521. [Google Scholar] [CrossRef]
- Gaetani, P.; Torre, M.L.; Klinger, M.; Faustini, M.; Crovato, F.; Bucco, M.; Marazzi, M.; Chlapanidas, T.; Levi, D.; Tancioni, F.; et al. Adipose-derived stem cell therapy for intervertebral disc regeneration: An in vitro reconstructed tissue in alginate capsules. Tissue Eng. Part A 2008, 14, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Tamone, C.; Sassi, F.; D’Amico, L.; Roato, I.; Patane, S.; Ravazzoli, M.; Veneziano, L.; Ferracini, R.; Pescarmona, G.P.; et al. Teriparatide increases the maturation of circulating osteoblast precursors. Osteoporos. Int. 2012, 23, 1245–1253. [Google Scholar] [CrossRef] [PubMed]




| Model | Equation | Proteins/Lipids | Coefficients (95% Confidence Bounds) | Sum of Squares | R2 | Degrees of Freedom | SE |
|---|---|---|---|---|---|---|---|
| Higuchi | F(t) = k × t0.5 | Proteins | k = 19.79 | 3,081,232 | 0.253 | 32 | 1.479 |
| (16.78, 22.80) | |||||||
| Lipids | k = 1.498 | 43,540 | −0.2396 | 32 | 0.1758 | ||
| (1.139, 1.856) | |||||||
| Higuchi (eq 2.12 from [32]) | F(t) = 100 × (1 − C × exp (−k × t)) | Proteins | C = −4.113 | 2,103,706 | 0.49 | 31 | C |
| (−5.216, −3.066) | 0.5425 | ||||||
| k = −0.0002381 | k | ||||||
| (−0.0003286, −0.0001524) | 0.00004424 | ||||||
| Lipids | C = 0.5763 | 28,621 | 0.1851 | 31 | C | ||
| (0.4179, 0.7419) | 0.07758 | ||||||
| k = 0.0002817 | k | ||||||
| (0.0000523, 0.0007763) | 0.0001296 | ||||||
| Peppas–Sahlin | F(t) = k1 × tm + k2 × t(2 × m) | Proteins | k1 = ~ | k1~ | |||
| k2 = ~ | k2~ | ||||||
| m = ~ | m~ | ||||||
| Lipids | k1= ~ | k1~ | |||||
| k2= ~ | k2~ | ||||||
| m = ~ | m~ | ||||||
| Ritger–Peppas | F(t) = k × tn | Proteins | k = 248.9 | 1,208,384 | 0.707 | 31 | k |
| (144.2, 394.5) | 59.38 | ||||||
| n = 0.1725 | n | ||||||
| (0.1076, 0.2459) | 0.03278 | ||||||
| Lipids | k = 33.89 | 21,454 | 0.3892 | 31 | k | ||
| (14.45, 63.50) | 11.55 | ||||||
| n = 0.09547 | n | ||||||
| (0.001254, 0.2032) | 0.04875 | ||||||
| Zero-order | F(t) = k × t | Proteins | k = 0.3309 | 5,985,916 | −0.4512 | 32 | 0.0378 |
| (0.2539, 0.4079) | |||||||
| Lipids | k = 0.02411 | 66,142 | −0.8831 | 32 | 0.003974 | ||
| (0.01602, 0.03221) | |||||||
| Korsmeyer–Peppas | F(t) = kKP × tn × Q0 | Proteins | kKP = 248.9 | 1,208,384 | 0.707 | 31 | kKP |
| (144.2, 394.5) | 59.38 | ||||||
| n = 0.1725 | n | ||||||
| (0.1076, 0.2459) | 0.03278 | ||||||
| Lipids | kKP = 33.89 | 21,454 | 0.3892 | 31 | kKP | ||
| (15.45, 63.50) | 11.55 | ||||||
| n = 0.09547 | n | ||||||
| (0.001254, 0.2032) | 0.04875 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, E.; Roato, I.; Perale, G.; Rossi, F.; Genova, T.; Mussano, F.; Ferracini, R.; Sorlini, M.; Torre, M.L.; Perteghella, S. Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 4064. https://doi.org/10.3390/ijms22084064
Bari E, Roato I, Perale G, Rossi F, Genova T, Mussano F, Ferracini R, Sorlini M, Torre ML, Perteghella S. Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration. International Journal of Molecular Sciences. 2021; 22(8):4064. https://doi.org/10.3390/ijms22084064
Chicago/Turabian StyleBari, Elia, Ilaria Roato, Giuseppe Perale, Filippo Rossi, Tullio Genova, Federico Mussano, Riccardo Ferracini, Marzio Sorlini, Maria Luisa Torre, and Sara Perteghella. 2021. "Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration" International Journal of Molecular Sciences 22, no. 8: 4064. https://doi.org/10.3390/ijms22084064
APA StyleBari, E., Roato, I., Perale, G., Rossi, F., Genova, T., Mussano, F., Ferracini, R., Sorlini, M., Torre, M. L., & Perteghella, S. (2021). Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration. International Journal of Molecular Sciences, 22(8), 4064. https://doi.org/10.3390/ijms22084064













