Quantitative Proteomics Analysis Reveals the Function of the Putative Ester Cyclase UvEC1 in the Pathogenicity of the Rice False Smut Fungus Ustilaginoidea virens
Abstract
:1. Introduction
2. Results
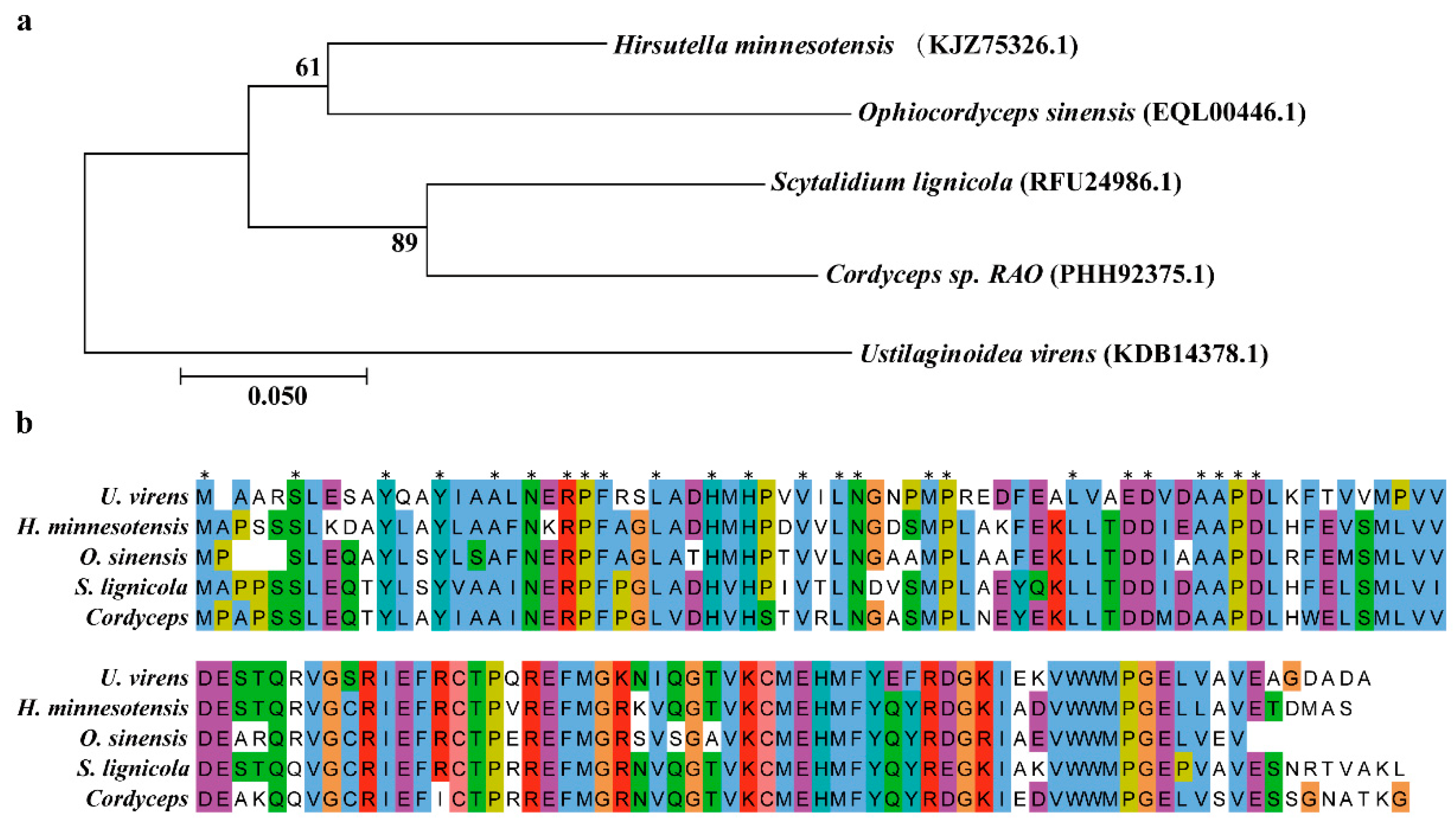
2.1. Identification and Characterization of UvEC1 in U. virens
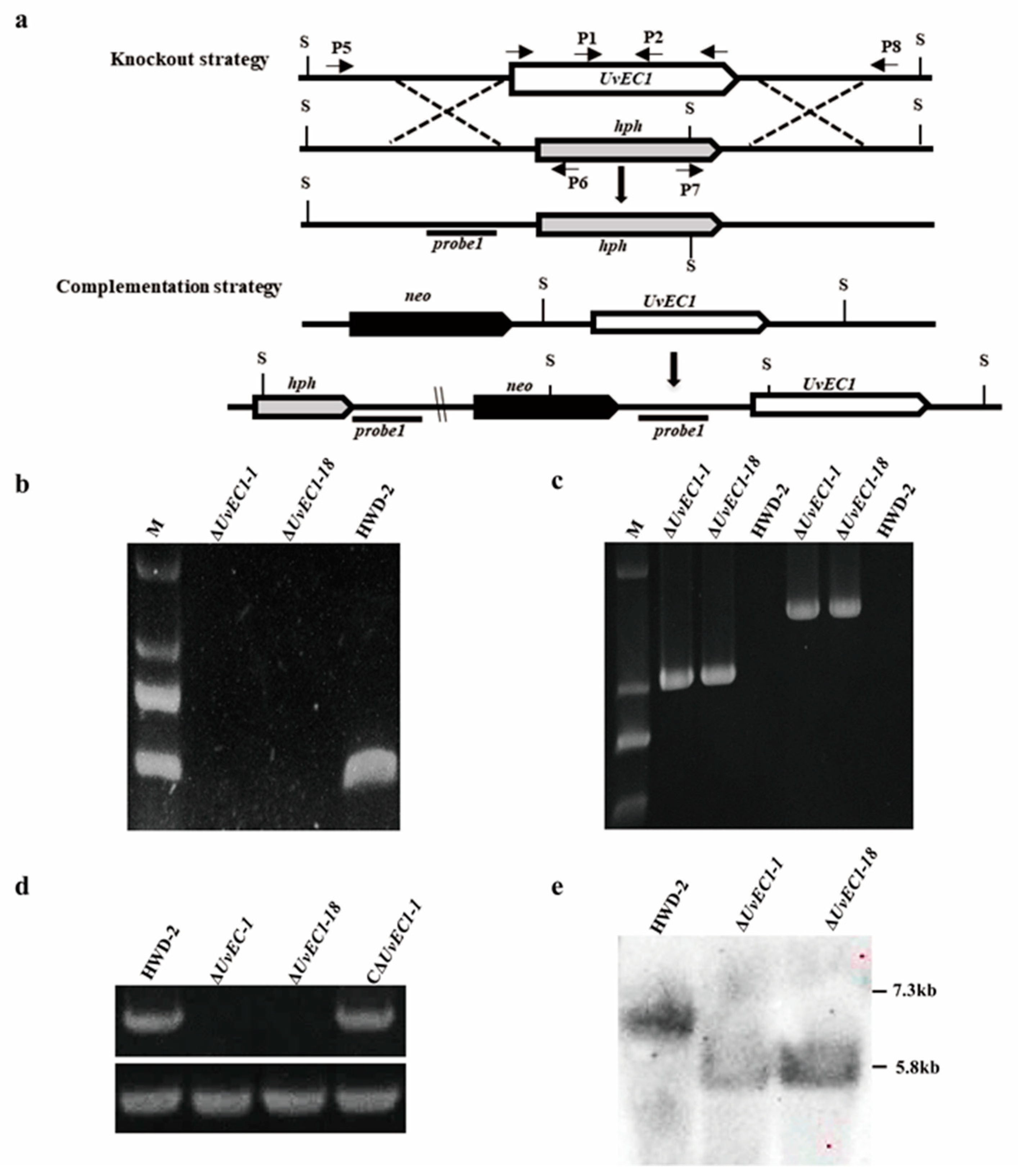
2.2. Genomic Deletion and Complementation of UvEC1 in U. virens
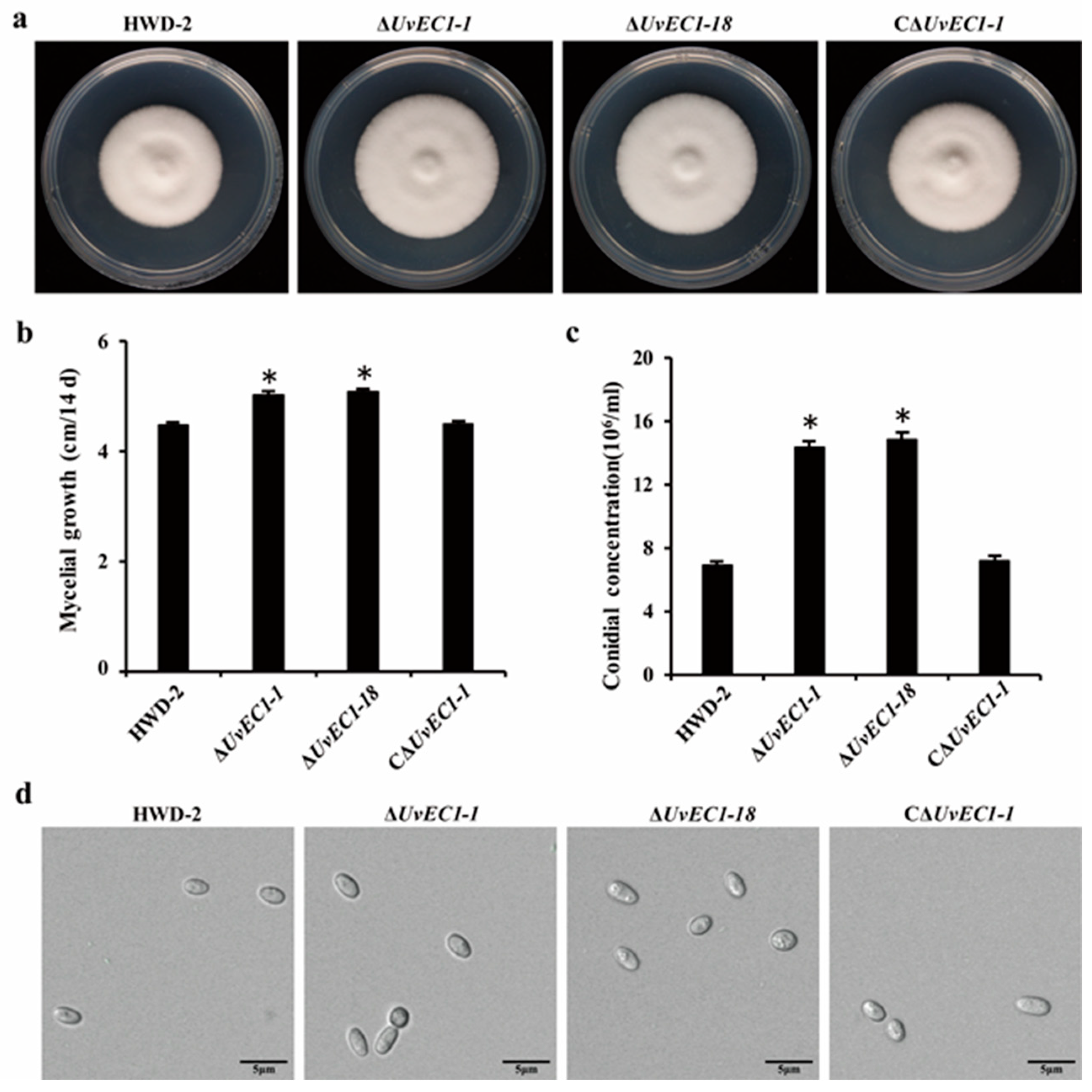
2.3. Deletion of UvEC1 Affects Vegetative Growth and Conidiation of U. virens
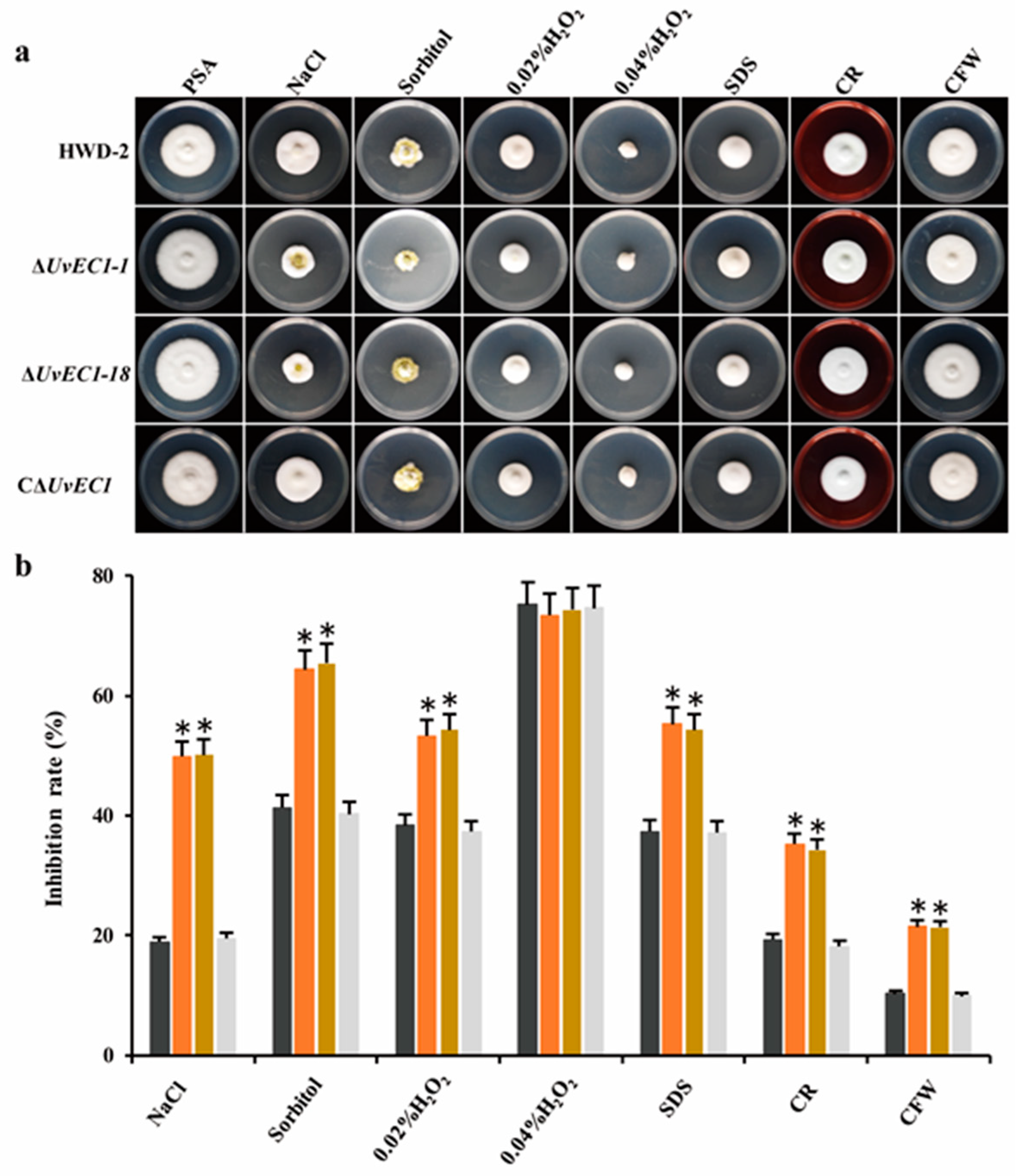
2.4. UvEC1 Is Important for Response to Various Stresses
2.5. Deletion of UvEC1 Affects the Pathogenicity of U. virens
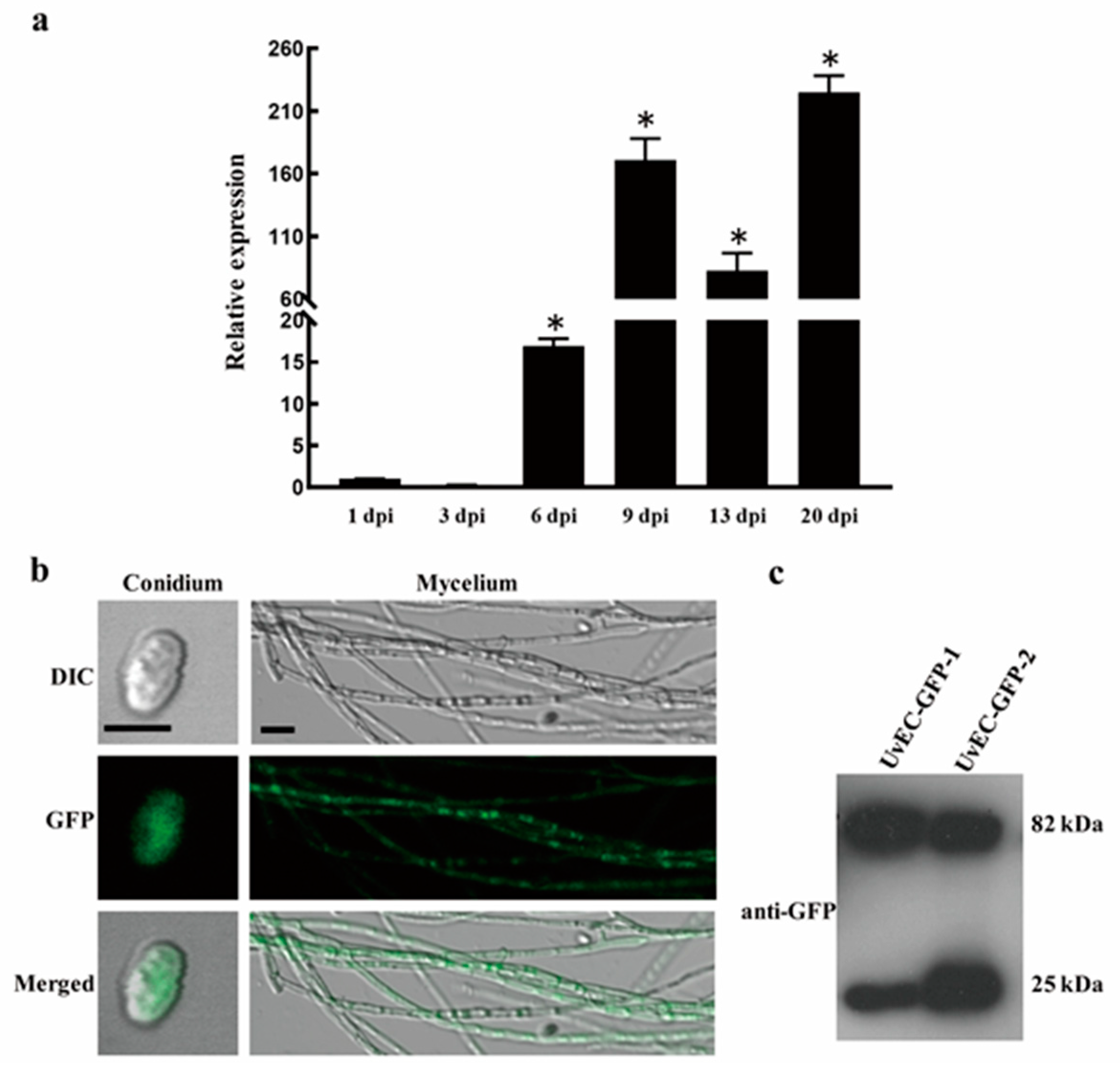
2.6. UvEC1 Is Highly Expressed during Infection by U. virens
2.7. Subcellular Localization of UvEC1
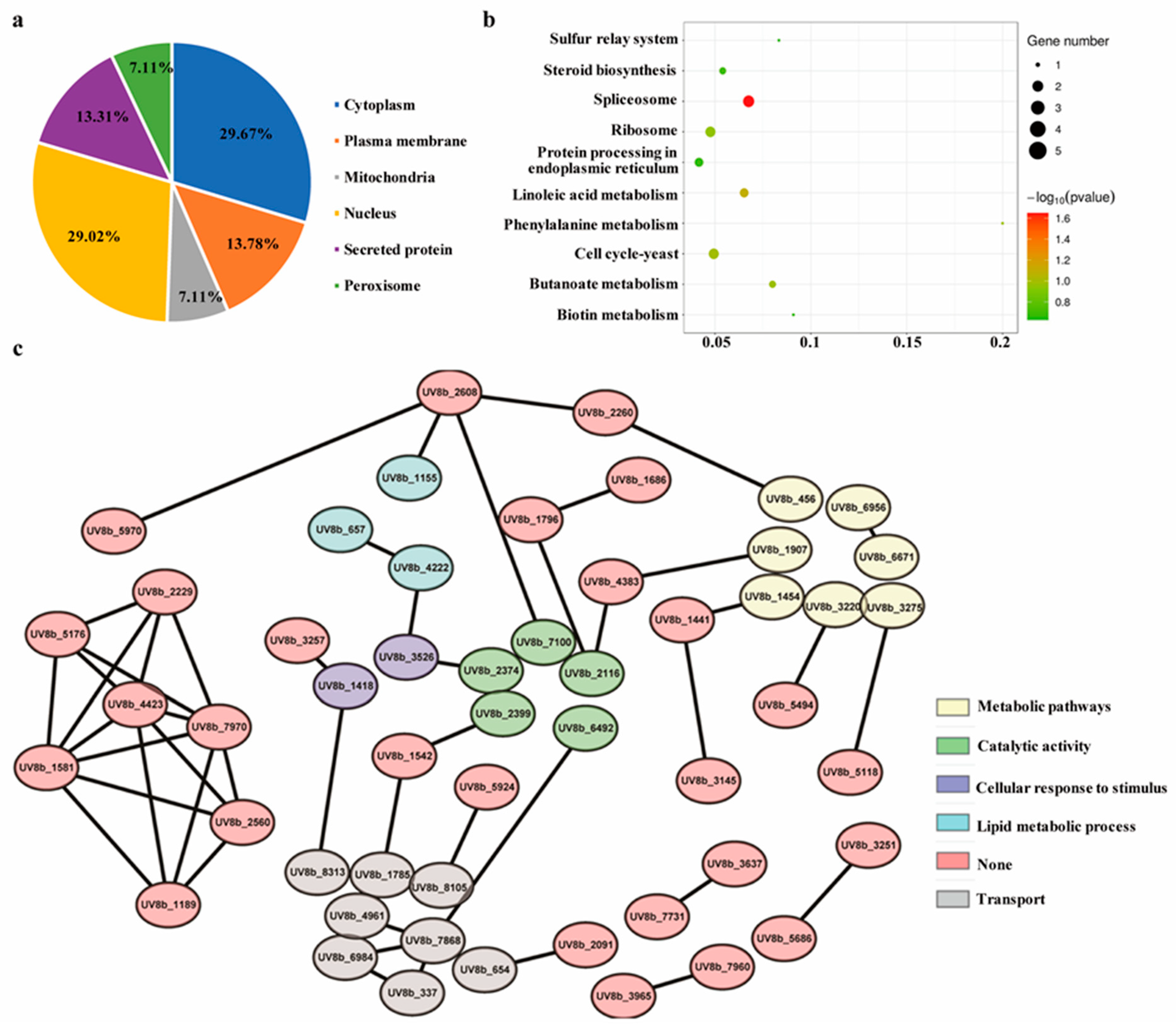
2.8. Functional Enrichment Analysis of the Differentially Accumulating Proteome of the ∆UvEC1-1 Mutant Relative to HWD-2
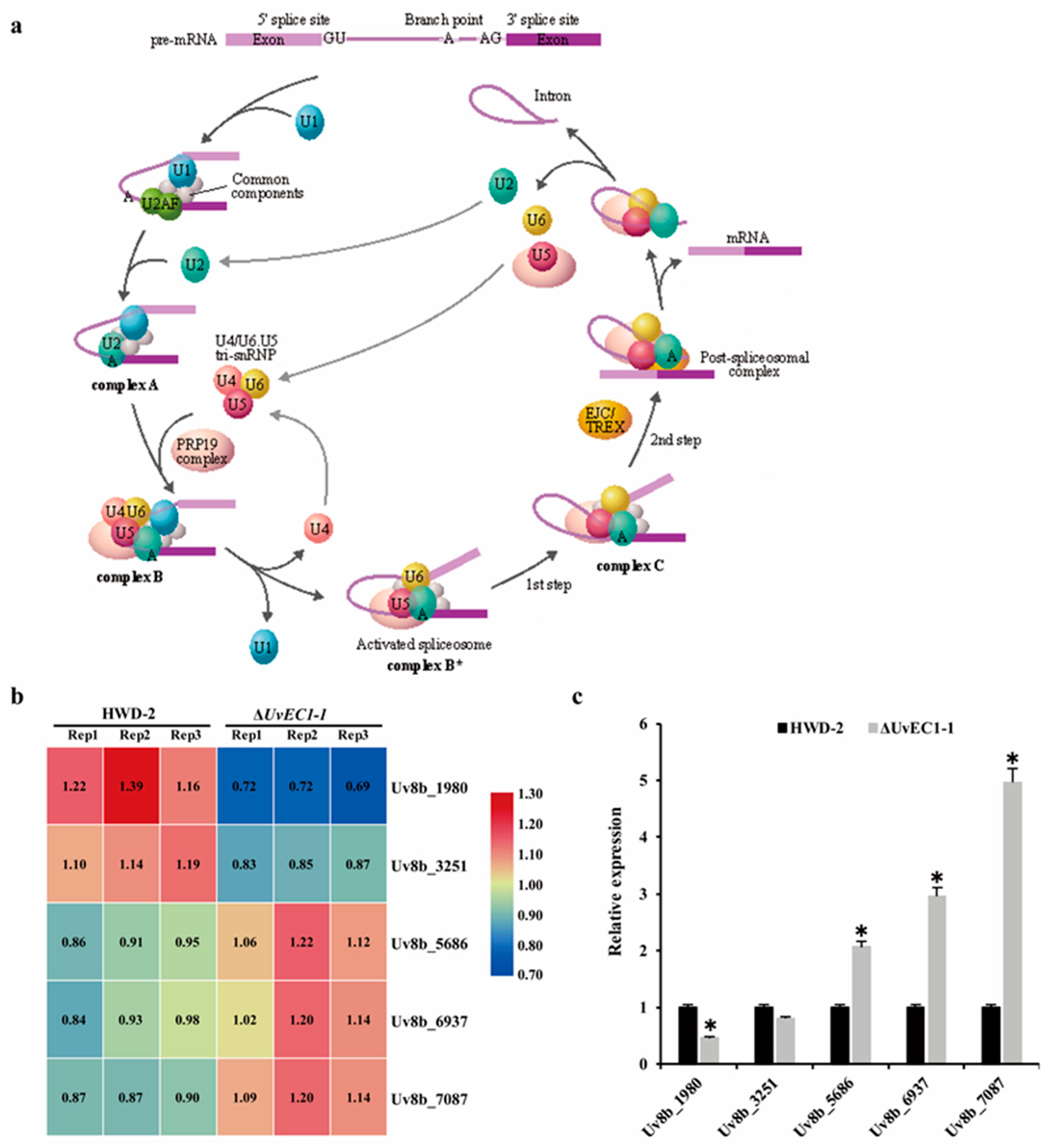
2.9. UvEC1 Is Involved in the Spliceosome
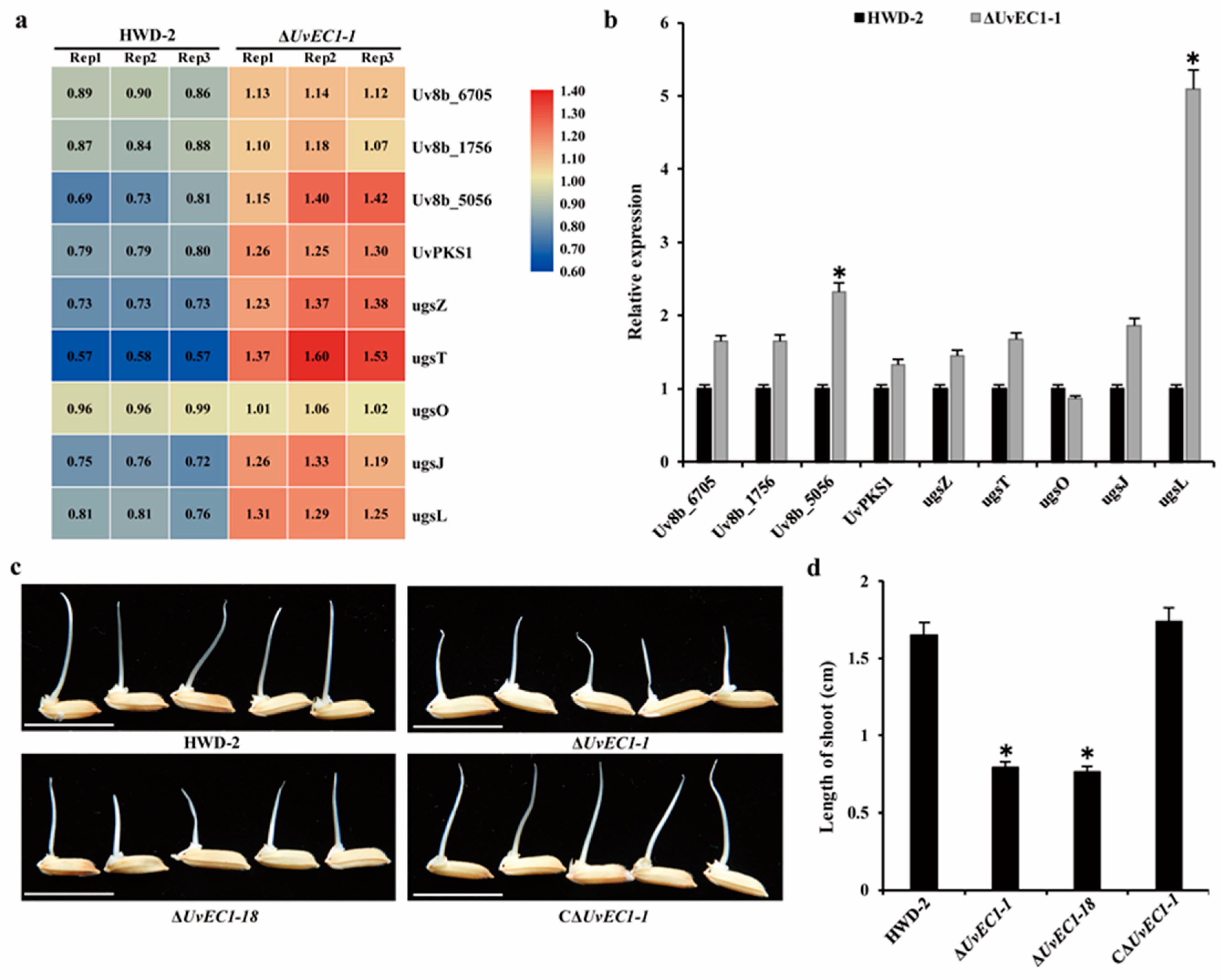
2.10. UvEC1 Is Associated with Toxin Production
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Growth Conditions
4.2. Gene Deletion and Complementation in U. virens
4.3. DNA/RNA Manipulation, Southern Blotting and RT-qPCR
4.4. Vegetative Growth, Conidiation and Pathogenicity
4.5. Stress Adaptation Assays
4.6. Immunoblotting
4.7. Protein Extraction and Digestion
4.8. LC-MS/MS Analysis
4.9. Bioinformatics Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.Y.; Li, Y.; Fan, J.; Li, L.; Huang, F.; Wang, W.M. Progress in the study of false smut disease in rice. J. Agric. Sci. Technol. 2012, 2, 1211–1217. [Google Scholar]
- Ashizawa, T.; Takahashi, M.; Arai, M.; Arie, T. Rice false smut pathogen, Ustilaginoidea virens, invades through small gap at the apex of a rice spikelet before heading. J. Gen. Plant Pathol. 2012, 78, 255–259. [Google Scholar] [CrossRef]
- Tang, Y.X.; Jin, J.; Hu, D.W.; Yong, M.L.; Xu, Y.; He, L.P. Elucidation of the infection process of Ustilaginoidea virens (teleomorph: Villosiclava virens) in rice spikelets. Plant Pathol. 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Song, J.H.; Wei, W.; Lv, B.; Lin, Y.; Yin, W.X.; Peng, Y.L.; Schnabel, G.; Huang, J.B.; Jiang, D.H.; Luo, C.X. Rice false smut fungus hijacks the rice nutrients supply by blocking and mimicking the fertilization of rice ovary. Environ. Microbiol. 2016, 18, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, J.; Gong, Z.Y.; Xu, P.Z.; Hu, X.H.; Wu, J.L.; Li, J.B.; Yang, J.; Wang, Y.Q.; Zhou, Y.F.; et al. The false smut pathogen Ustilaginoidea virens requires rice stamens for false smut ball formation. Environ. Microbiol. 2020, 2, 646–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koiso, Y.; Li, Y.; Iwasaki, S.; Hanaoka, K.; Kobayashi, T.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea Virens. J. Antibiot. 1994, 47, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Koyama, K.; Natori, S. Further characterization of seven bis (naphtho-γ-pyrone) congeners of ustilaginoidins, pigments of Claviceps virens (Ustilaginoidea virens). Chem. Pharm. Bull. 1988, 36, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Lai, D.W.; Meng, J.J.; Zhang, X.P.; Xu, D.; Dai, J.G.; Zhou, L.G. Ustilobisorbicillinol A, a cytotoxic sorbyl-containing aromatic polyketide from Ustilaginoidea virens. Org. Lett. 2019, 21, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhao, S.; Dang, P.; Zhou, Z.; Lai, D.; Zhou, L. Ustilaginoidin M1, a new bis-naphtho-γ-pyrone from the fungus Villosiclava virens. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Wang, J.; Lai, D.W.; Wang, W.X.; Dai, J.G.; Zhou, L.; Liu, Y. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins 2017, 9, 54. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Fang, A.F.; Han, Y.Q.; Yang, J.; Xue, M.F.; Bao, J.D.; Hu, D.W.; Zhou, B.; Sun, X.; et al. Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat. Commun. 2014, 5, 3849. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Y.; Tang, J.T.; Pei, Z.X.; Liu, H.; Huang, J.B.; Luo, C.X.; Hsiang, T.; Zheng, L. The “pears and lemons” protein UvPal1 regulates development and virulence of Ustilaginoidea virens. Environ. Microbiol. 2020, 22, 5414–5432. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Biosynthesis of polyketide metabolites. Nat. Prod. Rep. 1992, 9, 447–479. [Google Scholar] [CrossRef]
- Schuemann, J.; Hertweck, C. Advances in cloning, functional analysis and heterologous expression of fungal polyketide synthase genes. J. Biotechnol. 2006, 124, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, L.; Davis, C.R.; Roach, C.; Aldrich, T.; McAda, P.C.; Reeves, C.D. Lavastatin biosynthesis in Aspergillus terreus: Characterization of blocked mutants, enzyme activities and a multifunctional polyketide synthase gene. Chem. Biol. 1999, 6, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Keatinge-Clay, A. Crystal Structure of the Erythromycin Polyketide Synthase Dehydratase. J. Mol. Biol. 2008, 384, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.J.; Ames, B.D.; Tsai, S.-C.; Tang, Y. Engineered biosynthesis of a novel amidated polyketide, using the malonamyl-specific initiation module from the oxytetracycline polyketide synthase. Appl. Environ. Microbiol. 2006, 72, 2573–2580. [Google Scholar] [CrossRef] [Green Version]
- Chu, F.S. Mycotoxins: Food contamination, mechanism, carcinogenic potential and preventive measures. Mutat. Res. 1991, 259, 291–306. [Google Scholar] [CrossRef]
- Yang, G.; Rose, M.S.; Turgeon, B.G.; Yoder, O.C. A polyketide synthase is required for fungal virulence and production of the polyketide T toxin. Plant Cell 1996, 8, 2139–2150. [Google Scholar]
- Sweeney, M.J.; Dobson, A. Molecular biology of mycotoxin biosynthesis. FEMS Microbiol. Lett. 1999, 175, 149–163. [Google Scholar] [CrossRef]
- Proctor, R.H.; Desjardins, A.E.; Plattner, R.D.; Hohn, T.M. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi slating population A. Fungal Genet. Biol. 1999, 27, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, K.C.; Cony, P.J. An isolate of Aspergillus flavus used to reduce aflatoxin contamination in cottonseed has a defective polyketide synthase gene. Appl. Microbiol. Biotechnol. 2004, 65, 473–478. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, M.; Liu, Z.H.; Zhang, K.; Cui, F.H.; Sun, W.X. Towards understanding the biosynthetic pathway for ustilaginoidin mycotoxins in Ustilaginoidea virens. Environ. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.L.; Ye, J.; Strohl, W.R. Analysis of clustered genes encoding both early and late steps in daunomycin biosynthesis by Streptomyces sp. strain C5. J. Bacteriol. 1995, 177, 536–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallio, P.; Sultana, A.; Niemi, J.; Mäntsälä, P.; Schneider, G. Crystal structure of the polyketide cyclase AknH with bound substrate and product analogue: Implications for catalytic mechanism and product stereoselectivity. J. Mol. Biol. 2006, 357, 210–220. [Google Scholar] [CrossRef]
- Sultana, A.; Kallio, P.; Jansson, A.; Wang, J.S.; Niemi, J.; Mantsala, P.; Schneider, G. Structure of the polyketide cyclase SnoaL reveals a novel mechanism for enzymatic aldol condensation. EMBO J. 2004, 23, 1911–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, M.; Gallo, A.; Perrone, G.; Magistà, D.; Baker, S.E. Comparative genomic analysis of ochratoxin A biosynthetic cluster in producing fungi: New evidence of a cyclase gene involvement. Front. Microbiol. 2020, 11, 581309. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.T.; Chen, X.Y.; Yan, Y.Q.; Huang, J.B.; Luo, C.X.; Hsiang, T.; Zheng, L. Comprehensive transcriptome profiling reveals abundant long non-coding RNAs associated with development of the rice false smut fungus, Ustilaginoidea virens. Environ. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sun, W.; Meng, J.; Wang, A.; Wang, X.; Tian, J.; Fu, X.; Dai, J.; Liu, Y.; Lai, D.; et al. Bioactive bis-naphtho-λ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015, 63, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Zheng, L.; Liu, H.; Tang, J.T.; Hsiang, T.; Huang, J.B. Use of random T-DNA mutagenesis in identification of gene UvPRO1, a regulator of conidiation, stress response, and virulence in Ustilaginoidea virens. Front. Microbiol. 2016, 7, 2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Y.; Hai, D.; Tang, J.T.; Liu, H.; Huang, J.B.; Luo, C.X.; Hsiang, T.; Zheng, L. UvCom1 is an important regulator required for development and infection in the rice false smut fungus Ustilaginoidea virens. Phytopathology 2020, 110, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Yu, M.N.; Song, T.Q.; Cao, H.J.; Yong, M.L.; Qi, Z.Q.; Du, Y.; Zhang, R.S.; Yin, X.L.; Liu, Y.F. A homeobox transcription factor UvHOX2 regulates chlamydospore formation, conidiogenesis and pathogenicity in Ustilaginoidea virens. Front. Microbiol. 2019, 10, 1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.T.; Ding, H.; Huang, L.; Wang, Y.H.; Yu, M.N.; Zheng, R.; Yu, J.J.; Liu, Y.F. Low-affinity iron transport protein Uvt3277 is important for pathogenesis in the rice false smut fungus Ustilaginoidea virens. Curr. Genet. 2017, 63, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.L.; Wang, Y.F.; Wei, W.; Li, C.Y.; Liu, Y.; Qu, J.S.; Meng, Q.H.; Lin, Y.; Yin, W.X.; Yang, Y.N.; et al. The Bax inhibitor UvBI-1, a negative regulator of mycelial growth and conidiation, mediates stress response and is critical for pathogenicity of the rice false smut fungus Ustilaginoidea Virens. Curr. Genet. 2019, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Gao, Y.X.; Yu, Z.M.; Xiao, Y.H.; Zhang, Z.G.; Zhang, H.F. The adenylate cyclase UvAc1 and phosphodiesterase UvPdeH control the intracellular cAMP level, development, and pathogenicity of the rice false smut fungus Ustilaginoidea virens. Fungal Genet. Biol. 2019, 129, 65–73. [Google Scholar] [CrossRef]
- Tang, J.T.; Bai, J.; Chen, X.Y.; Zheng, L.; Liu, H.; Huang, J.B. Two protein kinases UvPmk1 and UvCDC2 with significant functions in conidiation, stress response and pathogenicity of rice false smut fungus Ustilaginoidea virens. Curr. Genet. 2020, 66, 409–420. [Google Scholar] [CrossRef]
- Meng, S.; Xiong, M.; Jagernath, J.S.; Wang, C.; Qiu, J.; Shi, H.; Kou, Y. UvAtg8-Mediated Autophagy Regulates Fungal Growth, Stress Responses, Conidiation, and Pathogenesis in Ustilaginoidea virens. Rice 2020, 13, 56. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, X.B.; Li, P.P.; Chen, X.L.; Liu, H.; Huang, J.B.; Luo, C.X.; Hsiang, T.; Zheng, L. Comprehensive identification of lysine 2-hydroxyisobutyrylated proteins in Ustilaginoidea virens reveals the involvement of lysine 2-hydroxyisobutyrylation in fungal virulence. J. Integr. Plant Biol. 2021, 63, 409–425. [Google Scholar] [CrossRef]
- Machado, A.K.; Brown, N.A.; Urban, M.; Kanyuka, K.; Hammond-Kosack, K.E. RNAi as an emerging approach to control Fusarium head blight disease and mycotoxin contamination in cereals. Pest Manag. Sci. 2018, 74, 790–799. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.H.; Wu, L.; Mei, Y.Z.; Zhao, Y.F.; Ma, Z.H.; Zhang, X.; Chen, Y. Host-induced gene silencing of multiple genes of Fusarium graminearum enhances resistance to Fusarium head blight in wheat. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef]
- Dou, T.; Shao, X.; Hu, C.; Liu, S.; Sheng, O.; Bi, F.; Gao, H.; He, W.; Peng, X.; Zhang, S.; et al. Host-induced gene silencing of Foc TR 4 ERG 6/11 genes exhibits superior resistance to Fusarium wilt of banana. Plant Biotechnol. J. 2020, 18, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Qi, T.; Yang, Q.; He, F.; Tan, C.; Ma, W.; Voegele, R.T.; Kang, Z.; Guo, J. Host-induced gene silencing of the MAPKK gene PsFUZ7 confers stable resistance to wheat stripe rust. Plant Physiol. 2017, 175, 1853–1863. [Google Scholar] [CrossRef] [Green Version]
- Panwar, V.; McCallum, B.; Bakkeren, G. Host-induced gene silencing of wheat leaf rust fungus Puccinia triticina pathogenicity genes mediated by the Barley stripe mosaic virus. Plant Mol. Biol. 2013, 81, 595–608. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.Y.; Li, Y.Q.; Zeng, J.G.; Wang, G.L.; Deng, C.Y.; Guo, W.Z. Host-induced gene silencing of a regulator of G protein signaling gene (VdRGS1) confers resistance to Verticillium wilt in cotton. Plant Biotechnol. J. 2018, 16, 1629–1643. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Zhu, X.; Tan, C.; Liu, P.; Guo, J.; Kang, Z.; Guo, J. Host-induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnol. J. 2018, 16, 797–807. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Lv, B.; Guo, M.Y.; Luo, C.X.; Zheng, L.; Hsiang, T.; Huang, J.B. Effect of rice growth stage, temperature, relative humidity and wetness duration on infection of rice panicles by Villosiclava virens. Eur. J. Plant Pathol. 2015, 141, 15–25. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring, NY, USA, 1989. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Pei, Z.; Li, P.; Li, X.; Duan, Y.; Liu, H.; Chen, X.; Zheng, L.; Luo, C.; Huang, J. Quantitative Proteomics Analysis Reveals the Function of the Putative Ester Cyclase UvEC1 in the Pathogenicity of the Rice False Smut Fungus Ustilaginoidea virens. Int. J. Mol. Sci. 2021, 22, 4069. https://doi.org/10.3390/ijms22084069
Chen X, Pei Z, Li P, Li X, Duan Y, Liu H, Chen X, Zheng L, Luo C, Huang J. Quantitative Proteomics Analysis Reveals the Function of the Putative Ester Cyclase UvEC1 in the Pathogenicity of the Rice False Smut Fungus Ustilaginoidea virens. International Journal of Molecular Sciences. 2021; 22(8):4069. https://doi.org/10.3390/ijms22084069
Chicago/Turabian StyleChen, Xiaoyang, Zhangxin Pei, Pingping Li, Xiabing Li, Yuhang Duan, Hao Liu, Xiaolin Chen, Lu Zheng, Chaoxi Luo, and Junbin Huang. 2021. "Quantitative Proteomics Analysis Reveals the Function of the Putative Ester Cyclase UvEC1 in the Pathogenicity of the Rice False Smut Fungus Ustilaginoidea virens" International Journal of Molecular Sciences 22, no. 8: 4069. https://doi.org/10.3390/ijms22084069
APA StyleChen, X., Pei, Z., Li, P., Li, X., Duan, Y., Liu, H., Chen, X., Zheng, L., Luo, C., & Huang, J. (2021). Quantitative Proteomics Analysis Reveals the Function of the Putative Ester Cyclase UvEC1 in the Pathogenicity of the Rice False Smut Fungus Ustilaginoidea virens. International Journal of Molecular Sciences, 22(8), 4069. https://doi.org/10.3390/ijms22084069






