Diverse Roles of Mitochondria in Renal Injury from Environmental Toxicants and Therapeutic Drugs
Abstract
1. Introduction
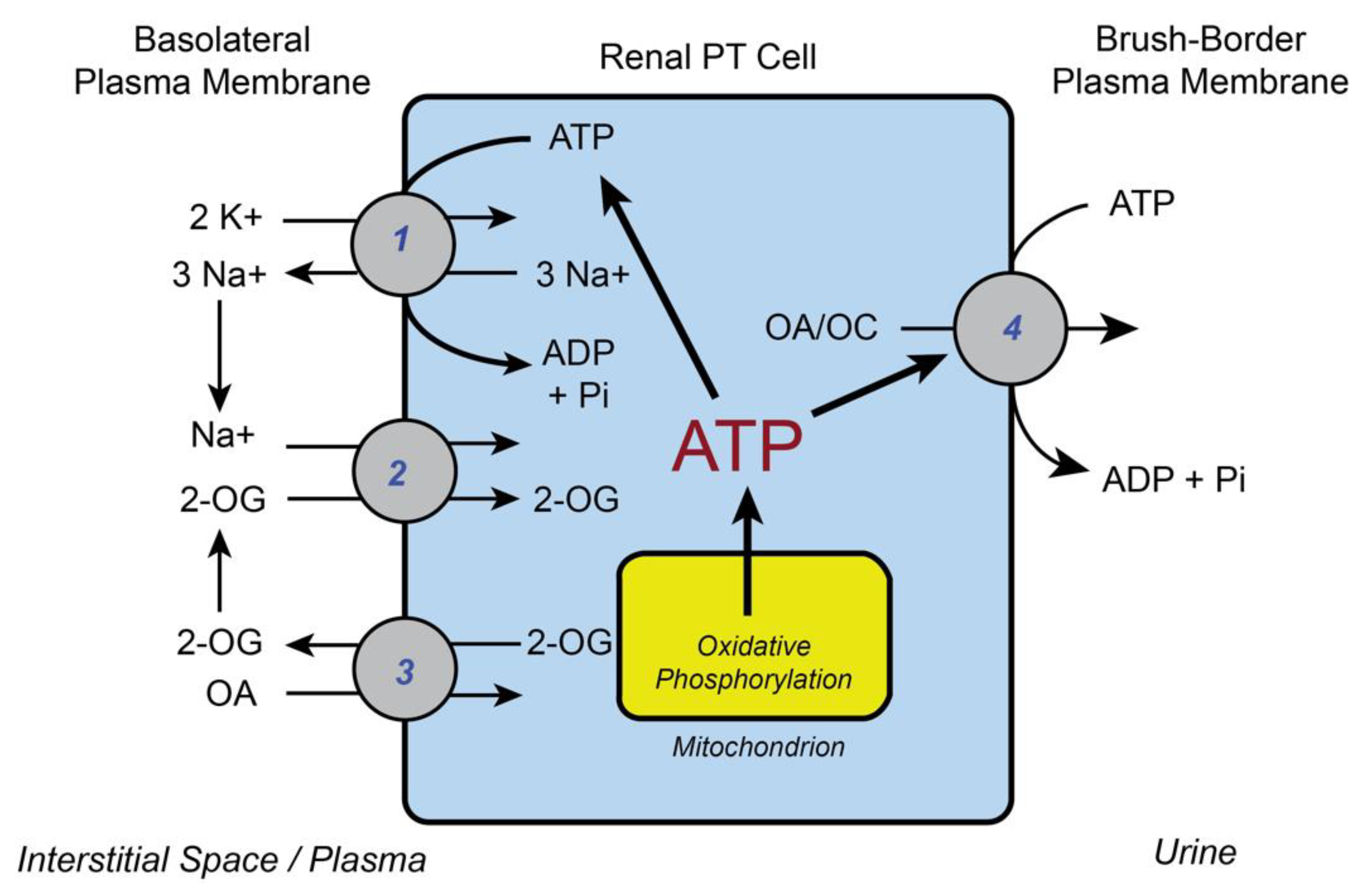
2. Basic Functions of Renal Mitochondria in Supplying ATP for Cellular Work
3. Renal Mitochondria as Redox Sensors
3.1. Susceptibility to Oxidative Stress
3.2. The Glutathione System as Both Protective Antioxidant and Activator for Nephrotoxicity
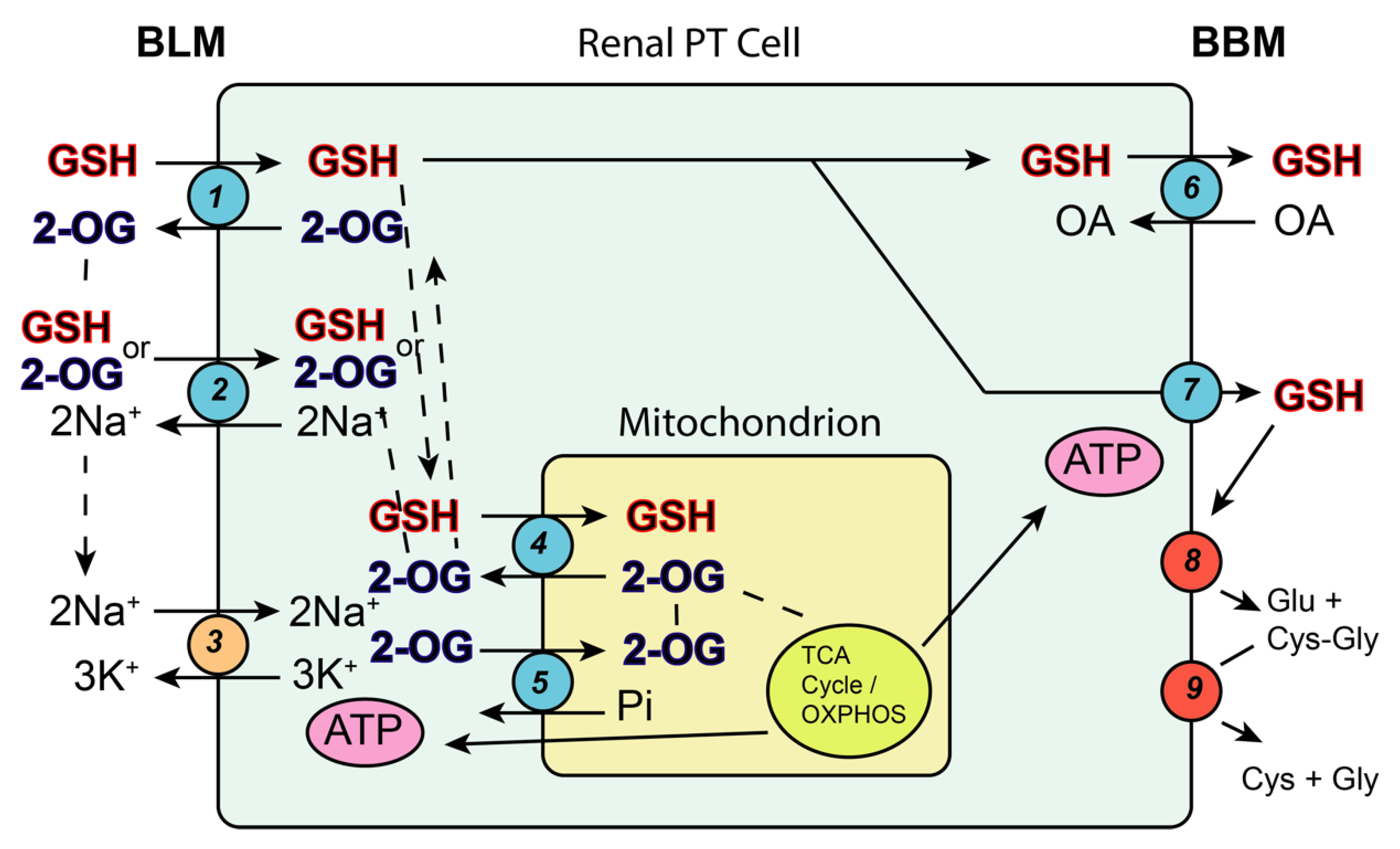
3.2.1. Mitochondrial Glutathione as a Protective Antioxidant
3.2.2. Glutathione-Dependent Bioactivation in Renal Mitochondria
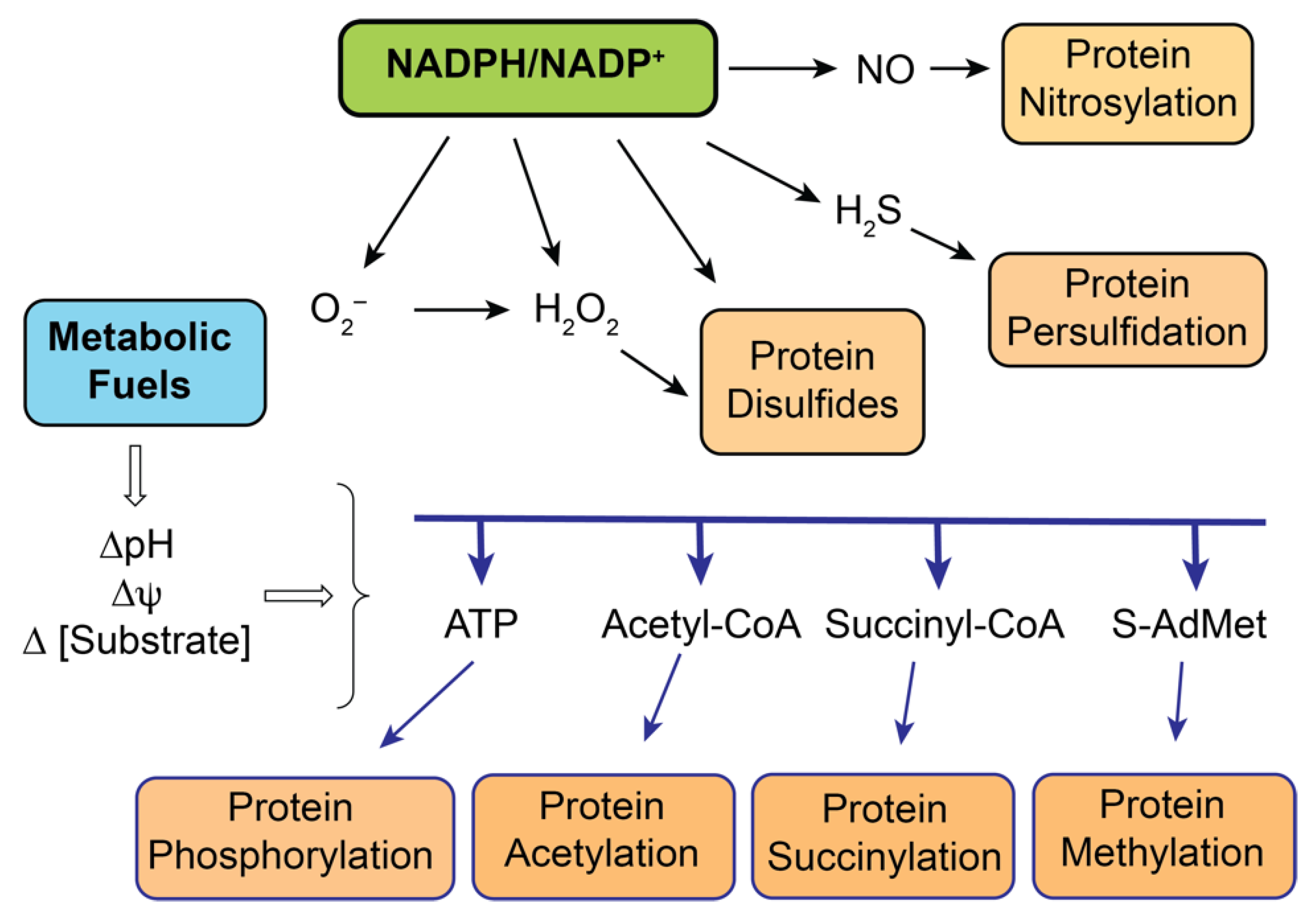
3.3. Other Redox Sensors in Renal Mitochondria
4. Renal Mitochondria in the Sequelae of Chemically-Induced Nephrotoxicity
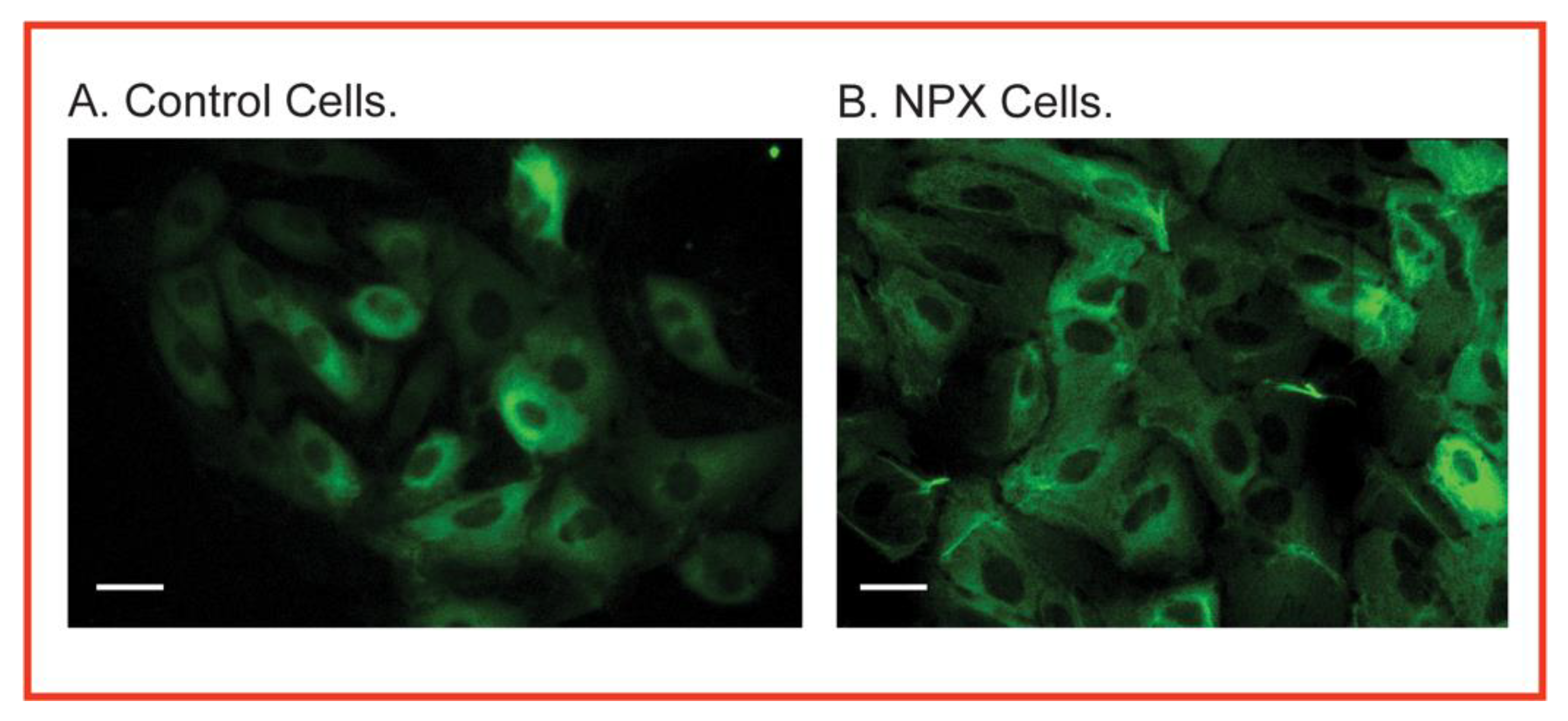
5. Renal Mitochondria as a Source of Biomarkers for Chemical Exposure
6. Summary and Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| S-AdMet | S-Adenosylmethionine |
| BBM | Brush-border membrane |
| BLM | Basolateral membrane |
| BUN | Blood urea nitrogen |
| CCBL | Cysteine conjugate beta-lyase |
| CKD | Chronic kidney disease |
| CRH | Compensatory renal hypertrophy |
| DIC | Dicarboxylate carrier |
| DCVC | S-(1,2-Dichlorovinyl)-L-cysteine |
| DP | Dipeptidase |
| ESRD | End-stage renal disease |
| FDA | Food and Drug Administration |
| FeNa | Fractional sodium excretion |
| GFR | Glomerular filtration rate |
| GGT | -Glutamyltransferase |
| GSH | Reduced glutathione |
| GSSG | Glutathione disulfide |
| hPT | Human proximal tubular |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| KIM-1 | Kidney Injury Molecule-1 |
| LC–MS/MS | Liquid chromatography—mass spectrometry/mass spectrometry |
| mtGSH | Mitochondrial GSH |
| Mrp | Multidrug resistance-associated protein |
| NAG | N-acetyl-β-D-glucosaminidase |
| NO | Nitric oxide |
| NPX | Uninephrectomy |
| NRK-52E | Normal Rat Kidney-52 Epithelial cell line |
| OA | Organic anion |
| OC | Organic cation |
| 2-OG | 2-Oxoglutarate |
| OGC | 2-Oxoglutarate carrier |
| OXPHOS | Oxidative phosphorylation |
| Pi | Inorganic phosphate |
| PT | Proximal tubular |
| QO2 | Oxygen consumption (respiration) rate |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SCr | Serum creatinine |
| tBH | tert-Butyl hydroperoxide |
| TCA | Tricarboxylic acid |
| Trx | Thioredoxin |
| TrxR | Thioredoxin reductase |
| WT | Wild type |
References
- Kiil, F. Renal energy metabolism and regulation of sodium reabsorption. Kidney Int. 1977, 11, 153–160. [Google Scholar] [CrossRef]
- Balaban, R.S.; Mandel, L.J.; Soltoff, S.P.; Storey, J.M. Coupling of active ion transport and aerobic respiratory rat in isolated renal tubules. Proc. Natl. Acad. Sci. USA 1980, 77, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.I.; Balaban, R.S.; Barrett, L.; Mandel, L.J. Mitochondrial respiratory capacity and Na+ - and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J. Biol. Chem. 1981, 256, 10319–10328. [Google Scholar] [CrossRef]
- Harris, S.I.; Patton, L.; Barrett, L.; Mandel, L.J. (Na+,K+)-ATPase kinetics within the intact renal cell: The role of oxidative metabolism. J. Biol. Chem. 1982, 257, 6996–7002. [Google Scholar] [CrossRef]
- Tessitore, N.; Sakhrani, L.M.; Massry, S.G. Quantitative requirement for ATP for active transport in isolated renal cells. Am. J. Physiol. Physiol. 1986, 251, C120–C127. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.P. Mitochondrial transport functions and renal metabolism. Kidney Int. 1983, 23, 785–793. [Google Scholar] [CrossRef]
- Brazy, P.C.; Mandel, L.J.; Gullans, S.R.; Soltoff, S.P. Interactions between phosphate and oxidative metabolism in proximal renal tubules. Am. J. Physiol. Content 1984, 247, F575–F581. [Google Scholar] [CrossRef]
- Soltoff, S. ATP and the Regulation of Renal Cell Function. Annu. Rev. Physiol. 1986, 48, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Fine, L. The biology of renal hypertrophy. Kidney Int. 1986, 29, 619–634. [Google Scholar] [CrossRef]
- Shirley, D.G.; Walter, S.J. Acute and chronic changes in renal function following unilateral nephrectomy. Kidney Int. 1991, 40, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Fine, L.G.; Badie-Dezfooly, B.; Lowe, A.G.; Hamzeh, A.; Wells, J.; Salehmoghaddam, S. Stimulation of Na/H antiport is an early event in hypertrophy of renal proximal tubular cells. Proc. Natl. Acad. Sci. USA. 1985, 82, 1736–1740. [Google Scholar] [CrossRef]
- Johnson, H.A.; Amendola, F. Mitochondrial proliferation in compensatory growth of the kidney. Am. J. Pathol. 1969, 54, 35–45. [Google Scholar] [PubMed]
- Schmidt, U.; Dubach, U.C. Induction of NaK ATPase in the proximal and distal convolution of the rat nephron after uninephrectomy. Pflug. Arch. 1974, 346, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.P.; Rodriguez, H.J.; Hogan, W.C.; Klahr, S. Mechanism of activation of renal Na+-K+-ATPase in the rat: Effects of reduction of renal mass. Am. J. Physiol. 1980, 239, F281–F288. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C.H.; Chan, L.; Schrier, R.W. Remnant kidney hypermetabolism and progression of chronic renal failure. Am. J. Physiol. 1988, 254, F267–F276. [Google Scholar] [CrossRef]
- Nath, K.A.; Croatt, A.J.; Hostetter, T.H. Oxygen consumption and oxidant stress in surviving nephrons. Am. J. Physiol. 1990, 258, F1354–F1362. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Lash, L.H. Effects of uninephrectomy and mercuric chloride on renal glutathione homeostasis. J. Pharm. Exp. 1990, 254, 962–970. [Google Scholar]
- Lash, L.H.; Zalups, R.K. Mercuric chloride-induced cytotoxicity and compensatory hypertrophy in rat kidney proximal tubular cells. J. Pharm. Exp. 1992, 261, 819–829. [Google Scholar]
- Lash, L.H.; Zalups, R.K. Activities of enzymes involved in renal cellular glutathione metabolism after uninephrectomy in the rat. Arch. Biochem. Biophys. 1994, 309, 129–138. [Google Scholar] [CrossRef]
- Lash, L.H.; Putt, D.A.; Zalups, R.K. Influence of exogenous thiols on mercury-induced cellular injury in isolated renal proximal tubular and distal tubular cells from normal and uninephrectomized rats. J. Pharm. Exp. 1999, 291, 492–502. [Google Scholar]
- Lash, L.H.; Putt, D.A.; Zalups, R.K. Biochemical and functional characteristics of cultured renal epithelial cells from uninephrectomized rats: Factors influencing nephrotoxicity. J. Pharm. Exp. 2001, 296, 243–251. [Google Scholar]
- Lash, L.H.; Putt, D.A.; Horky III, S.J.; Zalups, R.K. Functional and toxicological characteristics of isolated renal mitochondria: Impact of compensatory renal growth. Biochem. Pharm. 2001, 62, 383–395. [Google Scholar] [CrossRef]
- Lash, L.H.; Hueni, S.E.; Putt, D.A.; Zalups, R.K. Role of organic anion and amino acid carriers in transport of inorganic mercury in rat renal basolateral membrane vesicles: Influence of compensatory renal growth. Toxicol. Sci. 2005, 88, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H.; Putt, D.A.; Zalups, R.K. Influence of compensatory renal growth on susceptibility of primary cultures of renal cells to chemically induced injury. Toxicol. Sci. 2006, 94, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Benipal, B.; Lash, L.H. Influence of renal compensatory hypertrophy on mitochondrial energetics and redox status. Biochem. Pharm. 2011, 81, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Benipal, B.; Lash, L.H. Modulation of mitochondrial glutathione status and cellular energetics in primary cultures of proximal tubular cells from remnant kidney of uninephrectomized rats. Biochem. Pharm. 2013, 85, 1379–1388. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The redox code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.-M.; Fernandes, J.; Hu, X.; Uppal, K.; Jones, D.P. Mitochondrial network responses in oxidative physiology and disease. Free Radic. Biol. Med. 2018, 116, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Roede, J.R.; Go, Y.-M.; Jones, D.P. Redox equivalents and mitochondrial bioenergetics. Methods Mol. Biol. 2018, 1782, 197–227. [Google Scholar] [PubMed]
- Dennis, K.K.; Go, Y.-M.; Jones, D.P. Redox systems biology of nutrition and oxidative stress. J. Nutr. 2019, 194, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Cai, J.; Jones, D.P. Mitochondrial redox signaling during apoptosis. J. Bioenerg. Biomembr. 1999, 31, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Role of glutathione transport processes in kidney function. Toxicol. Appl. Pharm. 2005, 204, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006, 163, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Mitochondrial glutathione in toxicology and disease of the kidneys. Toxicol. Res. 2012, 1, 39–46. [Google Scholar] [CrossRef]
- McKernan, T.B.; Woods, E.B.; Lash, L.H. Uptake of glutathione by renal cortical mitochondria. Arch. Biochem. Biophys. 1991, 288, 653–663. [Google Scholar] [CrossRef]
- Schnellmann, R.G.; Mandel, L.J. Intracellular compartmentation of glutathione in rabbit renal proximal tubules. Biochem. Biophys. Res. Commun. 1985, 133, 1001–1005. [Google Scholar] [CrossRef]
- Schoolwerth, A.C.; LaNoue, K.F. Transport of metabolic substrates in renal mitochondria. Annu. Rev. Physiol. 1985, 47, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lash, L.H. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharm. Exp. 1998, 285, 608–618. [Google Scholar]
- Chen, Z.; Putt, D.A.; Lash, L.H. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: Further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch. Biochem. Biophys. 2000, 373, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Coll, O.; Garcia-Ruiz, C.; Kaplowitz, N.; Fernandez-Checa, J.C. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology 2003, 38, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Putt, D.A.; Xu, F.; Lash, L.H. Hepatic mitochondrial transport of glutathione: Studies in isolated rat liver mitochondria and H4IIE rat hepatoma cells. Arch. Biochem. Biophys. 2008, 474, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Guidot, D.M.; Brown, L.A.S. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin. Exp. Res. 2000, 24, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- O′Donovan, D.J.; Fernandes, C.J. Mitochondrial glutathione and oxidative stress: Implications for pulmonary oxygen toxicity in premature infants. Mol. Genet. Metab. 2000, 71, 352–358. [Google Scholar] [CrossRef]
- Ghosh, S.; Pulinilkunnil, T.; Yuen, G.; Kewalramani, G.; An, D.; Qi, D.; Abrahani, A.; Rodrigues, B. Cardiomycyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am. J. Physiol. 2005, 289, H768–H776. [Google Scholar]
- Circu, M.L.; Rodriguez, C.; Maloney, R.; Moyer, M.P.; Aw, T.Y. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic. Biol. Med. 2008, 44, 768–778. [Google Scholar] [CrossRef]
- Circu, M.L.; Moyer, M.P.; Harrison, L.; Aw, T.Y. Contribution of glutathione status to oxidant-induced mitochondrial DNA damage in colonic epithelial cells. Free Radic. Biol. Med. 2009, 47, 1190–1198. [Google Scholar] [CrossRef]
- Kamga, C.K.; Zhang, S.X.; Wang, Y. Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. Am. J. Physiol. 2010, 299, C497–C505. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Marquardt, K.; Lash, L.H.; Linseman, D.A. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic. Biol. Med. 2012, 52, 410–419. [Google Scholar] [CrossRef]
- Lash, L.H.; Putt, D.A.; Xu, F.; Matherly, L.H. Role of rat organic anion transporter 3 (Oat3) in the renal basolateral transport of glutathione. Chem. Biol. Interact. 2007, 170, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H.; Putt, D.A.; Hueni, S.E.; Cao, W.; Xu, F.; Kulidjian, S.J.; Horwitz, J.P. Cellular energetics and glutathione status in NRK-52E cells: Toxicological implications. Biochem. Pharm. 2002, 64, 1533–1546. [Google Scholar] [CrossRef]
- Lash, L.H.; Putt, D.A.; Matherly, L.H. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical induced apoptosis by overexpression of a mitochondrial glutathione transporter. J. Pharm. Exp. 2002, 303, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Putt, D.A.; Matherly, L.H.; Lash, L.H. Modulation of expression of rat mitochondrial 2-oxoglutarate carrier in NRK-52E cells alters mitochondrial transport and accumulation of glutathione and susceptibility to chemically induced apoptosis. J. Pharm. Exp. 2006, 316, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Renal glutathione transport systems: Roles in redox homeostasis, cytoprotection, and bioactivation. In Glutathione; Flohé, L., Ed.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 2; pp. 35–52. [Google Scholar]
- Lash, L.H. Glutathione-dependent bioactivation. In Current Protocols in Toxicology; Reed, D.J., Ed.; Unit 6.12; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Lash, L.H.; Chiu, W.A.; Guyton, K.Z.; Rusyn, I.I. Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target organ toxicity. Mutat. Res. Rev. 2014, 762, 22–36. [Google Scholar] [CrossRef]
- Parker, V.H. A biochemical study of the toxicity of S-dichlorovinyl-L-cysteine. Food Cosmet. Toxicol. 1965, 3, 75–84. [Google Scholar] [CrossRef]
- Stonard, M.D.; Parker, V.H. 2-Oxoacid dehydrogenases of rat liver mitochondria as the site of action of S-(1,2-dichlorovinyl)-L-cysteine and S-(1,2-dichlorovinyl)-3-mercaptopropionic acid. Biochem. Pharm. 1971, 20, 2417–2427. [Google Scholar] [CrossRef]
- Stonard, M.D.; Parker, V.H. The metabolism of S-(1,2-dichlorovinyl)-L-cysteine by rat liver mitochondria. Biochem. Pharm. 1971, 20, 2429–2437. [Google Scholar] [CrossRef]
- Lash, L.H.; Jones, D.P.; Anders, M.W. Glutathione homeostasis and glutathione S-conjugate toxicity in kidney. Rev. Biochem. Toxicol. 1988, 9, 29–67. [Google Scholar]
- Lash, L.H.; Anders, M.W. Uptake of nephrotoxic S-conjugates by isolated rat renal proximal tubular cells. J. Pharm. Exp. 1989, 248, 531–537. [Google Scholar]
- Lash, L.H.; Elfarra, A.A.; Anders, M.W. Renal cysteine conjugate b-lyase: Bioactivation of nephrotoxic cysteine S-conjugates in mitochondrial outer membrane. J. Biol. Chem. 1986, 261, 5930–5935. [Google Scholar] [CrossRef]
- Stevens, J.L.; Ayoubi, N.; Robbins, J.D. The role of mitochondrial matrix enzymes in the metabolism and toxicity of cysteine conjugates. J. Biol. Chem. 1988, 263, 3395–3401. [Google Scholar] [CrossRef]
- Banki, K.; Elfarra, A.A.; Lash, L.H.; Anders, M.W. Metabolism of S-(2-chloro-1,1,2-trifluoroethyl)-L-cysteine to hydrogen sulfide and the role of hydrogen sulfide in S-(2-chloro-1,1,2-trifluoroethyl)-L-cysteine-induced mitochondrial toxicity. Biochem. Biophys. Res. Commun. 1986, 138, 707–713. [Google Scholar] [CrossRef]
- Lash, L.H.; Anders, M.W. Mechanism of S-(1,2-dichlorovinyl)-L-cysteine- and S-(1,2-dichlorovinyl)-L-homocysteine-induced renal mitochondrial toxicity. Mol. Pharm. 1987, 32, 549–556. [Google Scholar]
- Hayden, P.J.; Stevens, J.L. Cysteine conjugate toxicity, metabolism, and binding to macromolecules in isolated rat kidney mitochondria. Mol. Pharm. 1990, 37, 468–476. [Google Scholar]
- Hayden, P.J.; Ichimura, T.; McCann, D.J.; Pohl, L.R.; Stevens, J.L. Detection of cysteine conjugate metabolite adduct formation with specific mitochondrial proteins using antibodies raised against halothane metabolite adducts. J. Biol. Chem. 1991, 266, 18415–18418. [Google Scholar] [CrossRef]
- Van de Water, B.; Zietbey, J.P.; de Bront, H.J.G.M.; Mulder, G.J.; Nagelkerke, J.F. The relationship between intracellular Ca2+ and the mitochondrial membrane potential in isolated proximal tubular cells from rat kidney exposed to the nephrotoxin 1,2-dichlorovinyl-cysteine. Biochem. Pharm. 1993, 45, 2259–2267. [Google Scholar] [CrossRef]
- Van de Water, B.; Zoeteweij, J.P.; de Bont, H.J.G.M.; Mulder, G.J.; Nagelkerke, J.F. Role of mitochondrial Ca2+ in the oxidative stress-induced dissipation of the mitochondrial membrane potential: Studies in isolated proximal tubular cells using the nephrotoxin 1,2-dichlorovinyl-L-cysteine. J. Biol. Chem. 1994, 269, 14546–14552. [Google Scholar] [CrossRef]
- Lash, L.H.; Xu, Y.; Elfarra, A.A.; Duescher, R.J.; Parker, J.C. Glutathione-dependent metabolism of trichloroethylene in isolated liver and kidney cells of rats and its role in mitochondrial and cellular toxicity. Drug Metab. Dispos. 1995, 23, 846–853. [Google Scholar] [PubMed]
- Van de Water, B.; Zoeteweij, J.P.; de Bont, H.J.G.M.; Nagelkerke, J.F. Inhibition of succinate:ubiquinone reductase and decrease of ubiquinol in nephrotoxic cysteine S-conjugate-induced oxidative cell injury. Mol. Pharm. 1995, 48, 928–937. [Google Scholar]
- Chen, Y.; Cai, J.; Anders, M.W.; Stevens, J.L.; Jones, D.P. Role of mitochondrial dysfunction in S-(1,2-dichlorovinyl)-L-cysteine-induced apoptosis. Toxicol. Appl. Pharm. 2001, 170, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H.; Putt, D.A.; Hueni, S.E.; Krause, R.J.; Elfarra, A.A. Roles of necrosis, apoptosis, and mitochondrial dysfunction in S-(1,2-dichlorovinyl)-L-cysteine sulfoxide-induced cytotoxicity in primary cultures of human renal proximal tubular cells. J. Pharm. Exp. 2003, 305, 1163–1172. [Google Scholar] [CrossRef]
- Xu, F.; Papanayotou, I.; Putt, D.A.; Wang, J.; Lash, L.H. Role of mitochondrial dysfunction in cellular responses to S-(1,2-dichlorovinyl)-L-cysteine in primary cultures of human proximal tubular cells. Biochem. Pharm. 2008, 76, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Jones, T.W.; Vercesi, A.E.; Cotgreave, I.; Ormstad, K.; Orrenius, S. Toxicity of S-pentachlorobutadienyl-L-cysteine studied with isolated rat renal cortical mitochondria. Arch. Biochem. Biophys. 1987, 258, 365–372. [Google Scholar] [CrossRef]
- Schnellmann, R.G.; Cross, T.J.; Lock, E.A. Pentachlorobutadienyl-L-cysteine uncouples oxidative phosphorylation by dissipating the proton gradient. Toxicol. Appl. Pharm. 1989, 100, 498–505. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Bruschi, S.A.; Anders, M.W. Toxic, halogenated cysteine S-conjugates and targeting of mitochondrial enzymes of energy metabolism. Biochem. Pharm. 2002, 64, 553–564. [Google Scholar] [CrossRef]
- James, E.A.; Gygi, S.P.; Adams, M.L.; Pierce, R.H.; Fausto, N.; Aebersold, R.H.; Nelson, S.D.; Bruschi, S.A. Mitochondrial aconitase modification, functional inhibition, and evidence for a supramolecular complex of the TCA cycle by the renal toxicant S-(1,1,2,2-tetrafluoroethyl)-L-cysteine. Biochemistry 2002, 41, 6789–6797. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.H.; Yang, X.; Choi, Y.E.; Jones, D.P.; Kehrer, J.P. Thioredoxin and its role in toxicology. Toxicol. Sci. 2004, 78, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Go, Y.-M.; Jones, D.P. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch. Biochem. Biophys. 2007, 465, 119–126. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Cai, J.; Go, Y.-M.; Johnson, J.M.; Martin, W.D.; Hansen, J.M.; Jones, D.P. Identification of thioredoxin-2 as a regulator of the mitochondrial permeability transition. Toxicol. Sci. 2008, 105, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Go, Y.-M.; Jones, D.P. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu. Rev. Pharm. Toxicol. 2006, 46, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.; Go, Y.-M.; Jones, D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic. Biol. Med. 2008, 44, 921–937. [Google Scholar] [CrossRef]
- Go, Y.-M.; Jones, D.P. Thiol/disulfide redox states in signaling and sensing. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Brooks, C.; Liu, F.; Sun, L.; Dong, Z. Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013, 83, 568–581. [Google Scholar] [CrossRef]
- Sharma, K. Mitochondrial dysfunction in the diabetic kidney. Adv. Exp. Med. Biol. 2017, 982, 553–562. [Google Scholar]
- Duann, P.; Lin, P.-H. Mitochondrial damage and kidney disease. Adv. Exp. Med. Biol. 2017, 982, 529–552. [Google Scholar] [PubMed]
- Quadri, M.M.; Fatima, S.-S.; Che, R.-C.; Zhang, A.-H. Mitochondria and renal fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 501–524. [Google Scholar] [PubMed]
- Inoue, T.; Maekawa, H.; Inagi, R. Organelle crosstalk in the kidney. Kidney Int. 2019, 95, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Schnellmann, R.G.; Ewell, F.P.Q.; Sgambati, M.; Mandel, L.J. Mitochondrial toxicity of 2-bromohydroquinone in rabbit renal proximal tubules. Toxicol. Appl. Pharm. 1987, 90, 420–426. [Google Scholar] [CrossRef]
- Hill, B.A.; Monks, T.J.; Lau, S.S. The effects of 2,3,5-triglutathion-S-yl)hydroquinone on renal mitochondrial respiratory function in vivo and in vitro: Possible role in cytotoxicity. Toxicol. Appl. Pharm. 1992, 117, 165–171. [Google Scholar] [CrossRef]
- Tune, B.M.; Fravert, D.; Hsu, C.-Y. Oxidative and mitochondrial toxic effects of cephalosporin antibiotics in the kidney: A comparative study of cephaloridine and cephaloglycin. Biochem. Pharm. 1989, 38, 795–802. [Google Scholar] [CrossRef]
- Tune, B.M.; Hsu, C.-Y. The renal mitochondrial toxicity of cephalosporins: Specificity of the effect on anionic substrate uptake. J. Pharm. Exp. 1990, 252, 65–69. [Google Scholar]
- Mela-Riker, L.M.; Widener, L.L.; Houghton, D.C.; Bennett, W.M. Renal mitochondrial integrity during continuous gentamicin treatment. Biochem. Pharm. 1986, 35, 979–984. [Google Scholar] [CrossRef]
- Walker, P.D.; Shah, S.V. Gentamicin enhanced production of hydrogen peroxide by renal cortical mitochondria. Am. J. Physiol. 1987, 253, C495–C499. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Guidet, B.; Shah, S.V. Gentamicin-induced mobilization of iron from renal cortical mitochondria. Am. J. Physiol. 1993, 265, F435–F439. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.J.; Hosseini, S.H.; Hoying-Brandt, A.; Green, E.; Johnson, D.M.; Russ, R.; Tran, D.; Raper, C.M.; Santoianni, R.; Lewis, W. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab. Investig. 2009, 89, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, L.C.; Mohan, S.; Stokes, M.B.; Radhakrishnan, J.; D’Agati, V.D.; Markowitz, G.S. Tenofovir nephrotoxicity: Acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010, 78, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Tenofovir-induced kidney disease: An acquired renal tubular mitochondriopathy. Kidney Int. 2010, 78, 1060–1063. [Google Scholar] [CrossRef]
- Milián, L.; Peris, J.E.; Gandía, P.; Andújar, I.; Pallardó, L.; Górriz, J.L.; Blas-García, A. Tenofovir-induced toxicity in renal proximal tubular epithelial cells: Involvement of mitochondria. AIDS 2017, 31, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, H.; Abraham, P.; Isaac, B.; Selvakumar, D. Mitochondrial pathway of apoptosis and necrosis contribute to tenofovir disproxil fumarate-induced renal damage in rats. Hum. Exp. Toxicol. 2019, 38, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Brady, H.R.; Kone, B.C.; Stromski, M.E.; Zeidel, M.L.; Giebisch, G.; Gullans, S.R. Mitochondrial injury: An early event in cisplatin toxicity to renal proximal tubules. Am. J. Physiol. 1990, 258, F1181–F1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-G.; Lindup, W.E. Role of mitochondria in cisplatin-induced oxidative damage exhibited by rat renal cortical slices. Biochem. Pharm. 1993, 45, 2215–2222. [Google Scholar]
- Zhang, J.-G.; Lindup, W.E. Cisplatin nephrotoxicity: Decreases in mitochondrial protein sulfhydryl concentration and calcium uptake by mitochondria from rat renal cortical slices. Biochem. Pharm. 1994, 47, 1127–1135. [Google Scholar] [CrossRef]
- Kruidering, M.; van de Water, B.; de Heer, E.; Mulder, G.J.; Nagelkerke, J.F. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: Mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J. Pharm. Exp. 1997, 280, 638–649. [Google Scholar]
- Jiang, M.; Wang, C.; Huang, S.; Yang, T.; Dong, Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am. J. Physiol. 2009, 296, F983–F993. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-C.; Honeyman, T.W.; Cooney, R.; Kennington, L.; Scheid, C.R.; Jonassen, J.A. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int. 2004, 66, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.-O.; Miller, D.M.; Woods, J.S. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem. Pharm. 1993, 45, 2017–2024. [Google Scholar] [CrossRef]
- Scaduto, R.C., Jr.; Fattone, V.H., II.; Grotyohann, L.W.; Wertz, J.; Martin, L.F. Effect of an altered glutathione content on renal ischemic injury. Am. J. Physiol. 1988, 255, F911–F921. [Google Scholar] [CrossRef]
- Feldkamp, T.; Kribben, A.; Weinberg, J.M. Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am. J. Physiol. 2005, 288, F1092–F1102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feldkamp, T.; Kribben, A.; Weinberg, J.M. F1FO-ATPase activity and ATP dependence of mitochondrial energization in proximal tubules after hypoxia/reoxygenation. J. Am. Soc. Nephrol. 2005, 16, 1742–1751. [Google Scholar] [CrossRef]
- Feldkamp, T.; Park, J.S.; Pasupulati, R.; Amora, D.; Roeser, N.F.; Venkatachalam, M.A.; Weinberg, J.M. Regulation of the mitochondrial permeability transition in kidney proximal tubules and its alteration during hypoxia-reoxygenation. Am. J. Physiol. 2009, 297, F1632–F1646. [Google Scholar] [CrossRef] [PubMed]
- Szeto, H.H.; Liu, S.; Soong, Y.; Birk, A.V. Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am. J. Physiol. Ren. Physiol. 2015, 308, F11–F21. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020. [Google Scholar]
- National Research Council. Biologic Markers in Urinary Toxicology; The National Academies Press: Washington, DC, USA, 1995. [Google Scholar] [CrossRef]
- Siwy, J.; Mischak, H. Novel biomarkers in chronic kidney disease. In Kidney Update: Biomarkers for Kidney Disease; Science/AAAS: Washington, DC, USA, 2019; pp. 4–8. [Google Scholar]
- FDA Biomarkers. List of Qualified Biomarkers. 2020. Available online: https://www.fda.gov/drugs/cder-biomarker-qualification-program/list-qualified-biomarkers (accessed on 25 February 2021).


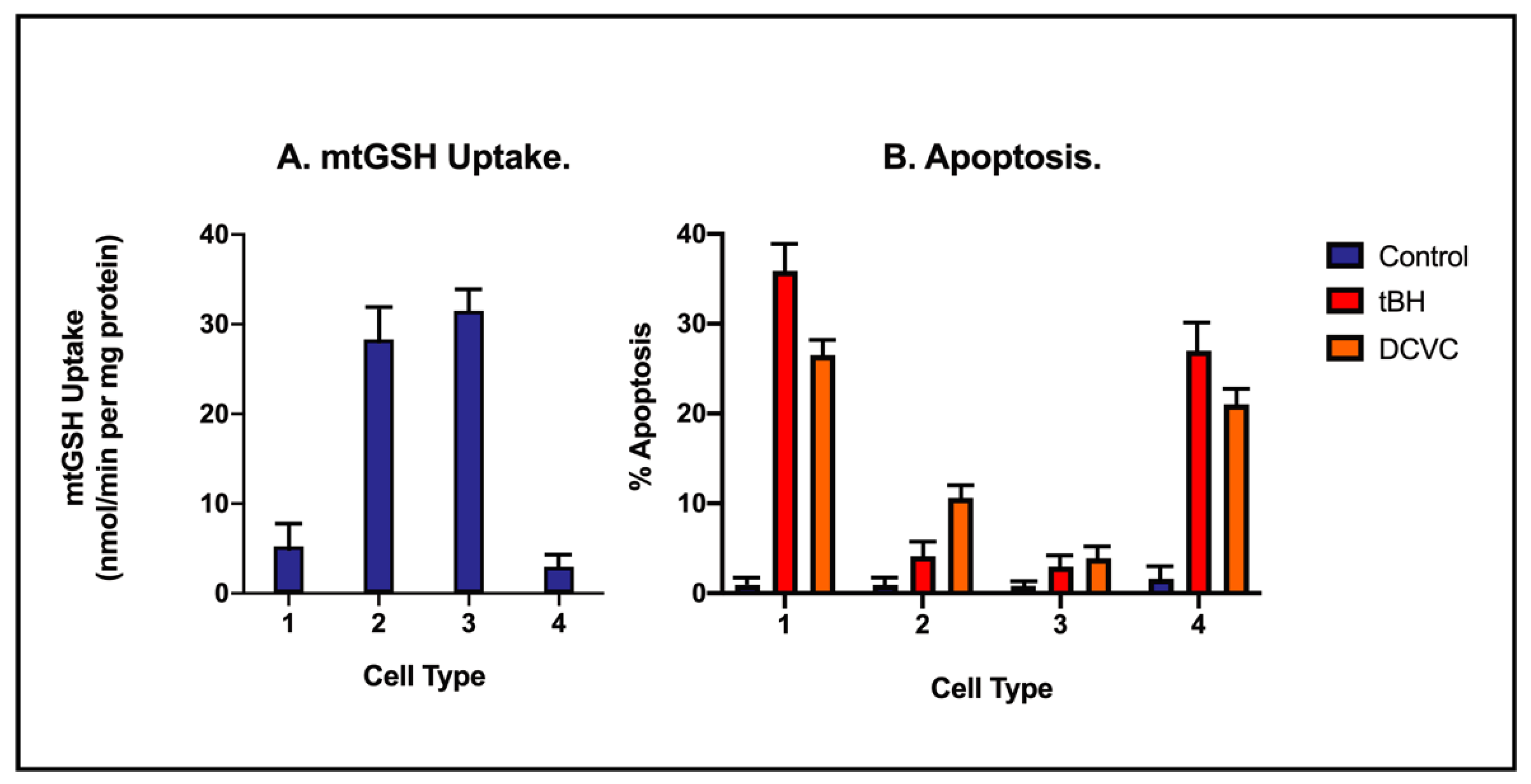
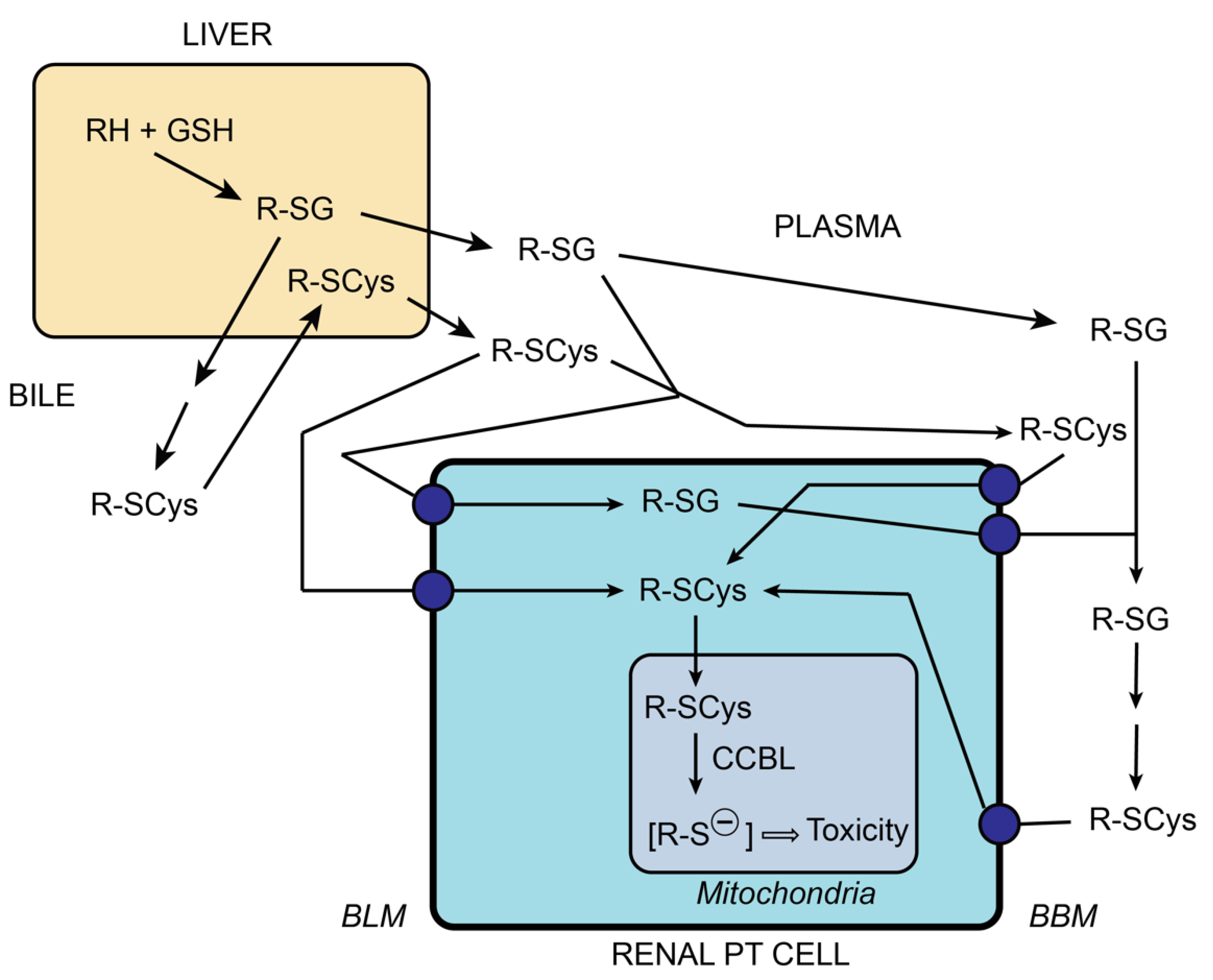
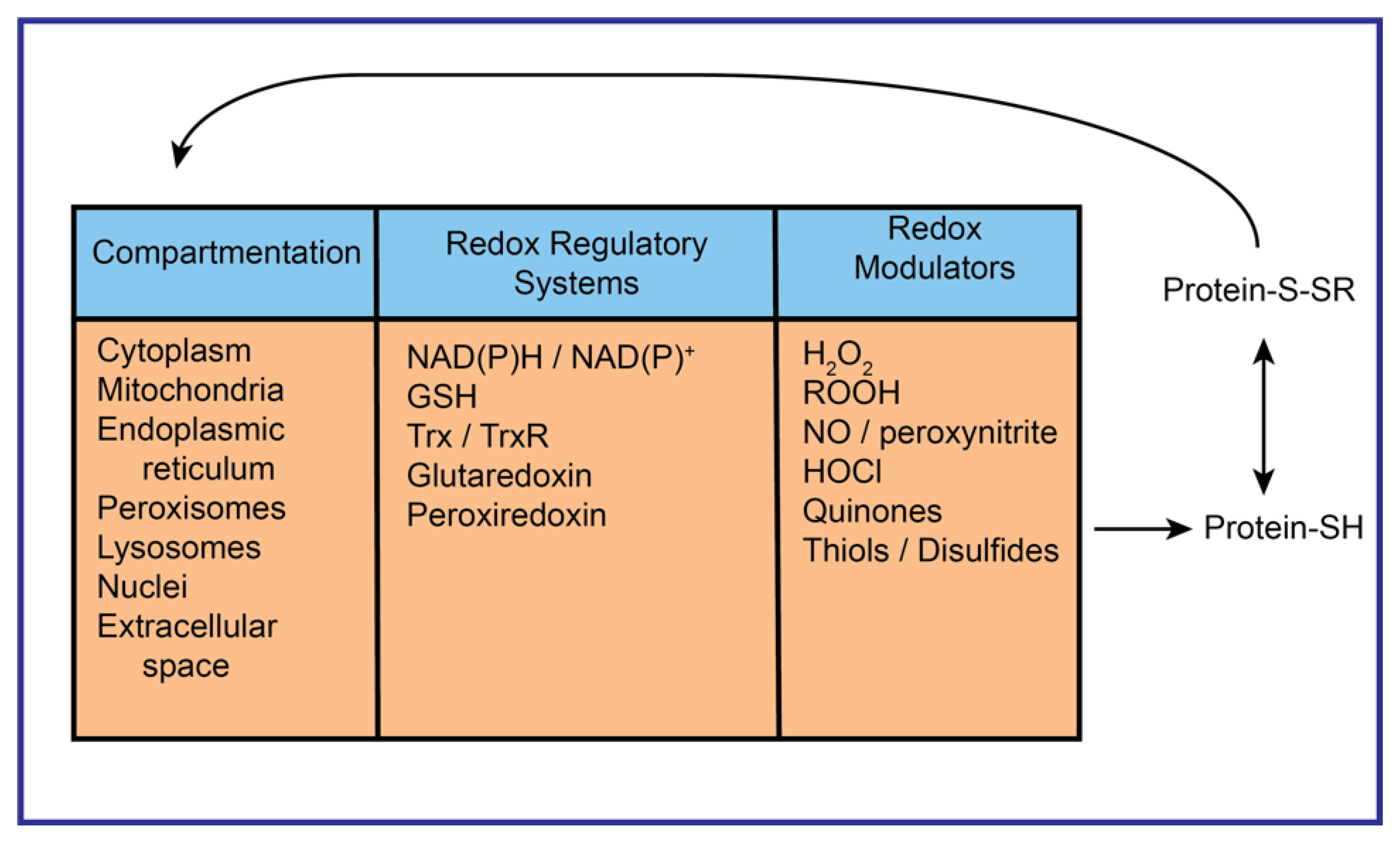
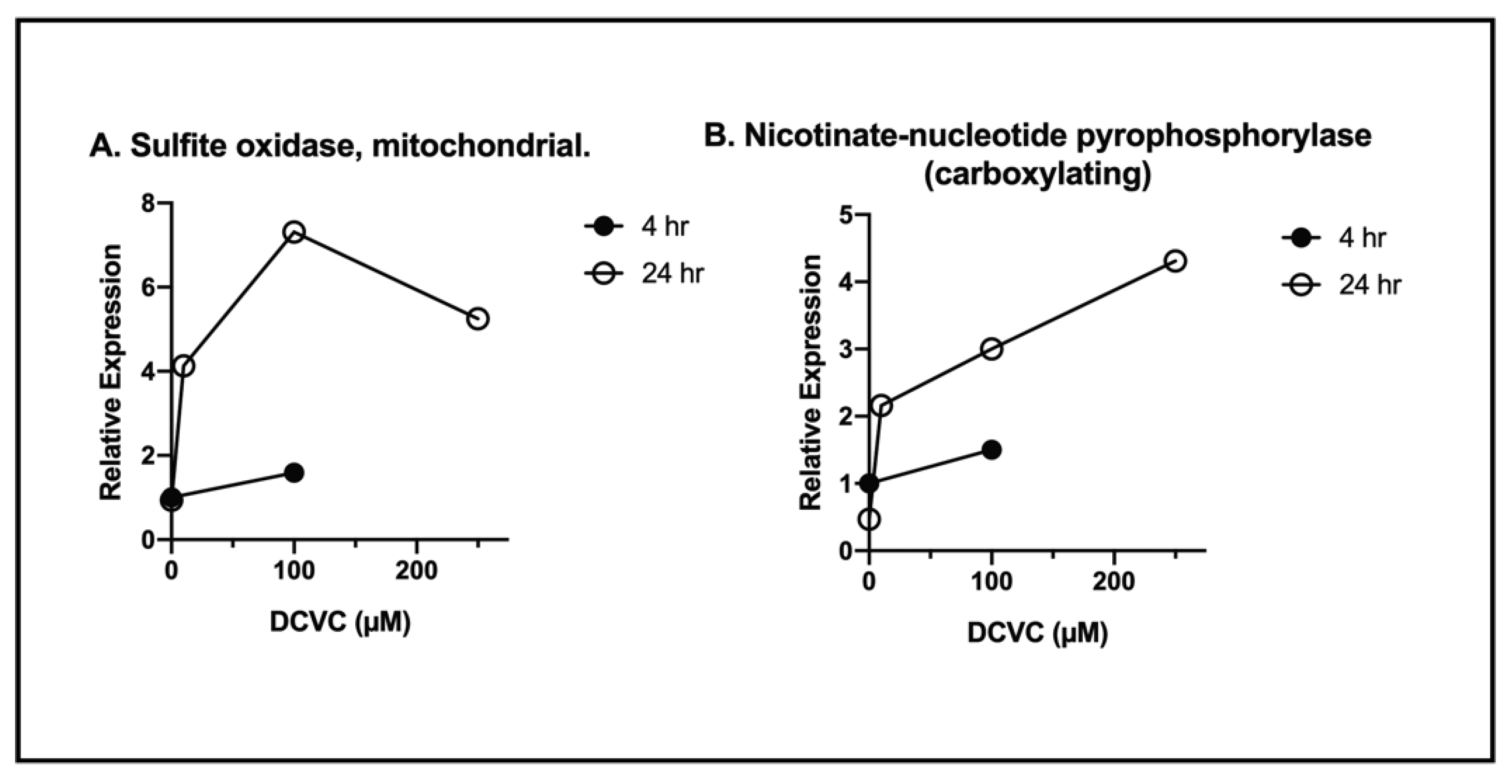
| Accession No. | Classification/Protein | NPX/Control (Mean ± SD) |
|---|---|---|
| ATP Synthesis, Transport and Metabolic Processes | ||
| P15999 | Atp5a1 ATP synthase F1 subunit alpha precursor, mitochondrial | 1.91 ± 0.05 |
| P10719 | Atp5b ATP synthase F1 subunit beta precursor, mitochondrial | 1.33 ± 0.71 |
| P05141 | ADP/ATP translocase 2, Slc25a4 | 1.03 ± 0.36 |
| Tricarboxylic Acid Cycle (Citrate Cycle) | ||
| P56574 | Isocitrate dehydrogenase (NADP) (oxalosuccinate decarboxylase) | 1.88 ± 0.56 |
| Q9ER34 | Aconitate hydratase (aconitase) | 2.54 ± 0.32 |
| Respiratory Electron Transport Chain | ||
| P13803 | Electron transfer flavoprotein subunit alpha | 1.00 ± 0.11 |
| Q66HF1 | NADH-ubiquinone oxidoreductase 75 kDa subunit | 1.90 ± 0.20 |
| Amino Acid and Fatty Acid Metabolic Pathways | ||
| P50442 | Glycine amidotransferase (transamidinase) | 1.92 ± 0.15 |
| Q64565 | Alanine-glyoxylate aminotransferase 2 (beta-alanine-pyruvate aminotransferase) | 2.13 ± 0.15 |
| Q02253 | Methylmalonate-semialdehyde dehydrogenase (malonate-semialdehyde dehydrogenase) | 1.32 ± 0.31 |
| P08503 | Medium-chain specific acyl-CoA dehydrogenase | 2.62 ± 0.35 |
| Q64428 | Trifunctional enzyme subunit alpha (3-hydroxyacyl-CoA dehydrogenase) | 0.70 ± 0.22 |
| P10860 | Glutamate dehydrogenase 1 | 1.94 ± 0.41 |
| Anion Transport (Transmembrane Transport) | ||
| Q9Z2L0 | Voltage-dependent anion-selective channel protein 1 (VDAC-1) | 1.12 ± 0.08 |
| Oxidoreductase Activity | ||
| Q6AYT0 | Quinone oxidoreductase (zeta-crystallin) | 1.21 ± 0.23 |
| Q07523 | Hydroxyacid oxidase 2 | 1.47 ± 0.74 |
| Q68FT3 | Pyridine nucleotide-disulfide oxidoreductase domain-containing protein 2 | 1.92 ± 0.22 |
| Chemical/Drug or Pathological State | Classification | References |
|---|---|---|
| DCVC | Cysteine conjugate of environmental pollutant trichloroethylene | [54,62,63,69,70,72,73,74,78] |
| PCBC | Cysteine conjugate of occupational contaminant hexachloro-butadiene | [76,77] |
| Bromohydroquinone and its cysteine conjugate | Cysteine conjugate of chemical synthetic intermediate bromohydroquinone | [91,92] |
| Cephalosporins | Antibiotics | [93,94] |
| Gentamicin | Antibiotic | [95,96,97] |
| Tenofovir | Antiviral | [98,99,100,101,102] |
| Cisplatin | Chemotherapeutic agent | [103,104,105,106,107] |
| Oxalate | Metabolite | [108] |
| HgCl2 | Environmental contaminant | [109] |
| Hypoxia/ischemia | Pathological states of oxygen deprivation with or without blood flow disruption | [110,111,112,113,114] |
| Subcellular Compartment | # Proteins Identified | % Total |
|---|---|---|
| Mitochondria | 118 | 43.2 |
| Cytosol | 30 | 11.0 |
| Vesicles | 21 | 7.7 |
| Soluble | 18 | 6.6 |
| Vacuoles | 13 | 4.8 |
| Lysosomes | 12 | 4.4 |
| Melanosomes | 12 | 4.4 |
| Cell Surface | 11 | 4.0 |
| Microsomes | 9 | 3.3 |
| Nucleoid | 9 | 3.3 |
| Vesicular | 9 | 3.3 |
| Outer membrane | 6 | 2.2 |
| Peroxisomes | 5 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lash, L.H. Diverse Roles of Mitochondria in Renal Injury from Environmental Toxicants and Therapeutic Drugs. Int. J. Mol. Sci. 2021, 22, 4172. https://doi.org/10.3390/ijms22084172
Lash LH. Diverse Roles of Mitochondria in Renal Injury from Environmental Toxicants and Therapeutic Drugs. International Journal of Molecular Sciences. 2021; 22(8):4172. https://doi.org/10.3390/ijms22084172
Chicago/Turabian StyleLash, Lawrence H. 2021. "Diverse Roles of Mitochondria in Renal Injury from Environmental Toxicants and Therapeutic Drugs" International Journal of Molecular Sciences 22, no. 8: 4172. https://doi.org/10.3390/ijms22084172
APA StyleLash, L. H. (2021). Diverse Roles of Mitochondria in Renal Injury from Environmental Toxicants and Therapeutic Drugs. International Journal of Molecular Sciences, 22(8), 4172. https://doi.org/10.3390/ijms22084172






