Genome-Wide Identification and Expression Patterns of the C2H2-Zinc Finger Gene Family Related to Stress Responses and Catechins Accumulation in Camellia sinensis [L.] O. Kuntze
Abstract
1. Introduction
2. Results and Discussion
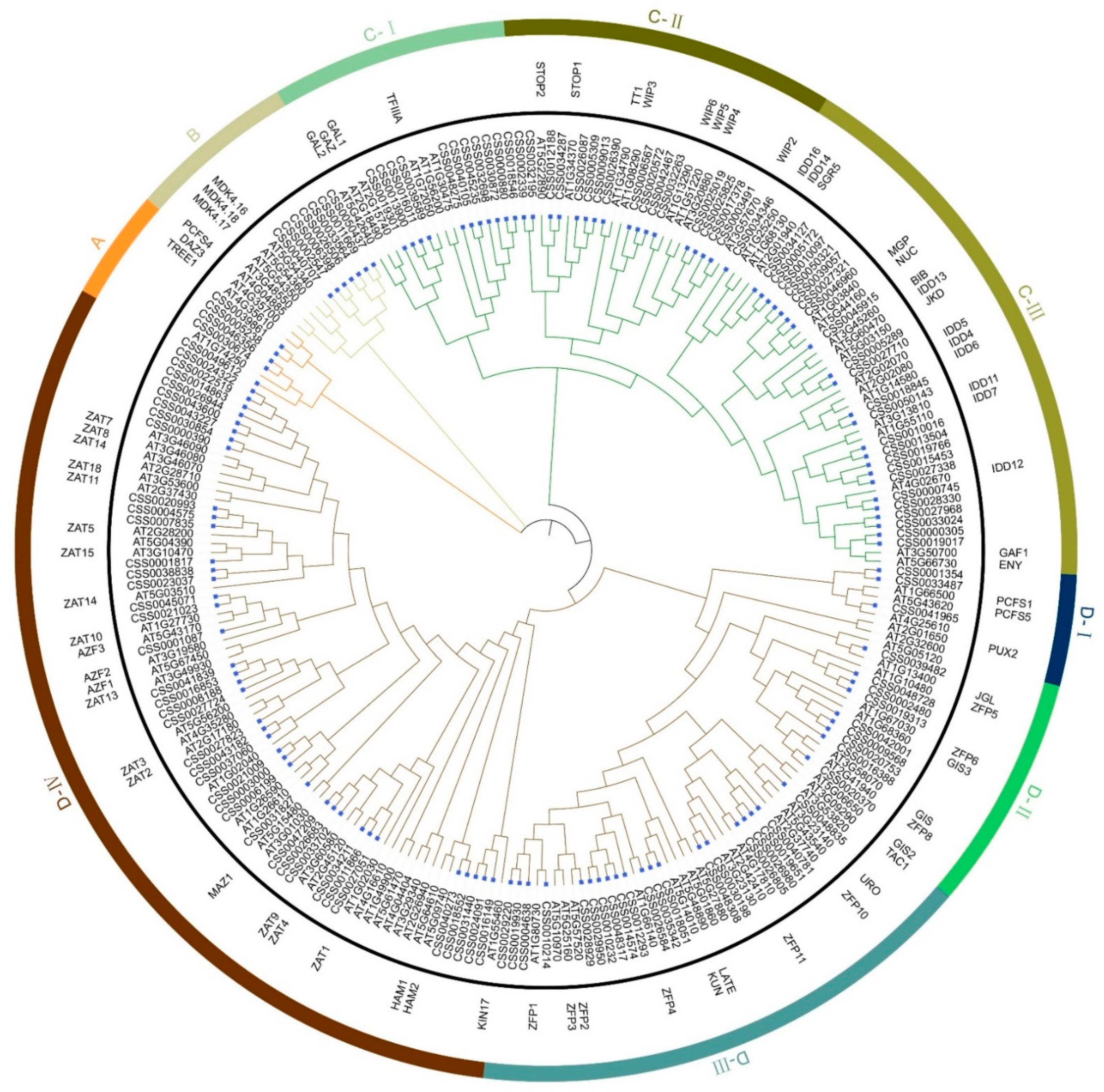
2.1. Identification and Phylogenetic Analysis of C2H2-Zinc Finger (C2H2-ZFP) Gene Family in C. sinensis
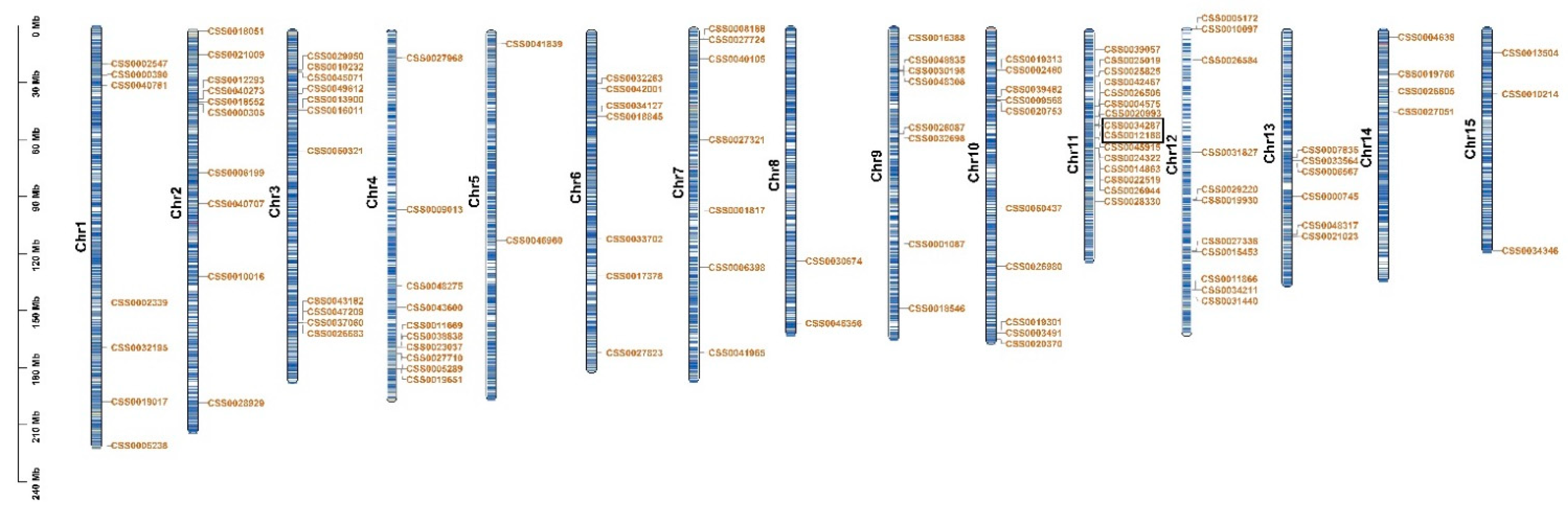
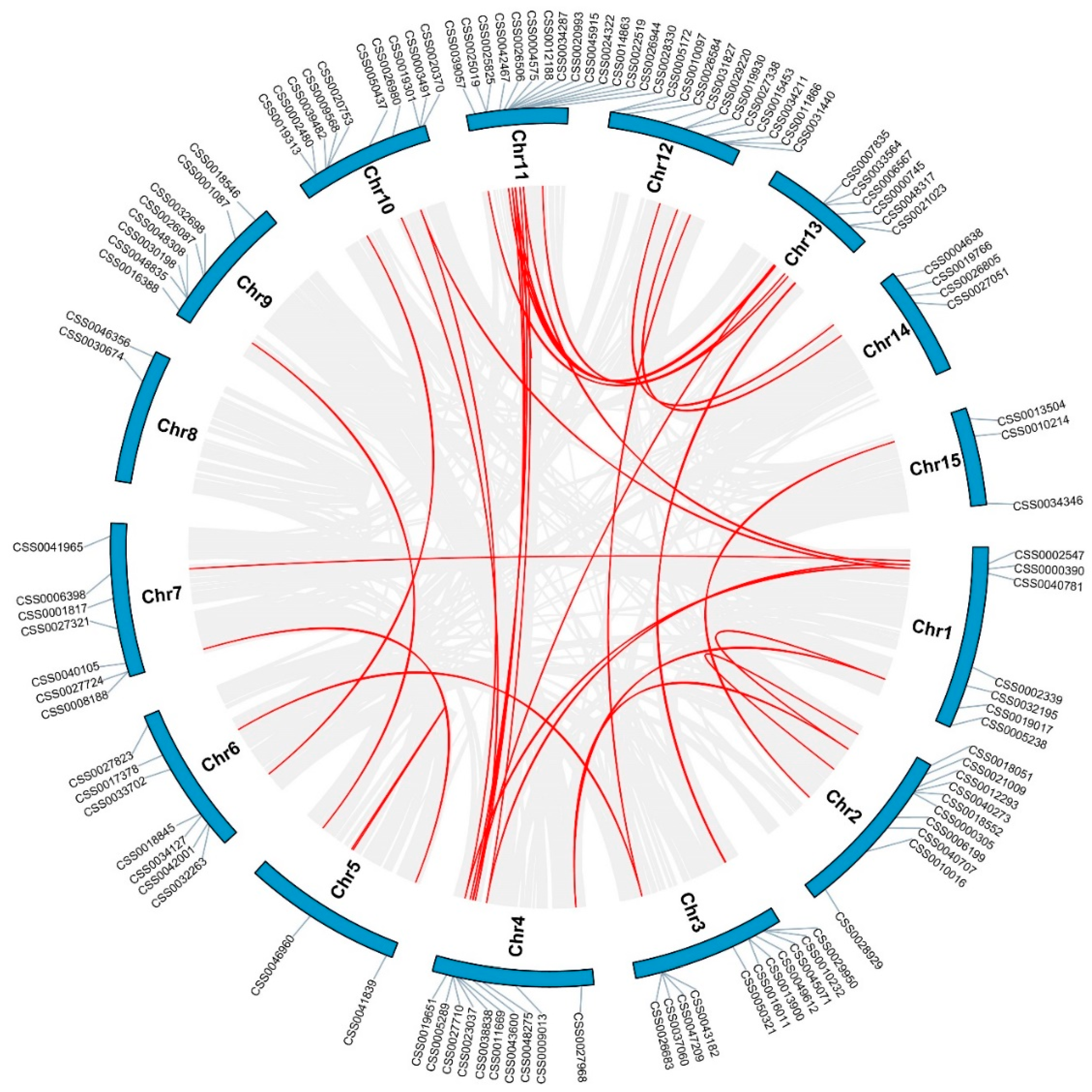
2.2. Distribution on Chromosome and Gene Duplication Events of C2H2-ZFP Gene Family in C. sinensis
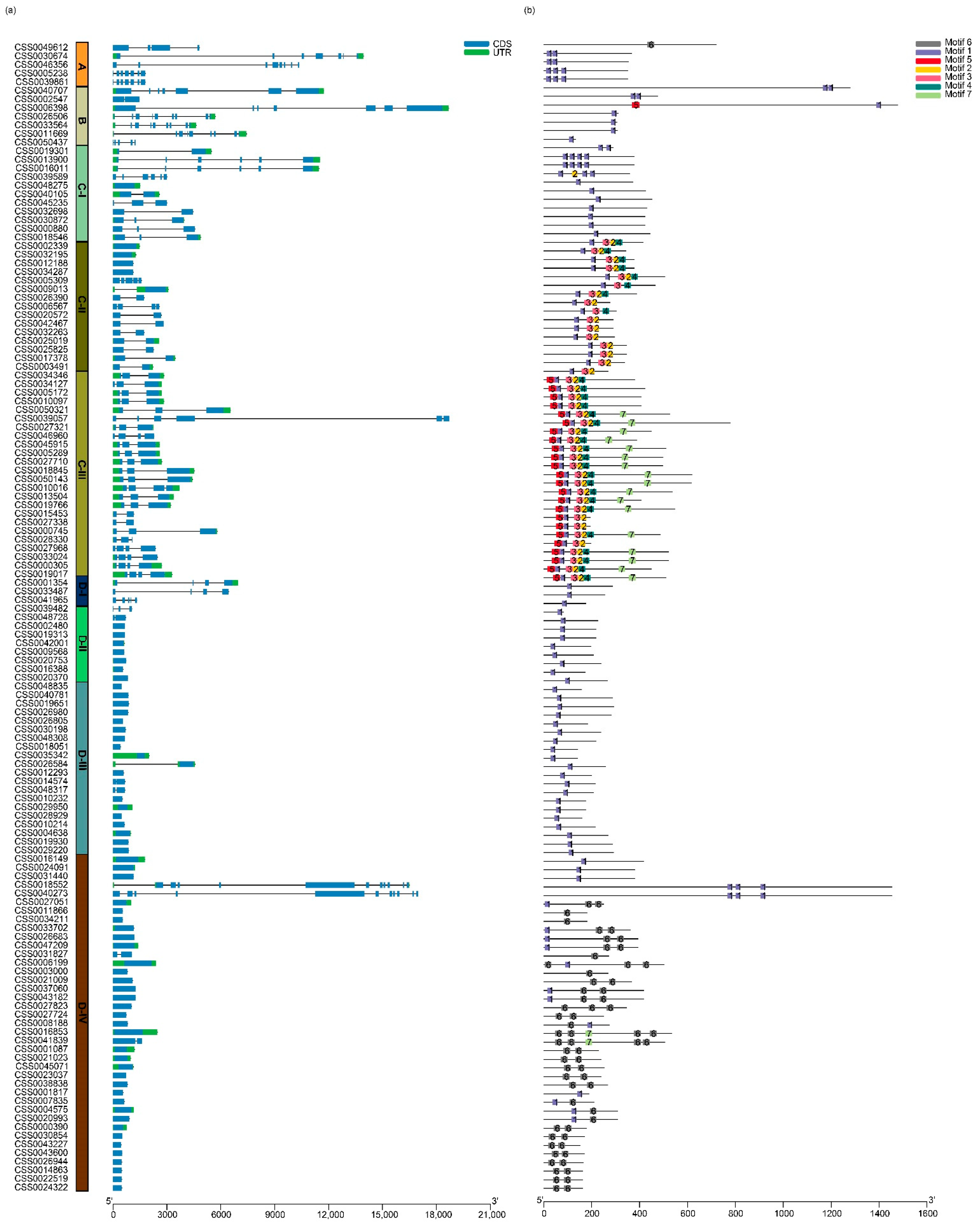
2.3. Gene Features and Conserved Motifs of the C2H2-ZFP Gene Family in C. sinensis
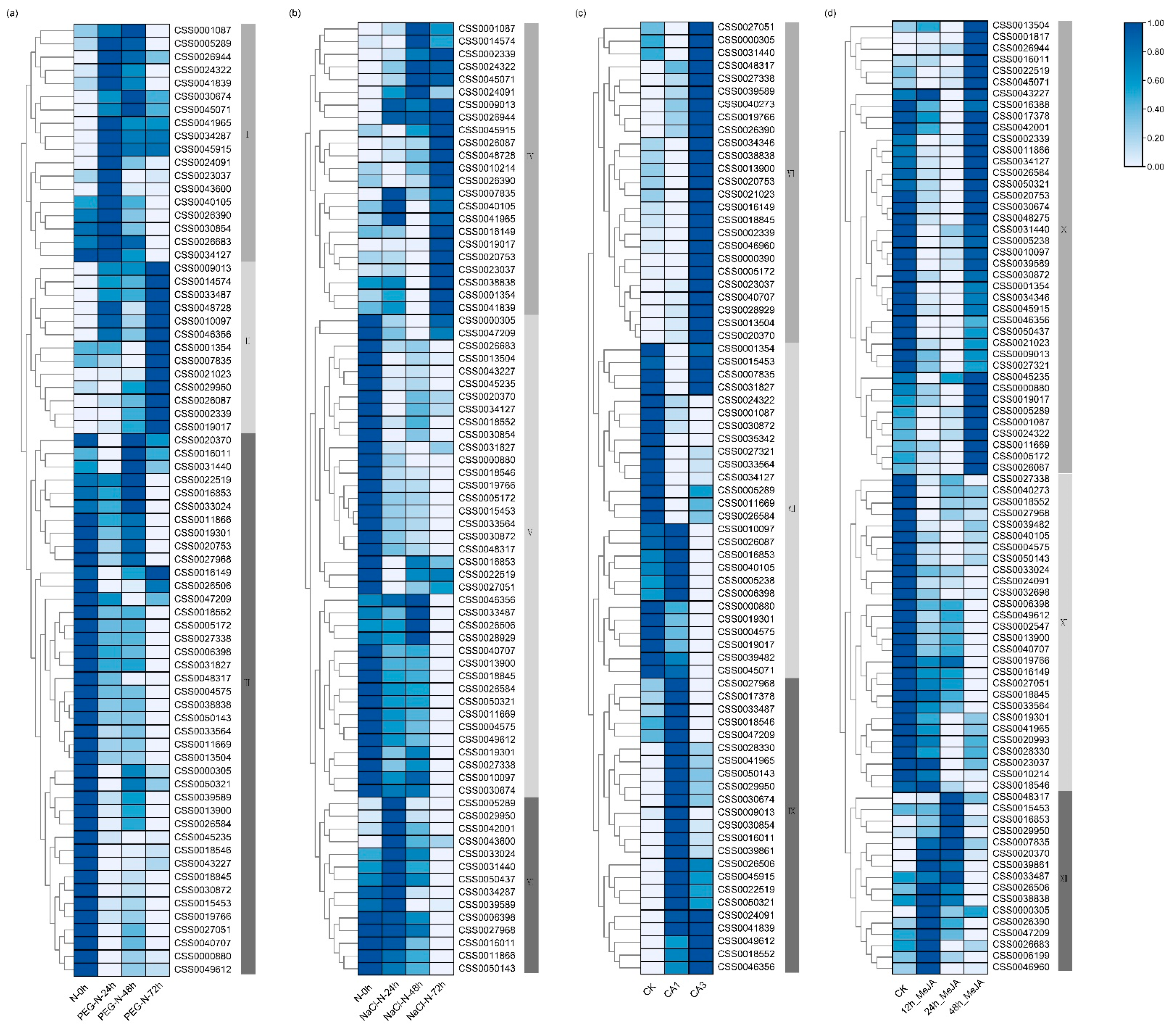
2.4. Responses of CsC2H2-ZFP Genes under Stress
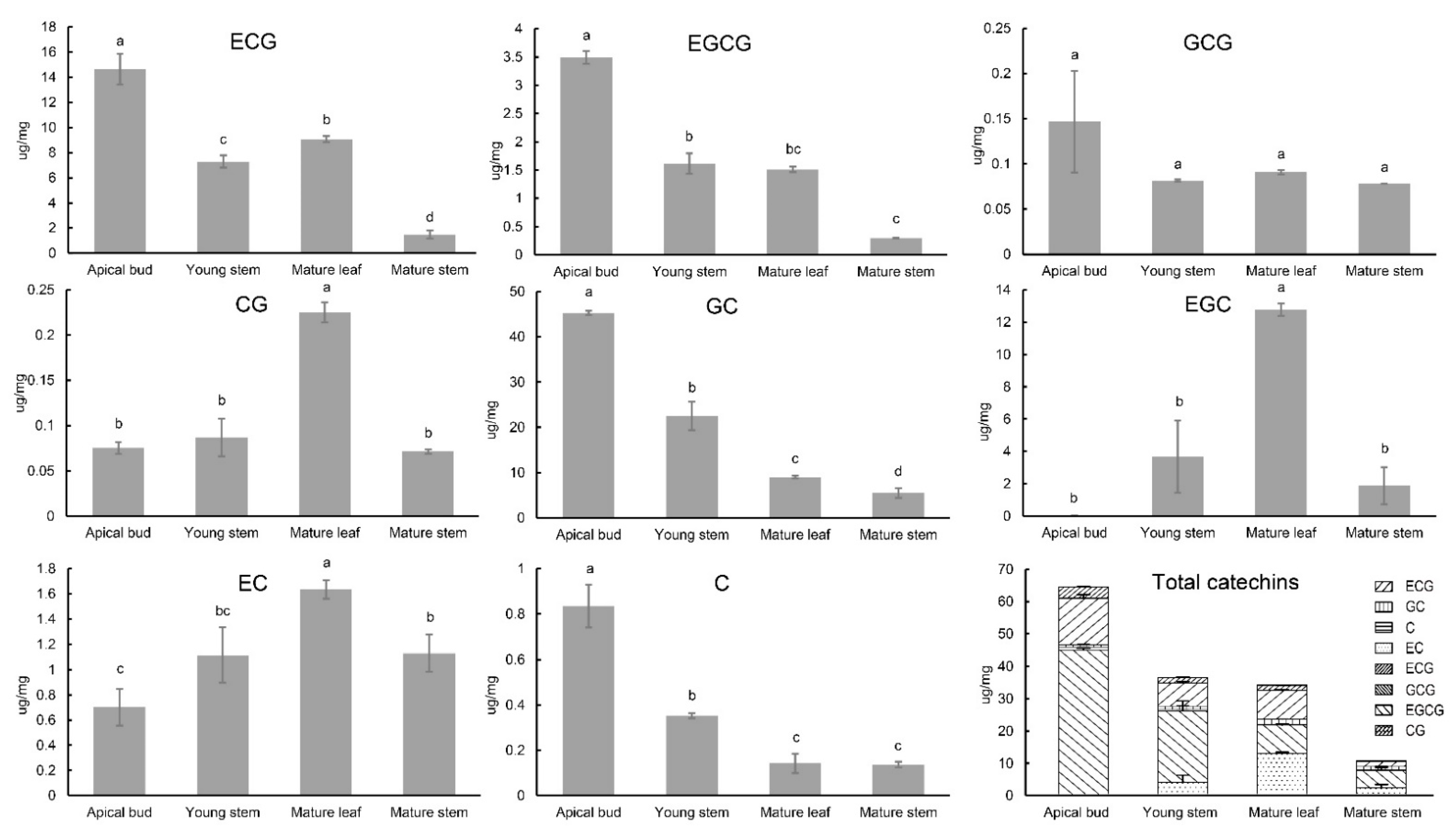
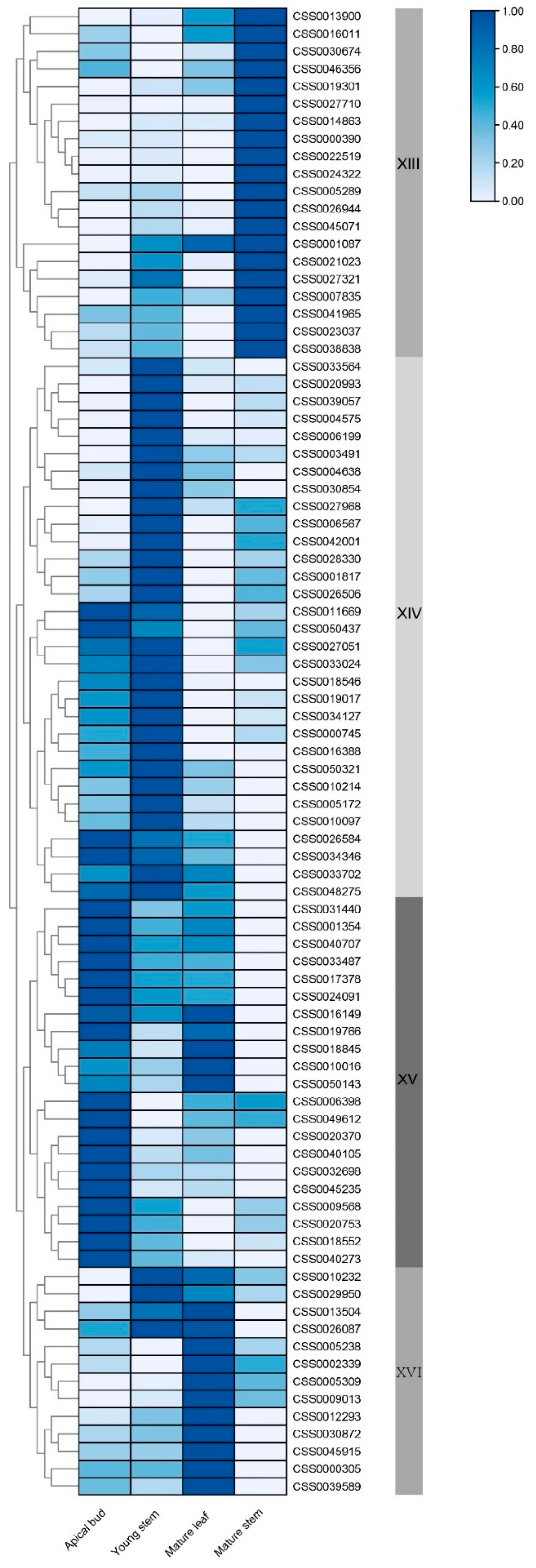
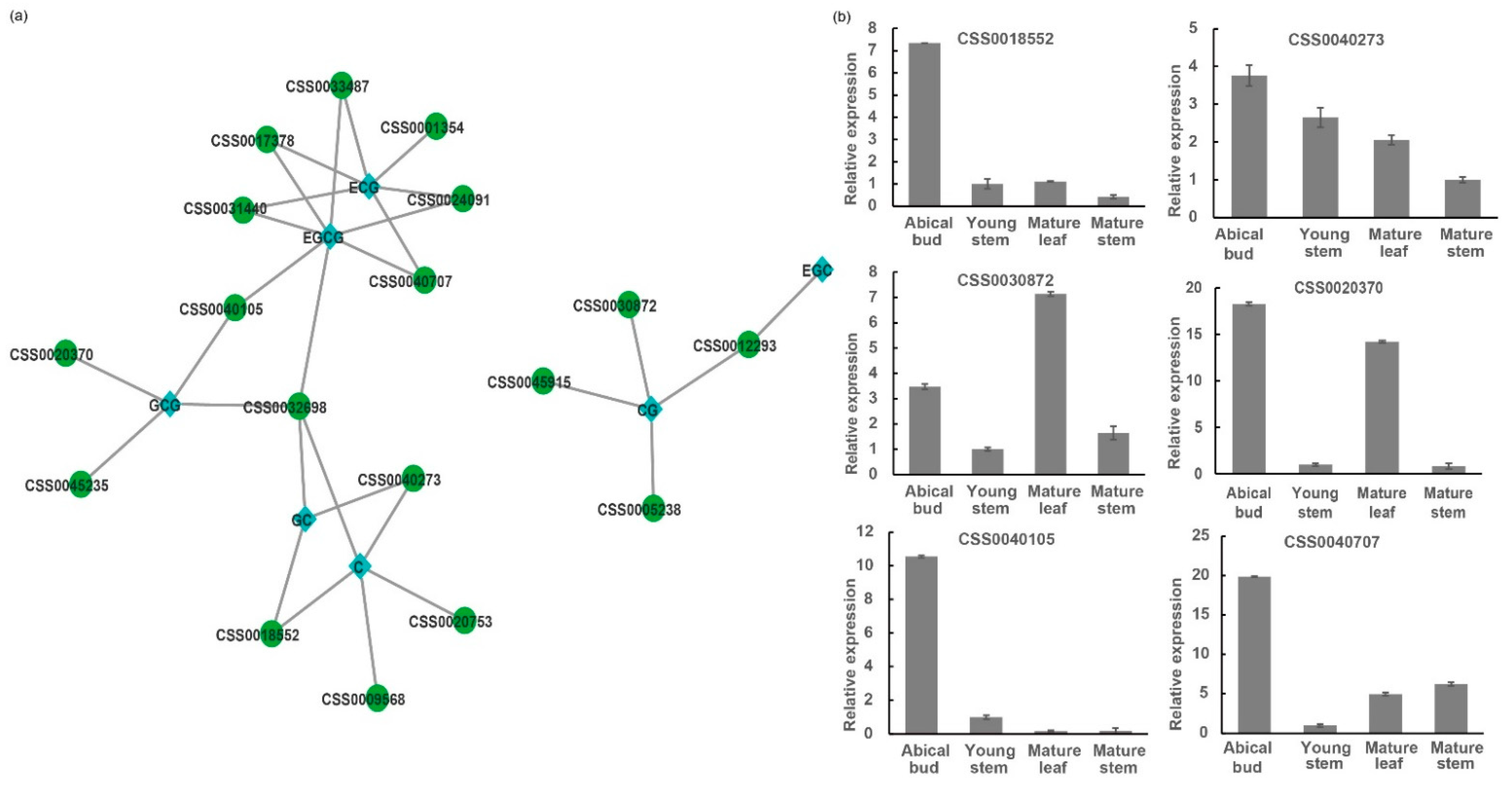
2.5. Combined Analysis of Catechin Content and Expression of CsC2H2-ZFP Genes in Young Tissues in C. sinensis
3. Materials and Methods
3.1. Identification and Characteristics of the C2H2-ZFP Gene Family in C. sinensis
3.2. Chromosomal Location of C2H2-ZFP Gene Family in C. sinensis
3.3. Phylogenetic Analysis of C2H2-ZFP Gene Family in C. sinensis
3.4. Exon/Intron Structure Analysis and Identification of Conserved Motifs
3.5. Plant Materials
3.6. Expression Analysis of the C2H2-ZFP Gene Family in C. sinensis
3.7. HPLC Analysis of Catechins
3.8. RNA Isolation and Quantitative Real-Time PCR Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimeld, S.M. C2H2 zinc finger genes of the Gli, Zic, KLF, SP, Wilms’ tumour, Huckebein, Snail, Ovo, Spalt, Odd, Blimp-1, Fez and related gene families from Branchiostoma floridae. Dev. Genes Evol. 2008, 218, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Pavletich, N.P.; Pabo, C.O. Zinc finger-DNA recognition: Crystal structure of a Zif268-DNA complex at 2.1 A. Science 1991, 252, 809. [Google Scholar] [CrossRef]
- Gan, Y.; Liu, C.; Yu, H.; Broun, P. Integration of cytokinin and gibberellin signalling by Arabidopsis transcription factors GIS, ZFP8 and GIS2 in the regulation of epidermal cell fate. Development 2007, 134, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zhou, Z.; Su, S.; Yan, A.; Gan, Y. Glabrous Inflorescence Stems (GIS) is required for trichome branching through gibberellic acid signaling in Arabidopsis. Plant Cell Physiol. 2012, 53, 457–469. [Google Scholar] [CrossRef]
- Sun, L.L.; Zhou, Z.J.; An, L.J.; An, Y.; Zhao, Y.Q.; Meng, X.F.; Steele-King, C.; Gan, Y.B. GLABROUS INFLORESCENCE STEMS regulates trichome branching by genetically interacting with SIM in Arabidopsis. J. Zhejiang Univ. Sci. B 2013, 14, 563–569. [Google Scholar] [CrossRef]
- Sakai, H.; Krizek, B.A.; Jacobsen, S.E.; Meyerowitz, E.M. Regulation of SUP expression identifies multiple regulators involved in Arabidopsis floral meristem development. Plant Cell 2000, 12, 1607–1618. [Google Scholar] [CrossRef]
- Morita, M.T.; Sakaguchi, K.; Kiyose, S.-I.; Taira, K.; Kato, T.; Nakamura, M.; Tasaka, M. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006, 47, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, E.S.; Min, Y.; Edwards, M.B.; Kramer, E.M.; Hodges, S.A. POPOVICH, encoding a C2H2 zinc-finger transcription factor, plays a central role in the development of a key innovation, floral nectar spurs, in Aquilegia. Proc. Natl. Acad. Sci. USA 2020, 117, 22552. [Google Scholar] [CrossRef]
- Sagasser, M.; Lu, G.-H.; Hahlbrock, K.; Weisshaar, B. A. thaliana TRANSPARENT TESTA 1 is involved in seed coat development and defines the WIP subfamily of plant zinc finger proteins. Genes Dev. 2002, 16, 138–149. [Google Scholar] [CrossRef]
- Appelhagen, I.; Lu, G.H.; Huep, G.; Schmelzer, E.; Weisshaar, B.; Sagasser, M. TRANSPARENT TESTA 1 interacts with R2R3-MYB factors and affects early and late steps of flavonoid biosynthesis in the endothelium of Arabidopsis thaliana seeds. Plant J. 2011, 67, 406–419. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Lian, J.; Lu, X.; Yin, N.; Ma, L.; Lu, J.; Liu, X.; Li, J.; Lu, J.; Lei, B.; Wang, R.; et al. Silencing of BnTT1 family genes affects seed flavonoid biosynthesis and alters seed fatty acid composition in Brassica napus. Plant Sci. 2017, 254, 32–47. [Google Scholar] [CrossRef]
- He, F.; Li, H.-G.; Wang, J.-J.; Su, Y.; Wang, H.-L.; Feng, C.-H.; Yang, Y.; Niu, M.-X.; Liu, C.; Yin, W.; et al. PeSTZ1, a C2H2-type zinc finger transcription factor from Populus euphratica, enhances freezing tolerance through modulation of ROS scavenging by directly regulating PeAPX2. Plant Biotechnol. J. 2019, 17, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, Z.; Luo, J.; Zhou, Y.; Cai, Y.; Lu, Y.; Xia, J.; Kuang, H.; Ye, Z.; Ouyang, B. The C2H2 zinc-finger protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol. J. 2018, 16, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, H.; Sun, C.; Ma, Q.; Bu, H.; Chong, K.; Xu, Y. A C2H2 zinc-finger protein OsZFP213 interacts with OsMAPK3 to enhance salt tolerance in rice. J. Plant Physiol. 2018, 229, 100–110. [Google Scholar] [CrossRef]
- Yu, G.-H.; Jiang, L.-L.; Ma, X.-F.; Xu, Z.-S.; Liu, M.-M.; Shan, S.-G.; Cheng, X.-G. A soybean C2H2-type zinc finger gene GmZF1 enhanced cold tolerance in transgenic Arabidopsis. PLoS ONE 2014, 9, e109399. [Google Scholar] [CrossRef]
- Sakamoto, H.; Maruyama, K.; Sakuma, Y.; Meshi, T.; Iwabuchi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004, 136, 2734. [Google Scholar] [CrossRef]
- Kodaira, K.-S.; Qin, F.; Tran, L.-S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol. 2011, 157, 742. [Google Scholar] [CrossRef] [PubMed]
- Ciftci-Yilmaz, S.; Morsy, M.R.; Song, L.; Coutu, A.; Krizek, B.A.; Lewis, M.W.; Warren, D.; Cushman, J.; Connolly, E.L.; Mittler, R. The EAR-motif of the Cys2/His2-type zinc finger protein Zat7 plays a key role in the defense response of Arabidopsis to salinity stress. J. Biol. Chem. 2007, 282, 9260–9268. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.D.; Novak, N.G.; Jones, R.W.; Farrar, R.R.; Blackburn, M.B. Herbivory responsive C2H2 zinc finger transcription factor protein StZFP2 from potato. Plant Physiol. Biochem. 2014, 80, 226–233. [Google Scholar] [CrossRef]
- Pauwels, L.; Goossens, A. Fine-tuning of early events in the jasmonate response. Plant Signal. Behav. 2008, 3, 846–847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Englbrecht, C.C.; Schoof, H.; Böhm, S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Batool, K.; Cui, D.-L.; Yang, Y.-Q.; Lu, Y.-H. Comprehensive genomic survey, structural classification and expression analysis of C2H2 zinc finger protein gene family in Brassica rapa L. PLoS ONE 2019, 14, e0216071. [Google Scholar] [CrossRef] [PubMed]
- Salih, H.; Odongo, M.R.; Gong, W.; He, S.; Du, X. Genome-wide analysis of cotton C2H2-zinc finger transcription factor family and their expression analysis during fiber development. BMC Plant Biol. 2019, 19, 400. [Google Scholar] [CrossRef]
- Ming, N.; Ma, N.; Jiao, B.; Lv, W.; Meng, Q. Genome wide identification of C2H2-type zinc finger proteins of tomato and expression analysis under different abiotic stresses. Plant Mol. Biol. Report. 2020, 38, 75–94. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Zhao, J.; Li, Y.; Huang, R.; Jiang, X.; Zhou, X.; Zhu, X.; He, Y.; He, Y.; et al. Genome-wide characterization of the C2H2 zinc-finger genes in Cucumis sativus and functional analyses of four CsZFPs in response to stresses. BMC Plant Biol. 2020, 20, 359. [Google Scholar] [CrossRef]
- Liu, Z.; Coulter, J.A.; Li, Y.; Zhang, X.; Meng, J.; Zhang, J.; Liu, Y. Genome-wide identification and analysis of the Q-type C2H2 gene family in potato (Solanum tuberosum L.). Int. J. Biol. Macromol. 2020, 153, 327–340. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Ban, Q.; Zhu, X.; Jiang, C.; Wei, C.; Bennetzen, J.L. Comparative transcriptomic analysis reveals gene expression associated with cold adaptation in the tea plant Camellia sinensis. BMC Genom. 2019, 20, 624. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, K.; Wang, J.; Ding, Z.-T.; Wang, H.; Bi, C.-H.; Zhang, Y.-W.; Sun, H.-W. Proteomic analysis of Camellia sinensis (L.) reveals a synergistic network in the response to drought stress and recovery. J. Plant Physiol. 2017, 219, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, M.; Yu, X.; Wang, L.; Guo, C.; Ming, R.; Zhang, J. Transcriptome dynamics of Camellia sinensis in response to continuous salinity and drought stress. Tree Genet. Genomes 2017, 13, 78. [Google Scholar] [CrossRef]
- Xia, E.; Li, F.; Tong, W.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Tai, Y.; She, G.; et al. The tea plant reference genome and improved gene annotation using long-read and paired-end sequencing data. Sci. Data 2019, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ko, E.E.; Tran, J.; Qiao, H. TREE1-EIN3–mediated transcriptional repression inhibits shoot growth in response to ethylene. Proc. Natl. Acad. Sci. USA 2020, 117, 29178. [Google Scholar] [CrossRef]
- Xing, D.; Zhao, H.; Xu, R.; Li, Q.Q. Arabidopsis PCFS4, a homologue of yeast polyadenylation factor Pcf11p, regulates FCA alternative processing and promotes flowering time. Plant J. 2008, 54, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Jang, S.; Yoon, E.K.; Heo, J.-O.; Chang, K.S.; Choi, J.W.; Dhar, S.; Kim, G.; Choe, J.-E.; Heo, J.B.; et al. Interplay between ABA and GA modulates the timing of asymmetric cell divisions in the Arabidopsis root ground tissue. Mol. Plant. 2016, 9, 870–884. [Google Scholar] [CrossRef]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ohyama, Y.; Kobayashi, Y.; Ito, H.; Iuchi, S.; Fujita, M.; Zhao, C.R.; Tanveer, T.; Ganesan, M.; Kobayashi, M.; et al. STOP2 activates transcription of several genes for Al- and low pH-tolerance that are regulated by STOP1 in Arabidopsis. Mol. Plant 2014, 7, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Appelhagen, I.; Huep, G.; Lu, G.H.; Strompen, G.; Weisshaar, B.; Sagasser, M. Weird fingers: Functional analysis of WIP domain proteins. FEBS Lett. 2010, 584, 3116–3122. [Google Scholar] [CrossRef]
- Prochetto, S.; Reinheimer, R. Step by step evolution of Indeterminate Domain (IDD) transcriptional regulators: From algae to angiosperms. Ann. Bot. 2020, 126, 85–101. [Google Scholar] [CrossRef]
- Chandran, D.; Rickert, J.; Cherk, C.; Dotson, B.R.; Wildermuth, M.C. Host cell ploidy underlying the fungal feeding site is a determinant of powdery mildew growth and reproduction. Mol. Plant Microbe Interact. 2013, 26, 537–545. [Google Scholar] [CrossRef]
- Zhou, Z.; An, L.; Sun, L.; Gan, Y. ZFP5 encodes a functionally equivalent GIS protein to control trichome initiation. Plant Signal. Behav. 2012, 7, 28–30. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, L.; Zhao, Y.; An, L.; Yan, A.; Meng, X.; Gan, Y. Zinc Finger Protein 6 (ZFP6) regulates trichome initiation by integrating gibberellin and cytokinin signaling in Arabidopsis thaliana. New Phytol. 2013, 198, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Dinneny, J.R.; Weigel, D.; Yanofsky, M.F. NUBBIN and JAGGED define stamen and carpel shape in Arabidopsis. Development 2006, 133, 1645–1655. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, A.; Zhou, Z.; Zhao, Y.; Yan, A.; Bao, S.; Yu, H.; Gan, Y. Glabrous Inflorescence Stems3 (GIS3) regulates trichome initiation and development in Arabidopsis. New Phytol. 2015, 206, 220–230. [Google Scholar] [CrossRef]
- Joseph, M.P.; Papdi, C.; Kozma-Bognár, L.; Nagy, I.; López-Carbonell, M.; Rigó, G.; Koncz, C.; Szabados, L. The Arabidopsis ZINC FINGER PROTEIN3 Interferes with abscisic acid and light signaling in seed germination and plant development. Plant Physiol. 2014, 165, 1203–1220. [Google Scholar] [CrossRef]
- Borg, M.; Rutley, N.; Kagale, S.; Hamamura, Y.; Gherghinoiu, M.; Kumar, S.; Sari, U.; Esparza-Franco, M.A.; Sakamoto, W.; Rozwadowski, K.; et al. An EAR-dependent regulatory module promotes male germ cell division and sperm fertility in Arabidopsis. Plant Cell 2014, 26, 2098–2113. [Google Scholar] [CrossRef]
- Lyu, T.; Hu, Z.; Liu, W.; Cao, J. Arabidopsis Cys2/His2 zinc-finger protein MAZ1 is essential for intine formation and exine pattern. Biochem. Biophys. Res. Commun. 2019, 518, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Eungwanichayapant, P.D.; Popluechai, S. Accumulation of catechins in tea in relation to accumulation of mRNA from genes involved in catechin biosynthesis. Plant Physiol. Biochem. 2009, 47, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Cheng, C.; Lin, Y.; Zhu, Q.; Lin, J.; Lai, Z. Combined small RNA and degradome sequencing reveals complex microRNA regulation of catechin biosynthesis in tea (Camellia sinensis). PLoS ONE 2017, 12, e0171173. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tian, H.-l.; Wu, J.-H.; Cang, R.-R.; Wang, R.-X.; Qi, X.-H.; Xu, Q.; Chen, X.-H. Relationship between gene expression and the accumulation of catechin during spring and autumn in tea plants (Camellia sinensis L.). Hortic. Res. 2015, 2, 15011. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, 14. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhao, X.-Q.; Wang, J.; Wong, G.K.-S.; Yu, J. KaKs_calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Xia, E.; Tong, W.; Hou, Y.; An, Y.; Chen, L.; Wu, Q.; Liu, Y.; Yu, J.; Li, F.; Li, R.; et al. The reference genome of tea plant and resequencing of 81 diverse accessions provide insights into its genome evolution and adaptation. Mol. Plant 2020, 13, 1013–1026. [Google Scholar] [CrossRef]
- Chen, G.; Liang, H.; Zhao, Q.; Wu, A.-M.; Wang, B. Exploiting MATE efflux proteins to improve flavonoid accumulation in Camellia sinensis in silico. Int. J. Biol. Macromol. 2020, 143, 732–743. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Novelli, S.; Gismondi, A.; Di Marco, G.; Canuti, L.; Nanni, V.; Canini, A. Plant defense factors involved in Olea europaea resistance against Xylella fastidiosa infection. J. Plant Res. 2019, 132, 439–455. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, J.; Zhong, G.; Wang, B. Genome-Wide Identification and Expression Patterns of the C2H2-Zinc Finger Gene Family Related to Stress Responses and Catechins Accumulation in Camellia sinensis [L.] O. Kuntze. Int. J. Mol. Sci. 2021, 22, 4197. https://doi.org/10.3390/ijms22084197
Zhang S, Liu J, Zhong G, Wang B. Genome-Wide Identification and Expression Patterns of the C2H2-Zinc Finger Gene Family Related to Stress Responses and Catechins Accumulation in Camellia sinensis [L.] O. Kuntze. International Journal of Molecular Sciences. 2021; 22(8):4197. https://doi.org/10.3390/ijms22084197
Chicago/Turabian StyleZhang, Shiyang, Junjie Liu, Guixian Zhong, and Bo Wang. 2021. "Genome-Wide Identification and Expression Patterns of the C2H2-Zinc Finger Gene Family Related to Stress Responses and Catechins Accumulation in Camellia sinensis [L.] O. Kuntze" International Journal of Molecular Sciences 22, no. 8: 4197. https://doi.org/10.3390/ijms22084197
APA StyleZhang, S., Liu, J., Zhong, G., & Wang, B. (2021). Genome-Wide Identification and Expression Patterns of the C2H2-Zinc Finger Gene Family Related to Stress Responses and Catechins Accumulation in Camellia sinensis [L.] O. Kuntze. International Journal of Molecular Sciences, 22(8), 4197. https://doi.org/10.3390/ijms22084197





