Role of Adipose Tissue in Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Obesity and IBD
3. Adipose Tissue as an Active Endocrine Organ Visceral Adipose Tissue and IBD
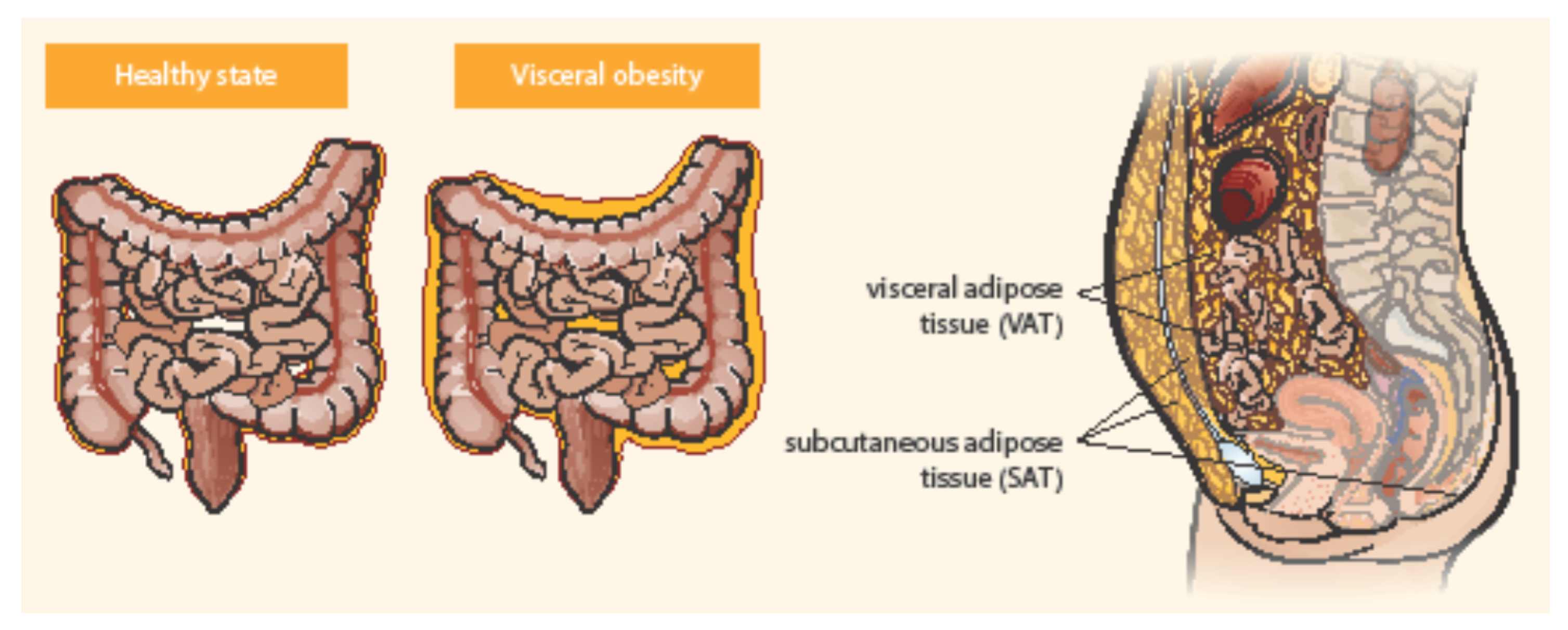
3.1. Mesenteric Adipose Tissue
3.2. Creeping Fat (Fat Wrapping)
3.3. Intestinal Microbiota, Visceral Obesity, and IBD
3.4. Mesenteric Fat as a Source of Inflammatory Peptides
4. Adipokines
4.1. Adiponectin
4.2. Leptin
4.3. Resistin
4.4. Chemerin
4.5. Visfatin
4.6. Apelin
4.7. Other Adipokines and Hormones Related to IBD
5. Adipokines and IBD Treatment
5.1. Adipokines for Therapeutic Use
5.2. Adipokines and Anti-TNF Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bousvaros, A. Use of immunomodulators and biologic therapies in children with inflammatory bowel disease. Expert Rev. Clin. Immunol. 2010, 6, 659–666. [Google Scholar] [CrossRef]
- Sýkora, J.; Pomahačová, R.; Kreslová, M.; Cvalínová, D.; Štych, P.; Schwarz, J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 2741–2763. [Google Scholar] [CrossRef]
- Gonçalves, P.; Magro, F.; Martel, F. Metabolic inflammation in inflammatory bowel disease: Crosstalk between adipose tissue and bowel. Inflamm. Bowel Dis. 2015, 21, 453–467. [Google Scholar] [CrossRef]
- Morshedzadeh, N.; Rahimlou, M.; Asadzadeh Aghdaei, H.; Shahrokh, S.; Reza Zali, M.; Mirmiran, P. Association Between Adipokines Levels with Inflammatory Bowel Disease (IBD): Systematic Reviews. Dig. Dis. Sci. 2017, 62, 3280–3286. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Sokal, A.; Filip, R. What was First, Obesity or Inflammatory Bowel Disease? What Does the Gut Microbiota Have to Do with It? Nutrients 2020, 12, 3073. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.B.; Ängquist, L.H.; Mendall, M.A.; Sørensen, T.I.A.; Baker, J.L.; Jess, T. Childhood body mass index and risk of inflammatory bowel disease in adulthood: A population-based cohort study. Am. J. Gastroenterol. 2018, 113, 694–701. [Google Scholar] [CrossRef]
- Bhagavathula, A.S.; Clark, C.C.T.; Rahmani, J.; Chattu, V.K. Impact of Body Mass Index on the Development of Inflammatory Bowel Disease: A Systematic Review and Dose-Response Analysis of 15.6 Million Participants. Healthcare 2021, 9, 35. [Google Scholar] [CrossRef]
- Barroso, T.; Conway, F.; Emel, S.; McMillan, D.; Young, D.; Karteszi, H.; Gaya, D.R.; Gerasimidis, K. Patients with inflammatory bowel disease have higher abdominal adiposity and less skeletal mass than healthy controls. Ann. Gastroenterol. 2018, 31, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Schultz, C.G.; Ooi, S.; Goess, C.; Costello, S.P.; Vincent, A.D.; Schoeman, S.N.; Lim, A.; Bartholomeusz, F.D.; Travis, S.P.L.; et al. Obesity in Inflammatory Bowel Disease: Gains in Adiposity despite High Prevalence of Myopenia and Osteopenia. Nutrients 2018, 10, 1192. [Google Scholar] [CrossRef] [Green Version]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef]
- Holt, D.Q.; Moore, G.T.; Strauss, B.J.; Hamilton, A.L.; De Cruz, P.; Kamm, M.A. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment Pharmacol. Ther. 2017, 45, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.W.; Gurwara, S.; Silver, H.J.; Horst, S.N.; Beaulieu, D.B.; Schwartz, D.A.; Seidner, D.L. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflamm. Bowel Dis. 2017, 7, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, C.; Xie, T.; Yang, J.; Dai, X.; Lv, T.; Li, Y.; Gu, L.; Wei, Y.; Gong, J.; et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin. Nutr. 2017, 36, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Pavelock, N.; Masood, U.; Minchenberg, S.; Heisih, D. Effects of obesity on the course of inflammatory bowel disease. Bayl. Univ. Med. Center Proc. 2019, 32, 14–17. [Google Scholar] [CrossRef]
- Yerushalmy-Feler, A.; Ben-Tov, A.; Weintraub, Y.; Amir, A.; Galai, T.; Moran-Lev, H.; Cohen, S. High and low body mass index may predict severe disease course in children with inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 708–713. [Google Scholar] [CrossRef]
- Malik, T.A.; Manne, A.; Oster, R.A.; Eckhoff, A.; Inusah, S.; Gutierrez, A.M. Obesity is Associated With Poor Surgical Outcome in Crohn’s Disease. Gastroenterol. Res. 2013, 6, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Hass, D.J.; Brensinger, C.M.; Lewis, J.D.; Lichtenstein, G.R. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 482–488. [Google Scholar] [CrossRef]
- Johnson, A.M.; Loftus, E.V. Impact of obesity on the management of inflammatory bowel disease. Gastroenterol. Hepatol. 2020, 16, 350–359. [Google Scholar]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Crandall, W.V.; Leibowitz, I.H.; Duffy, L.; del Rosario, F.; Kim, S.C.; Integlia, M.J.; Berman, J.; Grunow, J.; Colletti, R.B.; et al. ImproveCareNow Collaborative for Pediatric IBD. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 2162–2168. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Ren, J.; Li, G.; Wu, X.; Li, J. The Impact of Obesity on the Clinical Course of Inflammatory Bowel Disease: A Meta-Analysis. Med. Sci. Monit. 2017, 23, 2599–2606. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Nguyen, N.H.; Proudfoot, J.A.; Martin, C.F.; Sandborn, W.J.; Kappelman, M.D.; Long, M.D.; Singh, S. Impact of Obesity on Disease Activity and Patient-Reported Outcomes Measurement Information System (PROMIS) in Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2019, 114, 630–639. [Google Scholar] [CrossRef]
- Blain, A.; Cattan, S.; Beaugerie, L.; Carbonnel, F.; Gendre, J.P.; Cosnes, J. Crohn’s disease clinical course and severity in obese patients. Clin. Nutr. 2002, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Burstein, E.; Cipher, D.J.; Feagins, L.A. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig. Dis. Sci. 2015, 60, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.; Adler, M.; Dobrowolska, A.; Kamhieh-Milz, J.; Witowski, J. The Role of Adipose Tissue in the Pathogenesis and Therapeutic Outcomes of Inflammatory Bowel Disease. Cells 2019, 8, 628. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.W.; Sinanan, M.N.; Zisman, T.L. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2118–2224. [Google Scholar] [CrossRef] [PubMed]
- Bultman, E.; de Haar, C.; van Liere-Baron, A.; Verhoog, H.; West, R.L.; Kuipers, E.J.; Zelinkova, Z.; van der Woude, C.J. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn’s disease patients. Aliment Pharmacol. Ther. 2012, 35, 335–341. [Google Scholar] [CrossRef]
- Dreesen, E.; Verstockt, B.; Bian, S.; de Bruyn, M.; Compernolle, G.; Tops, S.; Noman, M.; Van Assche, G.; Ferrante, M.; Gils, A.; et al. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1937–1946. [Google Scholar] [CrossRef]
- Rodin, I.; Chan, J.; Meleady, L.; Hii, C.; Lawrence, S.; Jacobson, K. High body mass index is not associated with increased treatment failure in infliximab treated pediatric patients with inflammatory bowel disease. JGH Open 2019, 23, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Proudfoot, J.; Xu, R.; Sandborn, W.J. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am. J. Gastroenterol. 2018, 113, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Facciorusso, A.; Singh, A.G.; Vande Casteele, N.; Zarrinpar, A.; Prokop, L.J.; Grunvald, E.L.; Curtis, J.R.; Sandborn, W.J. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195123. [Google Scholar] [CrossRef] [Green Version]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Surmiak, M.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Danielak, A.; Ptak-Belowska, A.; et al. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules 2019, 9, 780. [Google Scholar] [CrossRef] [Green Version]
- Klopfenstein, B.J.; Kim, M.S.; Krisky, C.M.; Szumowski, J.; Rooney, W.D.; Purnell, J.Q. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br. J. Radiol. 2012, 85, e826–e830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.W.; Fu, S.H.; Dong, J.L.; Chien, M.W.; Liu, Y.W.; Hsu, C.Y.; Sytwu, H.K. Adipokine-Modulated Immunological Homeostasis Shapes the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 9564. [Google Scholar] [CrossRef]
- Bryant, R.V.; Schultz, C.G.; Ooi, S.; Goess, C.; Costello, S.P.; Vincent, A.D.; Schoeman, S.; Lim, A.; Bartholomeusz, F.D.; Travis, S.P.L.; et al. Visceral Adipose Tissue Is Associated With Stricturing Crohn’s Disease Behavior, Fecal Calprotectin, and Quality of Life. Inflamm. Bowel Dis. 2019, 25, 592–600. [Google Scholar] [CrossRef]
- Uko, V.; Vortia, E.; Achkar, J.P.; Karakas, P.; Fiocchi, C.; Worley, S.; Kay, M.H. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sloot, K.W.; Joshi, A.D.; Bellavance, D.R.; Gilpin, K.K.; Stewart, K.O.; Lochhead, P.; Garber, J.J.; Giallourakis, C.; Yajnik, V.; Ananthakrishnan, A.N.; et al. Visceral Adiposity, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Drouet, M.; Dubuquoy, L.; Desreumaux, P.; Bertin, B. Visceral fat and gut inflammation. Nutrition 2012, 28, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Chamaillard, M.; Gonzalez, F.; Beclin, E.; Decourcelle, C.; Antunes, L.; Gay, J.; Neut, C.; Colombel, J.F.; Desreumaux, P. Mesenteric fat in Crohn’s disease: A pathogenetic hallmark or an innocent bystander? Gut 2007, 56, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Gonzalez, F.; Dubuquoy, L.; Rousseaux, C.; Dubuquoy, C.; Decourcelle, C.; Saudemont, A.; Tachon, M.; Béclin, E.; Odou, M.F.; et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 2012, 61, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Solem, C.A.; Sandborn, W.J.; Booya, F.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Bodily, K.D.; Fletcher, J.G. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: Correlation with endoscopic severity and C reactive protein. Gut 2006, 55, 1561–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crohn, B.B.; Ginzburg, L.; Oppenheimer, G.D. Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Burril B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. JAMA 1984, 251, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Kurada, S.; Gordon, I.O.; Baker, M.E.; Gandhi, N.; McDonald, C.; Coffey, J.C.; Rieder, F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, R.; Doyon, G.; Gordon, I.O.; Li, J.; Lin, S.; Wang, J.; Le, T.H.N.; Elias, M.; Kurada, S.; Southern, B.; et al. Activated intestinal muscle cells promote preadipocyte migration: A novel mechanism for creeping fat formation in Crohn’s disease. Gut 2021. [Google Scholar] [CrossRef]
- Sheehan, A.L.; Warren, B.F.; Gear, M.W.; Shepherd, N.A. Fat-wrapping in Crohn’s disease: Pathological basis and relevance to surgical practice. Br. J. Surg. 1992, 79, 955–958. [Google Scholar] [CrossRef]
- Kredel, L.I.; Jödicke, L.J.; Scheffold, A.; Gröne, J.; Glauben, R.; Erben, U.; Kühl, A.A.; Siegmund, B. T-cell Composition in Ileal and Colonic Creeping Fat—Separating Ileal from Colonic Crohn’s Disease. J. Crohn’s Colitis 2019, 13, 79–91. [Google Scholar] [CrossRef]
- Mattacks, C.A.; Sadler, D.; Pond, C.M. The effects of dietary lipids on dendritic cells in perinodal adipose tissue during chronic mild inflammation. Br. J. Nutr. 2004, 91, 883–892. [Google Scholar] [CrossRef]
- Harvey, N.L.; Srinivasan, R.S.; Dillard, M.E.; Johnson, N.C.; Witte, M.H.; Boyd, K.; Sleeman, M.W.; Oliver, G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005, 37, 1072–1081. [Google Scholar] [CrossRef]
- Heatley, R.V.; Bolton, P.M.; Hughes, L.E.; Owen, E.W. Mesenteric lymphatic obstruction in Crohn’s disease. Digestion 1980, 20, 307–313. [Google Scholar] [CrossRef]
- von der Weid, P.Y.; Rainey, K.J. Review article: Lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Aliment Pharmacol. Ther. 2010, 32, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Guedj, K.; Abitbol, Y.; Cazals-Hatem, D.; Morvan, M.; Maggiori, L.; Panis, Y.; Bouhnik, Y.; Caligiuri, G.; Corcos, O.; Nicoletti, A. Adipocytes orchestrate the formation of tertiary lymphoid organs in the creeping fat of Crohn’s disease affected mesentery. J. Autoimmun. 2019, 103, 102281. [Google Scholar] [CrossRef]
- da Silva, F.A.R.; Pascoal, L.B.; Dotti, I.; Setsuko Ayrizono, M.L.; Aguilar, D.; Rodrigues, B.L.; Arroyes, M.; Ferrer-Picon, E.; Milanski, M.; Velloso, L.A.; et al. Whole transcriptional analysis identifies markers of B, T and plasma cell signaling pathways in the mesenteric adipose tissue associated with Crohn’s disease. J. Transl. Med. 2020, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A. Relationship(s) between obesity and inflammatory bowel diseases: Possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 2020, 13, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Fan, X.; Ying, J.; Chen, S. Emerging trends and research foci in gastrointestinal microbiome. J. Trans Med. 2019. [Google Scholar] [CrossRef]
- Zulian, A.; Cancello, R.; Ruocco, C.; Gentilini, D.; Di Blasio, A.M.; Danelli, P.; Micheletto, G.; Cesana, E.; Invitti, C. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn’s disease. An in vivo and in vitro study. PLoS ONE 2013, 8, e78495. [Google Scholar] [CrossRef]
- Kiernan, M.G.; Coffey, J.C.; McDermott, K.; Cotter, P.D.; Cabrera-Rubio, R.; Kiely, P.A.; Dunne, C.P. The Human Mesenteric Lymph Node Microbiome Differentiates between Crohn’s Disease and Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 58–66. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Kreuter, R.; Wankell, M.; Ahlenstiel, G.; Hebbard, L. The role of obesity in inflammatory bowel disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Queipo-Ortuño, M.; Millan, M.; Sanchez-Alcoholado, L.; Caro, A.; Espina, B.; Menacho, M.; Bautista, M.; Monfort-Ferré, D.; Terrón-Puig, M.; et al. Microbial Signature in Adipose Tissue of Crohn’s Disease Patients. J. Clin. Med. 2020, 9, 2448. [Google Scholar] [CrossRef] [PubMed]
- Anty, R.; Bekri, S.; Luciani, N.; Saint-Paul, M.C.; Dahman, M.; Iannelli, A.; Amor, I.B.; Staccini-Myx, A.; Huet, P.M.; Gugenheim, J.; et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am. J. Gastroenterol. 2006, 101, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Schölmerich, J. Innate immunity and adipose tissue biology. Trends Immunol. 2010, 31, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, T.; Schaeffler, A. Adipokines and the role of visceral adipose tissue in inflammatory bowel disease. Ann. Gastroenterol. 2016, 29, 424–438. [Google Scholar] [CrossRef]
- Bertin, B.; Desreumaux, P.; Dubuquoy, L. Obesity, visceral fat and Crohn’s disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 574–580. [Google Scholar] [CrossRef]
- Batra, A.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Glauben, R.; Kunkel, D.; Scheffold, A.; Erben, U.; Kühl, A.; Loddenkemper, C.; et al. Mesenteric fat—control site for bacterial translocation in colitis? Mucosal. Immunol. 2012, 5, 580–591. [Google Scholar] [CrossRef] [Green Version]
- Goldmannova, D.; Spurna, J.; Krystynik, O.; Schovanek, J.; Cibickova, L.; Karasek, D.; Zadražil, J. Adipocytokines and new onset diabetes mellitus after transplantation. J. Appl. Biomed. 2018, 16, 247–254. [Google Scholar] [CrossRef]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and Their Role in Intestinal Inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Spurná, J.; Karásek, D.; Kubíčková, V.; Goldmannová, D.; Krystyník, O.; Schovánek, J.; Zadražil, J. Relationship of Selected Adipokines with Markers of Vascular Damage in Patients with Type 2 Diabetes. Metab. Syndr. Relat. Disord. 2018, 16, 246–253. [Google Scholar] [CrossRef]
- Rodrigues, V.S.; Milanski, M.; Fagundes, J.J.; Torsoni, A.S.; Ayrizono, M.L.; Nunez, C.E.; Dias, C.B.; Meirelles, L.R.; Dalal, S.; Coy, C.S.; et al. Serum levels and mesenteric fat tissue expression of adiponectin and leptin in patients with Crohn’s disease. Clin. Exp. Immunol. 2012, 170, 358–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Kiyohara, T.; Murayama, Y.; Kihara, S.; Okamoto, Y.; Funahashi, T.; Ito, T.; Nezu, R.; Tsutsui, S.; Miyagawa, J.I.; et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut 2005, 54, 789–796. [Google Scholar] [CrossRef]
- Valentini, L.; Wirth, E.K.; Schweizer, U.; Hengstermann, S.; Schaper, L.; Koernicke, T.; Dietz, E.; Norman, K.; Buning, C.; Winklhofer-Roob, B.M.; et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition 2009, 25, 172–181. [Google Scholar] [CrossRef]
- Kahraman, R.; Calhan, T.; Sahin, A.; Ozdil, K.; Caliskan, Z.; Bireller, E.S.; Cakmakoglu, B. Are adipocytokines inflammatory or metabolic mediators in patients with inflammatory bowel disease? Ther. Clin. Risk Manag. 2017, 13, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 100–105. [Google Scholar] [CrossRef]
- Weigert, J.; Obermeier, F.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Aslanidis, C.; Rogler, G.; Ott, C.; Schäffler, A.; et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Waluga, M.; Hartleb, M.; Boryczka, G.; Kukla, M.; Zwirska-Korczala, K. Serum adipokines in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6912–6917. [Google Scholar] [CrossRef]
- Chouliaras, G.; Panayotou, I.; Margoni, D.; Mantzou, E.; Pervanidou, P.; Manios, Y.; Chrousos, G.P.; Roma, E. Circulating leptin and adiponectin and their relation to glucose metabolism in children with Crohn’s disease and ulcerative colitis. Pediatr. Res. 2013, 74, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Ortega Moreno, L.; Sanz-Garcia, A.; Fernández de la Fuente, M.J.; Arroyo Solera, R.; Fernández-Tomé, S.; Marin, A.C.; Mora-Gutierrez, I.; Fernández, P.; Baldan-Martin, M.; Chaparro, M.; et al. Serum adipokines as non-invasive biomarkers in Crohn’s disease. Sci. Rep. 2020, 10, 18027. [Google Scholar] [CrossRef]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Kouroumalis, E.A. The effect of infliximab on circulating levels of leptin, adiponectin and resistin in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2007, 19, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Wulster-Radcliffe, M.C.; Ajuwon, K.M.; Wang, J.; Christian, J.A.; Spurlock, M.E. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem. Biophys. Res. Commun. 2004, 316, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Beales, I.L. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul. Pept. 2006, 134, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sitaraman, S.; Liu, X.; Charrier, L.; Gu, L.H.; Ziegler, T.R.; Gewirtz, A.; Merlin, D. Colonic leptin: Source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004, 18, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, N.P.; Guan, H.; Busbee, B.; Price, R.L.; Taub, D.D.; Mishra, M.K.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. The emerging role of leptin antagonist as potential therapeutic option for inflammatory bowel disease. Int. Rev. Immunol. 2014, 33, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Barbier, M.; Vidal, H.; Desreumaux, P.; Dubuquoy, L.; Bourreille, A.; Colombel, J.F.; Cherbut, C.; Galmiche, J.P. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol. Clin. Biol. 2003, 27, 987–991. [Google Scholar] [CrossRef]
- Aurangzeb, B.; Leach, S.T.; Lemberg, D.A.; Day, A.S. Assessment of nutritional status and serum leptin in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 536–541. [Google Scholar] [CrossRef]
- Biesiada, G.; Czepiel, J.; Ptak-Belowska, A.; Targosz, A.; Krzysiek-Maczka, G.; Strzalka, M.; Konturek, S.J.; Brzozowski, T.; Mach, T. Expression and release of leptin and proinflammatory cytokines in patients with ulcerative colitis and infectious diarrhea. J. Physiol. Pharmacol. 2012, 63, 471–481. [Google Scholar]
- Tuzun, A.; Uygun, A.; Yesilova, Z.; Ozel, A.M.; Erdil, A.; Yaman, H.; Bagci, S.; Gulsen, M.; Karaeren, N.; Dagalp, K. Leptin levels in the acute stage of ulcerative colitis. J. Gastroenterol. Hepatol. 2004, 19, 429–432. [Google Scholar] [CrossRef]
- Nishi, Y.; Isomoto, H.; Ueno, H.; Ohnita, K.; Wen, C.Y.; Takeshima, F.; Mishima, R.; Nakazato, M.; Kohno, S. Plasma leptin and ghrelin concentrations in patients with Crohn’s disease. World J. Gastroenterol. 2005, 11, 7314–7317. [Google Scholar] [CrossRef]
- Hoppin, A.G.; Kaplan, L.M.; Zurakowski, D.; Leichtner, A.M.; Bousvaros, A. Serum leptin in children and young adults with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 500–505. [Google Scholar] [CrossRef]
- Trejo-Vazquez, F.; Garza-Veloz, I.; Villela-Ramirez, G.A.; Ortiz-Castro, Y.; Mauricio-Saucedo, P.; Cardenas-Vargas, E.; Diaz-Baez, M.; Cid-Baez, M.A.; Castañeda-Miranda, R.; Ortiz-Rodriguez, J.M.; et al. Positive association between leptin serum levels and disease activity on endoscopy in inflammatory bowel disease: A case-control study. Exp. Ther. Med. 2018, 15, 3336–3344. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Böttcher, C.; Letizia, M.; Yerinde, C.; Wu, H.; Freise, I.; Rodriguez-Sillke, Y.; Stoyanova, A.K.; Kreis, M.E.; Asbach, P.; et al. Leptin induces TNFα-dependent inflammation in acquired generalized lipodystrophy and combined Crohn’s disease. Nat. Commun. 2019, 10, 5629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaser, S.; Kaser, A.; Sandhofer, A.; Ebenbichler, C.F.; Tilg, H.; Patsch, J.R. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem. Biophys. Res. Commun. 2003, 309, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Lehrke, M.; Schachinger, V.; Seibold, F.; Stark, R.; Ochsenkühn, T.; Parhofer, K.G.; Göke, B.; Broedl, U.C. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur. J. Gastroenterol. Hepatol. 2007, 19, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, X.; Yue, W.; Xu, X.; Li, B.; Zou, L.; He, R. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell Mol. Immunol. 2014, 11, 355–366. [Google Scholar] [CrossRef]
- Terzoudis, S.; Malliaraki, N.; Damilakis, J.; Dimitriadou, D.A.; Zavos, C.; Koutroubakis, I.E. Chemerin, visfatin, and vaspin serum levels in relation to bone mineral density in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 814–819. [Google Scholar] [CrossRef]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, T.J.; Lee, W.J.; Chuang, L.M. The relationship of visfatin/pre-B-cell colony-enhancing factor/nicotinamide phosphoribosyltransferase in adipose tissue with inflammation, insulin resistance, and plasma lipids. Metabolism 2010, 59, 93–99. [Google Scholar] [CrossRef]
- Dogan, S.; Guven, K.; Celikbilek, M.; Deniz, K.; Saraymen, B.; Gursoy, S. Serum Visfatin Levels in Ulcerative Colitis. J. Clin. Lab. Anal. 2016, 30, 552–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starr, A.E.; Deeke, S.A.; Ning, Z.; Chiang, C.K.; Zhang, X.; Mottawea, W.; Singleton, R.; Benchimol, E.I.; Wen, M.; Mack, D.R.; et al. Proteomic analysis of ascending colon biopsies from a paediatric inflammatory bowel disease inception cohort identifies protein biomarkers that differentiate Crohn’s disease from UC. Gut 2017, 66, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Li, M.Z.; Xu, H.; Wang, Q.; Song, J.; Lin, P.; Zhang, L.; Liu, Q.; Huang, Q.X.; et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin. Med. J. 2012, 125, 3440–3444. [Google Scholar]
- Han, S.; Wang, G.; Qiu, S.; de la Motte, C.; Wang, H.Q.; Gomez, G.; Englander, E.W.; Greeley, G.H., Jr. Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul. Pept. 2007, 142, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Masoud, A.G.; Lin, J.; Azad, A.K.; Farhan, M.A.; Fischer, C.; Zhu, L.F.; Zhang, H.; Sis, B.; Kassiri, Z.; Moore, R.B.; et al. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J. Clin. Investig. 2020, 130, 94–107. [Google Scholar] [CrossRef]
- Kwon, H.B.; Wang, S.; Helker, C.S.; Rasouli, S.J.; Maischein, H.M.; Offermanns, S.; Herzog, W.; Stainier, D.Y. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nat. Commun. 2016, 7, 11805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berta, J.; Hoda, M.A.; Laszlo, V.; Rozsas, A.; Garay, T.; Torok, S.; Grusch, M.; Berger, W.; Paku, S.; Renyi-Vamos, F.; et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget 2014, 5, 4426–4437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawane, M.; Kidoya, H.; Muramatsu, F.; Takakura, N.; Kajiya, K. Apelin attenuates UVB-induced edema and inflammation by promoting vessel function. Am. J. Pathol. 2011, 179, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Tiaka, E.K.; Manolakis, A.C.; Kapsoritakis, A.N.; Potamianos, S.P. Unraveling the link between leptin, ghrelin and different types of colitis. Ann. Gastroenterol. 2011, 24, 20–28. [Google Scholar] [PubMed]
- Peracchi, M.; Bardella, M.T.; Caprioli, F.; Massironi, S.; Conte, D.; Valenti, L.; Ronchi, C.; Beck-Peccoz, P.; Arosio, M.; Piodi, L. Circulating ghrelin levels in patients with inflammatory bowel disease. Gut 2006, 55, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Ates, Y.; Degertekin, B.; Erdil, A.; Yaman, H.; Dagalp, K. Serum ghrelin levels in inflammatory bowel disease with relation to disease activity and nutritional status. Dig. Dis. Sci. 2008, 53, 2215–2221. [Google Scholar] [CrossRef]
- Ghomraoui, F.A.; Alotaibi, S.T.; Alharthi, M.A.; Asiri, S.S.; Almadi, M.A.; Alharbi, O.R.; Azzam, N.A.; Aljebreen, A.M.; Saeed, M.; Hajkhder, B.; et al. Plasma ghrelin and leptin in patients with inflammatory bowel disease and its association with nutritional status. Saudi J. Gastroenterol. 2017, 23, 199–205. [Google Scholar] [CrossRef]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, L.; Liu, L.; Feng, Y.; Lu, L.; Ren, X.; Dong, X.; Sang, W. Serum omentin-1 as a disease activity marker for Crohn’s disease. Dis. Markers 2014, 2014, 162517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabesh, M.; Noroozi, A.; Amini, M.; Feizi, A.; Saraf-Bank, S.; Zare, M. Association of retinol-binding protein 4 with metabolic syndrome in first-degree relatives of type 2 diabetic patients. J. Res. Med. Sci. 2017, 22, 28. [Google Scholar]
- Roma, E.; Krini, M.; Hantzi, E.; Sakka, S.; Panayiotou, I.; Margeli, A.; Papassotiriou, I.; Kanaka-Gantenbein, C. Retinol Binding Protein 4 in children with Inflammatory Bowel Disease: A negative correlation with the disease activity. Hippokratia 2012, 16, 360–365. [Google Scholar]
- Gholamrezayi, A.; Mohamadinarab, M.; Rahbarinejad, P.; Fallah, S.; Barez, S.R.; Setayesh, L.; Moradi, N.; Fadaei, R.; Chamani, E.; Tavakoli, T. Characterization of the serum levels of Meteorin-like in patients with inflammatory bowel disease and its association with inflammatory cytokines. Lipids Health Dis. 2020, 19, 230. [Google Scholar] [CrossRef]
- Arsenescu, V.; Narasimhan, M.L.; Halide, T.; Bressan, R.A.; Barisione, C.; Cohen, D.A.; de Villiers, W.J.; Arsenescu, R. Adiponectin and plant-derived mammalian adiponectin homolog exert a protective effect in murine colitis. Dig. Dis. Sci. 2011, 56, 2818–2832. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, T.; Matsuda, M.; Araki, H.; Oshima, K.; Kihara, S.; Funahashi, T.; Shimomura, I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 2006, 131, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Chumanevich, A.; Fletcher, E.; Larsen, B.; Lattwein, K.; Kaur, K.; Fayad, R. Adiponectin deficiency: Role in chronic inflammation induced colon cancer. Biochim. Biophys. Acta 2012, 1822, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.J.; Shen, T.L.; Chen, Y.S.; Mersmann, H.J.; Liu, B.H.; Ding, S.T. Adiponectin and adiponectin receptor 1 overexpression enhance inflammatory bowel disease. J. Biomed. Sci. 2018, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, N.P.; Guan, H.; Busbee, B.; Price, R.L.; Taub, D.D.; Mishra, M.K.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Leptin antagonist ameliorates chronic colitis in IL-10−/− mice. Immunobiology 2013, 218, 1439–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Li, Y.; Chen, Q.; Zhu, W.; Zuo, L.; Guo, Z.; Gong, J.; Cao, L.; Gu, L.; Li, J. Adipokine apelin ameliorates chronic colitis in Il-10-/- mice by promoting intestinal lymphatic functions. Biochem. Pharmacol. 2018, 148, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Gerner, R.R.; Klepsch, V.; Macheiner, S.; Arnhard, K.; Adolph, T.E.; Grander, C.; Wieser, V.; Pfister, A.; Moser, P.; Hermann-Kleiter, N.; et al. NAD metabolism fuels human and mouse intestinal inflammation. Gut 2018, 67, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Franchimont, D.; Roland, S.; Gustot, T.; Quertinmont, E.; Toubouti, Y.; Gervy, M.C.; Deviere, J.; Van Gossum, A. Impact of infliximab on serum leptin levels in patients with Crohn’s disease. J. Clin. Endocrinol. Metab. 2005, 90, 3510–3516. [Google Scholar] [CrossRef] [Green Version]
- Frivolt, K.; Schwerd, T.; Schatz, S.B.; Freudenberg, F.; Prell, C.; Werkstetter, K.J.; Bufler, P.; Koletzko, S. Hyperadiponectinemia During Infliximab Induction Therapy in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 915–919. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaskova, E.; Velganova-Veghova, M.; Geryk, M.; Foltenova, H.; Kucerova, V.; Karasek, D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 4226. https://doi.org/10.3390/ijms22084226
Karaskova E, Velganova-Veghova M, Geryk M, Foltenova H, Kucerova V, Karasek D. Role of Adipose Tissue in Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2021; 22(8):4226. https://doi.org/10.3390/ijms22084226
Chicago/Turabian StyleKaraskova, Eva, Maria Velganova-Veghova, Milos Geryk, Hana Foltenova, Veronika Kucerova, and David Karasek. 2021. "Role of Adipose Tissue in Inflammatory Bowel Disease" International Journal of Molecular Sciences 22, no. 8: 4226. https://doi.org/10.3390/ijms22084226





