Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far
Abstract
:1. Introduction
2. Immunological Regulation in Healthy Pregnancy and Its Dysregulation in GDM
2.1. Innate Immune Response
2.1.1. Neutrophils
2.1.2. NK and NKT Cells
2.1.3. Monocytes
2.1.4. Macrophages
2.1.5. Dendritic Cells
2.1.6. Platelets
2.2. Adaptive Immune Response
2.2.1. T Cells
2.2.2. B Cells
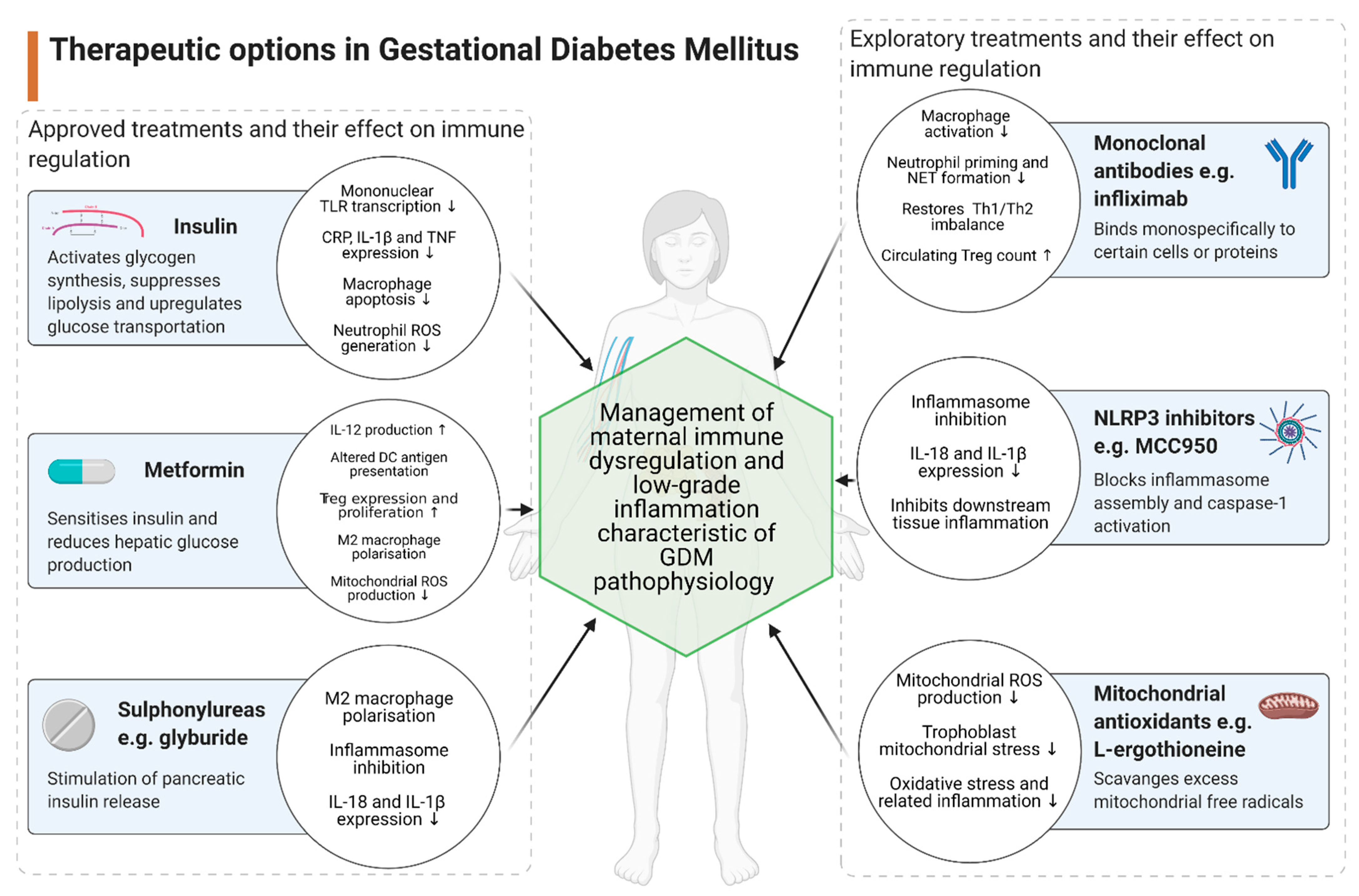
3. Therapeutic Implications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GDM | gestational diabetes mellitus |
| NK cell | natural killer cell |
| DC | dendritic cell |
| Treg | regulatory T cell |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| IL | interleukin |
| TGF | transforming growth factor |
| cAMP | cyclic adenosine monophosphate |
| dNK cell | decidual NK cell |
| Th17 | T-helper 17 |
| PE | pre-eclampsia |
| NET | neutrophil extracellular trap |
| SGA | small for gestational age |
| NCR+ILC3 | natural cytotoxic receptors+ group 3 innate lymphoid cells |
| IFN | interferon |
| TCR | T cell receptor |
| APC | antigen-presenting cell |
| HLA | human leukocyte antigen |
| VEGF | vascular endothelial growth factor |
| EVT | extravillous trophoblast cell |
| HBC | Hofbauer cell |
| MPS | mononuclear phagocyte system |
| mDC | myeloid DC |
| Th1 | T-helper 1 |
| Th2 | T-helper 2 |
| pDC | plasmacytoid DC |
| Tc | cytotoxic T cell |
| IC | intracellular |
| TNF | tumour necrosis factor |
| EC | extracellular |
| Breg | regulatory B cell |
| TXA2 | thromboxane A2 |
| TNF | tumour necrosis factor |
| EC | extracellular |
| Breg | regulatory B cell |
| TXA2 | thromboxane A2 |
| CCL5 | chemokine ligand 5 |
| CXCL4 | chemokine ligand 4 |
| PDGF | platelet-derived growth factor |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| NLR | neutrophil-to-lymphocyte ratio |
| DNI | delta neutrophil index |
| TLR | Toll-like receptor |
| MLR | monocyte-lymphocyte ratio |
| MCP-1 | monocyte chemoattractant protein-1 |
| MIF | macrophage migration-inhibitory factor |
| CRP | C-reactive protein |
| T2DM | type 2 diabetes mellitus |
| PLR | platelet to lymphocyte ratio |
| MPV | mean platelet volume |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| IRS1 | insulin receptor substrate 1 |
| GLUT4 | glucose transporter type 4 |
| FFA | free fatty acid |
| VAT | visceral adipose tissue |
| SAT | subcutaneous adipose tissue |
| LPS | lipopolysaccharide |
| ROS | reactive oxygen species |
| AGE | advanced glycation end product |
| AMPK | adenosine monophosphate-activated protein kinase |
| mTORC | mechanistic target of rapamycin complex 1 |
| mAb | monoclonal antibody |
| GC | glucocorticoid |
| IGF | insulin-like growth factor |
| DAMP | damage-associated molecular pattern |
| OCTN1 | organic cation transporter novel type 1 |
References
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billington, W.D. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to peter medawar. J. Reprod. Immunol. 2003, 60, 1–11. [Google Scholar] [CrossRef]
- Deshmukh, H.; Way, S.S. Immunological basis for recurrent fetal loss and pregnancy complications. Annu. Rev. Pathol. 2019, 14, 185–210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liao, X.; Kang, Y. Tregs: Where We Are and What Comes Next? Front. Immunol. 2017, 8, 1578. [Google Scholar] [CrossRef] [PubMed]
- Mjosberg, J.; Berg, G.; Ernerudh, J.; Ekerfelt, C. CD4+ CD25+ regulatory T cells in human pregnancy: Development of a Treg-MLC-ELISPOT suppression assay and indications of paternal specific Tregs. Immunology 2007, 120, 456–466. [Google Scholar] [CrossRef]
- Fu, B.; Li, X.; Sun, R.; Tong, X.; Ling, B.; Tian, Z.; Wei, H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2013, 110, E231–E240. [Google Scholar] [CrossRef] [Green Version]
- Kampmann, U.; Madsen, L.R.; Skajaa, G.O.; Iversen, D.S.; Moeller, N.; Ovesen, P. Gestational diabetes: A clinical update. World J. Diabetes 2015, 6, 1065–1072. [Google Scholar] [CrossRef]
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Almoros, A.; Hang, T.; Peiro, C.; Soriano-Guillen, L.; Egido, J.; Tunon, J.; Lorenzo, O. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc. Diabetol. 2019, 18, 140. [Google Scholar] [CrossRef]
- Sheu, A.; Chan, Y.; Ferguson, A.; Bakhtyari, M.B.; Hawke, W.; White, C.; Chan, Y.F.; Bertolino, P.J.; Woon, H.G.; Palendira, U.; et al. A proinflammatory CD4(+) T cell phenotype in gestational diabetes mellitus. Diabetologia 2018, 61, 1633–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daskalakis, G.; Marinopoulos, S.; Krielesi, V.; Papapanagiotou, A.; Papantoniou, N.; Mesogitis, S.; Antsaklis, A. Placental pathology in women with gestational diabetes. Acta Obstet. Gynecol. Scand. 2008, 87, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Edu, A.; Teodorescu, C.; Dobjanschi, C.G.; Socol, Z.Z.; Teodorescu, V.; Matei, A.; Albu, D.F.; Radulian, G. Placenta changes in pregnancy with gestational diabetes. Rom. J. Morphol. Embryol. 2016, 57, 507–512. [Google Scholar] [PubMed]
- Correa-Silva, S.; Alencar, A.P.; Moreli, J.B.; Borbely, A.U.; de, S. Lima, L.; Scavone, C.; Damasceno, D.C.; Rudge, M.V.C.; Bevilacqua, E.; Calderon, I.M.P. Hyperglycemia induces inflammatory mediators in the human chorionic villous. Cytokine 2018, 111, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.S.; Herrin, M.A.; Pitruzzello, M.C.; Mulla, M.J.; Werner, E.F.; Pettker, C.M.; Flannery, C.A.; Abrahams, V.M. Glucose and metformin modulate human first trimester trophoblast function: A model and potential therapy for diabetes-associated uteroplacental insufficiency. Am. J. Reprod. Immunol. 2015, 73, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Rocha, A.D.S.; Bernardi, J.R.; Matos, S.; Kretzer, D.C.; Schoffel, A.C.; Goldani, M.Z.; de Azevedo Magalhaes, J.A. Maternal visceral adipose tissue during the first half of pregnancy predicts gestational diabetes at the time of delivery—A cohort study. PLoS ONE 2020, 15, e0232155. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Catalano, P.M.; Nizielski, S.E.; Shao, J.; Preston, L.; Qiao, L.; Friedman, J.E. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: Relationship to FFA during pregnancy. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E522–E533. [Google Scholar] [CrossRef] [Green Version]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on neutrophil function in severe inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauvillain, C.; Delneste, Y.; Scotet, M.; Peres, A.; Gascan, H.; Guermonprez, P.; Barnaba, V.; Jeannin, P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood 2007, 110, 2965–2973. [Google Scholar] [CrossRef]
- Dabrowska, D.; Jablonska, E.; Garley, M.; Sawicka-Powierza, J.; Nowak, K. The Phenomenon of Neutrophil Extracellular Traps in Vascular Diseases. Arch. Immunol. Ther. Exp. 2018, 66, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, S.; Tripathi, A.K.; Mishra, S.; Amzarul, M.; Vaish, A.K. Physiological changes in hematological parameters during pregnancy. Indian J. Hematol. Blood Transfus. 2012, 28, 144–146. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Meng, F.; Zhao, H.; Yang, M.; Zhang, R.; Yu, Z.; Huang, X.; Ding, H.; Liu, J.; Zang, S. Elevated first-trimester neutrophil count is closely associated with the development of maternal gestational diabetes mellitus and adverse pregnancy outcomes. Diabetes 2020, 69, 1401–1410. [Google Scholar] [CrossRef]
- Aktulay, A.; Engin-Ustun, Y.; Ozkan, M.S.; Erkaya, S.; Kara, M.; Kaymak, O.; Danisman, N. Gestational diabetes mellitus seems to be associated with inflammation. Acta Clin. Croat. 2015, 54, 475–478. [Google Scholar]
- Yilmaz, H.; Celik, H.T.; Namuslu, M.; Inan, O.; Onaran, Y.; Karakurt, F.; Ayyildiz, A.; Bilgic, M.A.; Bavbek, N.; Akcay, A. Benefits of the neutrophil-to-lymphocyte ratio for the prediction of gestational diabetes mellitus in pregnant women. Exp. Clin. Endocrinol. Diabetes 2014, 122, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Lou, X.; Zhang, Z.; Chai, Y.; Yu, Q. Association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume with the risk of gestational diabetes mellitus. Gynecol. Endocrinol. 2020, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, C.; Ma, Q.; Long, Y.; Cheng, Z. Variations of blood cells in prediction of gestational diabetes mellitus. J. Perinat. Med. 2015, 43, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Sahin Uysal, N.; Eroglu, H.; Ozcan, C.; Sahin, D.; Yucel, A. Is the serum delta neutrophil index level different in gestational diabetic women? J. Matern. Fetal. Neonatal. Med. 2020, 33, 3349–3354. [Google Scholar] [CrossRef] [PubMed]
- Stoikou, M.; Grimolizzi, F.; Giaglis, S.; Schafer, G.; van Breda, S.V.; Hoesli, I.M.; Lapaire, O.; Huhn, E.A.; Hasler, P.; Rossi, S.W.; et al. Gestational diabetes mellitus is associated with altered neutrophil activity. Front. Immunol. 2017, 8, 702. [Google Scholar] [CrossRef] [Green Version]
- Croxatto, D.; Micheletti, A.; Montaldo, E.; Orecchia, P.; LoIacono, F.; Canegallo, F.; Calzetti, F.; Fulcheri, E.; Munari, E.; Zamò, A.; et al. Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal. Immunol. 2016, 9, 1372–1383. [Google Scholar] [CrossRef] [Green Version]
- de Castro Pernet Hara, C.; Franca, E.L.; Fagundes, D.L.; de Queiroz, A.A.; Rudge, M.V.; Honorio-Franca, A.C.; de Mattos Paranhos Calderon, I. Characterization of natural killer cells and cytokines in maternal placenta and fetus of diabetic mothers. J. Immunol. Res. 2016, 2016, 7154524. [Google Scholar]
- Vokalova, L.; Van Breda, S.V.; Ye, X.L.; Huhn, E.A.; Than, N.G.; Hasler, P.; Lapaire, O.; Hoesli, I.; Rossi, S.W.; Hahn, S. Excessive neutrophil activity in gestational diabetes mellitus: Could it contribute to the development of preeclampsia? Front. Endocrinol. 2018, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Le Gars, M.; Seiler, C.; Kay, A.W.; Bayless, N.L.; Starosvetsky, E.; Moore, L.; Shen-Orr, S.S.; Aziz, N.; Khatri, P.; Dekker, C.L.; et al. Pregnancy-induced alterations in NK cell phenotype and function. Front. Immunol. 2019, 10, 2469. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Oh, S.; Lim, S.; Shin, J.H.; Yoon, M.S.; Park, S.H. Invariant NKT cells facilitate cytotoxic T-cell activation via direct recognition of CD1d on T cells. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [Green Version]
- de Andres, C.; Fernandez-Paredes, L.; Tejera-Alhambra, M.; Alonso, B.; Ramos-Medina, R.; Sanchez-Ramon, S. Activation of blood CD3(+)CD56(+)CD8(+) T cells during pregnancy and multiple sclerosis. Front. Immunol. 2017, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Boyson, J.E.; Rybalov, B.; Koopman, L.A.; Exley, M.; Balk, S.P.; Racke, F.K.; Schatz, F.; Masch, R.; Wilson, S.B.; Strominger, J.L. CD1d and invariant NKT cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2002, 99, 13741–13746. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Karasawa, M.; Kawano, T.; Akasaka, T.; Koseki, H.; Akutsu, Y.; Kondo, E.; Sekiya, S.; Sekikawa, K.; Harada, M.; et al. Involvement of decidual Valpha14 NKT cells in abortion. Proc. Natl. Acad. Sci. USA 2000, 97, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, H.; Sakai, M.; Michimata, T.; Tanebe, K.; Hayakawa, S.; Saito, S. Characterization of NKT cells in human peripheral blood and decidual lymphocytes. Am. J. Reprod. Immunol. 2001, 45, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Southcombe, J.; Redman, C.; Sargent, I. Peripheral blood invariant natural killer T cells throughout pregnancy and in preeclamptic women. J. Reprod. Immunol. 2010, 87, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Lobo, T.F.; Borges, C.M.; Mattar, R.; Gomes, C.P.; de Angelo, A.G.S.; Pendeloski, K.P.T.; Daher, S. Impaired Treg and NK cells profile in overweight women with gestational diabetes mellitus. Am. J. Reprod. Immunol. 2018, 79. [Google Scholar] [CrossRef]
- Chiba, H.; Fukui, A.; Fuchinoue, K.; Funamizu, A.; Tanaka, K.; Mizunuma, H. Expression of natural cytotoxicity receptors on and intracellular cytokine production by NK cells in women with gestational diabetes mellitus. Am. J. Reprod. Immunol. 2016, 75, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.H.; Wang, D.P.; Zhang, L.L.; Zhang, F.; Wang, D.M.; Zhang, W.Y. Genomic expression profiles of blood and placenta reveal significant immune-related pathways and categories in Chinese women with gestational diabetes mellitus. Diabet Med. 2011, 28, 237–246. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Van Kaer, L. Contribution of lipid-reactive natural killer T cells to obesity-associated inflammation and insulin resistance. Adipocyte 2013, 2, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, F.; Abul, H.; Dashti, A.; Al-Jassar, W.; Omu, A. Trace elements and cell-mediated immunity in gestational and pre-gestational diabetes mellitus at third trimester of pregnancy. Acta Med. Acad. 2012, 41, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendeloski, K.P.; Mattar, R.; Torloni, M.R.; Gomes, C.P.; Alexandre, S.M.; Daher, S. Immunoregulatory molecules in patients with gestational diabetes mellitus. Endocrine 2015, 50, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Yougbare, I.; Tai, W.S.; Zdravic, D.; Oswald, B.E.; Lang, S.; Zhu, G.; Leong-Poi, H.; Qu, D.; Yu, L.; Dunk, C.; et al. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat. Commun. 2017, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef]
- Sojka, D.K.; Yang, L.; Plougastel-Douglas, B.; Higuchi, D.A.; Croy, B.A.; Yokoyama, W.M. Cutting edge: Local proliferation of uterine tissue-resident NK cells during decidualization in mice. J. Immunol. 2018, 201, 2551–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and During Viral Infection. Front. Immunol. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Sampath, P.; Moideen, K.; Ranganathan, U.D.; Bethunaickan, R. Monocyte subsets: Phenotypes and function in tuberculosis infection. Front. Immunol. 2018, 9, 1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ. Transplant. 2016, 21, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Melgert, B.N.; Spaans, F.; Borghuis, T.; Klok, P.A.; Groen, B.; Bolt, A.; de Vos, P.; van Pampus, M.G.; Wong, T.Y.; van Goor, H.; et al. Pregnancy and preeclampsia affect monocyte subsets in humans and rats. PLoS ONE 2012, 7, e45229. [Google Scholar] [CrossRef] [Green Version]
- Luppi, P.; Haluszczak, C.; Betters, D.; Richard, C.A.; Trucco, M.; DeLoia, J.A. Monocytes are progressively activated in the circulation of pregnant women. J. Leukoc. Biol. 2002, 72, 874–884. [Google Scholar]
- Angelo, A.G.S.; Neves, C.T.C.; Lobo, T.F.; Godoy, R.V.C.; Ono, E.; Mattar, R.; Daher, S. Monocyte profile in peripheral blood of gestational diabetes mellitus patients. Cytokine 2018, 107, 79–84. [Google Scholar] [CrossRef]
- Shive, C.L.; Jiang, W.; Anthony, D.D.; Lederman, M.M. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015, 29, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Q.W.; Cheng, X.Y.; Sha, C.X.; Cui, Y.B. Clinical significance of neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in women with hyperglycemia. Postgrad. Med. 2020, 132, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Telejko, B.; Kuzmicki, M.; Zonenberg, A.; Niedziolko-Bagniuk, K.; Nikolajuk, A.; Szamatowicz, J.; Gorska, M. Circulating monocyte chemoattractant protein-1 in women with gestational diabetes. Folia Histochem. Cytobiol. 2007, 45 (Suppl. 1), S153–S156. [Google Scholar]
- Klein, K.; Satler, M.; Elhenicky, M.; Brix, J.; Krzyzanowska, K.; Schernthaner, G.; Husslein, P.W.; Schernthaner, G.H. Circulating levels of MCP-1 are increased in women with gestational diabetes. Prenat. Diagn. 2008, 28, 845–851. [Google Scholar] [CrossRef]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 2018, 9, 17937–17950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the polarization of tumor-associated macrophages Into M1 and M2 phenotypes in human cancer tissue: Technicalities and challenges in routine clinical practice. Front. Oncol. 2019, 9, 1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [Green Version]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two unique human decidual macrophage populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal–maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, H. Macrophage subsets at the maternal-fetal interface. Cell. Mol. Immunol. 2020, 17, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Zulu, M.Z.; Martinez, F.O.; Gordon, S.; Gray, C.M. The elusive role of placental macrophages: The hofbauer cell. J. Innate Immun. 2019, 11, 447–456. [Google Scholar] [CrossRef]
- Reyes, L.; Golos, T.G. Hofbauer cells: Their role in healthy and complicated pregnancy. Front. Immunol. 2018, 9, 2628. [Google Scholar] [CrossRef] [Green Version]
- Dige, A.; Støy, S.; Thomsen, K.L.; Hvas, C.L.; Agnholt, J.; Dahlerup, J.F.; Møller, H.J.; Grønbaek, H.; Grønbæk, H. Soluble CD163, a specific macrophage activation marker, is Decreased by Anti-TNF-αAntibody treatment in active inflammatory bowel disease. Scand. J. Immunol. 2014, 80, 417–423. [Google Scholar] [CrossRef]
- Ueland, T.; Michelsen, A.E.; Aukrust, P.; Henriksen, T.; Bollerslev, J.; Lekva, T. Adipokines and macrophage markers during pregnancy–possible role for sCD163 in prediction and progression of gestational diabetes mellitus. Diabetes Metab. Res. Rev. 2018, 35, e3114. [Google Scholar] [CrossRef]
- Luo, N.; Liu, J.; Chung, B.H.; Yang, Q.; Klein, R.L.; Garvey, W.T.; Fu, Y. Macrophage Adiponectin Expression Improves Insulin Sensitivity and Protects Against Inflammation and Atherosclerosis. Diabetes 2010, 59, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Bari, M.F.; Weickert, M.O.; Sivakumar, K.; James, S.G.; Snead, D.R.J.; Tan, B.K.; Randeva, H.S.; Bastie, C.C.; Vatish, M. Elevated soluble CD163 in gestational diabetes mellitus: Secretion from human placenta and adipose tissue. PLoS ONE 2014, 9, e101327. [Google Scholar] [CrossRef]
- Yilmaz, O.; Kucuk, M.; Kebapcilar, L.; Altındag, T.; Yuksel, A.; Yuvanç, H.O.; Dal, T.; Savran, Y.; Altindag, T. Macrophage migration-inhibitory factor is elevated in pregnant women with gestational diabetes mellitus. Gynecol. Endocrinol. 2012, 28, 76–79. [Google Scholar] [CrossRef]
- Calandra, T.; Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef]
- Aslani, S.; Hossein-Nezhad, A.; Maghbooli, Z.; Mirzaei, K.; Karimi, F. Genetic variation in macrophage migration inhibitory factor associated with gestational diabetes mellitus and metabolic syndrome. Horm. Metab. Res. 2011, 43, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Li, C.; Chen, J.; Yu, S.; Gao, Q.; Wang, Y.; Liu, S. Association between macrophage migration inhibitory factor rs1007888 and GDM. Genet. Mol. Res. 2015, 14, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Schliefsteiner, C.; Peinhaupt, M.; Kopp, S.; Lögl, J.; Lang-Olip, I.; Hiden, U.; Heinemann, A.; Desoye, G.; Wadsack, C. Human placental hofbauer cells maintain an anti-inflammatory m2 phenotype despite the presence of gestational diabetes mellitus. Front. Immunol. 2017, 8, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barke, T.L.; Goldstein, J.A.; Sundermann, A.C.; Reddy, A.P.; Linder, J.E.; Correa, H.; Velez-Edwards, D.R.; Aronoff, D.M. Gestational diabetes mellitus is associated with increased CD163 expression and iron storage in the placenta. Am. J. Reprod. Immunol. 2018, 80, e13020. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Y.; Gui, J.; Li, A.-Z.; Su, X.-L.; Feng, L. Assessment of the number and function of macrophages in the placenta of gestational diabetes mellitus patients. Acta Acad. Med. Wuhan 2013, 33, 725–729. [Google Scholar] [CrossRef]
- Stirm, L.; Kovárová, M.; Perschbacher, S.; Michlmaier, R.; Fritsche, L.; Siegel-Axel, D.; Schleicher, E.; Peter, A.; Pauluschke-Fröhlich, J.; Brucker, S.; et al. BMI-Independent Effects of Gestational Diabetes on Human Placenta. J. Clin. Endocrinol. Metab. 2018, 103, 3299–3309. [Google Scholar] [CrossRef]
- Mrizak, I.; Grissa, O.; Henault, B.; Fekih, M.; Bouslema, A.; Boumaiza, I.; Zaouali, M.; Tabka, Z.; Khan, N.A. Placental infiltration of inflammatory markers in gestational diabetic women. Gen. Physiol. Biophys. 2014, 33, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Li, C.; Qi, W.H.; Qiao, B.L.; Zhao, H.; Zhou, Y.B.; Lu, C.X. Expression of macrophage migration inhibitory factor gene in placenta tissue and its correlation with gestational diabetes mellitus. Zhonghua Yi Xue Za Zhi 2017, 97, 3388–3391. [Google Scholar]
- Rogers, L.M.; Serezani, C.H.; Eastman, A.J.; Hasty, A.H.; Englund-Ögge, L.; Jacobsson, B.; Vickers, K.C.; Aronoff, D.M. Palmitate induces apoptotic cell death and inflammasome activation in human placental macrophages. Placenta 2020, 90, 45–51. [Google Scholar] [CrossRef]
- Harlev, A.; Aricha-Tamir, B.; Shaco-Levy, R.; Tarnovscki, T.; Bashan, N.; Rudich, A.; Sheiner, E.; Press, F.; Wiznitzer, A. Macrophage infiltration and stress-signaling in omental and subcutaneous adipose tissue in diabetic pregnancies. J. Matern. Neonatal Med. 2014, 27, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human dendritic cells: Their heterogeneity and clinical application potential in cancer immunotherapy. Front. Immunol. 2018, 9, 3176. [Google Scholar] [CrossRef]
- Bachy, V.; Williams, D.; Ibrahim, M. Altered dendritic cell function in normal pregnancy. J. Reprod. Immunol. 2008, 78, 11–21. [Google Scholar] [CrossRef]
- Shah, N.M.; Herasimtschuk, A.A.; Boasso, A.; Benlahrech, A.; Fuchs, D.; Imami, N.; Johnson, M.R. Changes in T cell and dendritic cell phenotype from mid to late pregnancy are indicative of a shift from immune tolerance to immune activation. Front. Immunol. 2017, 8, 1138. [Google Scholar] [CrossRef] [PubMed]
- Tagliani, E.; Erlebacher, A. Dendritic cell function at the maternal–fetal interface. Expert Rev. Clin. Immunol. 2011, 7, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Cappelletti, M.; Giannelli, S.; Martinelli, A.; Cetin, I.; Colombo, E.; Calcaterra, F.; Mavilio, D.; Della Bella, S. Lack of activation of peripheral blood dendritic cells in human pregnancies complicated by intrauterine growth restriction. Placenta 2013, 34, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-D.; Huang, L.-L.; Zhu, X.-M. An immunohistochemical study of CD83- and CD1a-positive dendritic cells in the decidua of women with recurrent spontaneous abortion. Eur. J. Med. Res. 2015, 20, 2. [Google Scholar] [CrossRef] [Green Version]
- Cornish, E.F.; Filipovic, I.; Åsenius, F.; Williams, D.J.; McDonnell, T. Innate Immune responses to acute viral infection during pregnancy. Front. Immunol. 2020, 11, 572567. [Google Scholar] [CrossRef]
- Friebe-Hoffmann, U.; Antony, L.; Kruessel, J.-S.; Pawlowski, B.; Hoffmann, T.K. Peripheral immunological cells in pregnant women and their change during diabetes. Exp. Clin. Endocrinol. Diabetes 2017, 125, 677–683. [Google Scholar] [CrossRef]
- Paccosi, S.; Pala, L.; Cresci, B.; Silvano, A.; Cecchi, M.; Caporale, R.; Rotella, C.M.; Parenti, A. Insulin resistance and obesity affect monocyte-derived dendritic cell phenotype and function. Diabetes Res. Clin. Pract. 2020, 170, 108528. [Google Scholar] [CrossRef]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef]
- Reese, J.A.; Peck, J.D.; Deschamps, D.R.; McIntosh, J.J.; Knudtson, E.J.; Terrell, D.R.; Vesely, S.K.; George, J.N. Platelet counts during pregnancy. N. Engl. J. Med. 2018, 379, 32–43. [Google Scholar] [CrossRef]
- Moser, G.; Guettler, J.; Forstner, D.; Gauster, M. Maternal platelets-friend or foe of the human placenta? Int. J. Mol. Sci. 2019, 20, 5639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, P.; Stefano, G.; Antonella, S.; Albana, C. Platelets in pregnancy. J. Prenat. Med. 2011, 5, 90–92. [Google Scholar] [PubMed]
- Sheu, J.R.; Hsiao, G.; Shen, M.Y.; Lin, W.Y.; Tzeng, C.R. The hyperaggregability of platelets from normal pregnancy is mediated through thromboxane A2 and cyclic AMP pathways. Clin. Lab. Haematol 2002, 24, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, P.F. Progesterone metabolites rapidly stimulate calcium influx in human platelets by a src-dependent pathway. Steroids 2008, 73, 738–750. [Google Scholar] [CrossRef]
- Babic, G.; Novokmet, S.; Jankovic, S. Changes of platelets’ function in preeclampsia. Open Med. 2011, 6, 696. [Google Scholar] [CrossRef]
- Sak, M.E.; Soydinc, H.E.; Ozler, A.; Evsen, M.S.; Turgut, A.; Sak, S.; Gul, T. Platelet profile in patients with gestational diabetes: A retrospective study. J. Turk. Gynecol. Assoc. 2012, 13, 223–226. [Google Scholar] [CrossRef]
- Blaschitz, A.; Siwetz, M.; Schlenke, P.; Gauster, M. Adhering maternal platelets can contribute to the cytokine and chemokine cocktail released by human first trimester villous placenta. Placenta 2015, 36, 1333–1336. [Google Scholar] [CrossRef] [Green Version]
- Morita, H.; Mizutori, M.; Takeuchi, K.; Motoyama, S.; Maruo, T. Abundant expression of platelet-derived growth factor in spiral arteries in decidua associated with pregnancy-induced hypertension and its relevance to atherosis. Eur. J. Endocrinol. 2001, 144, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Iyidir, O.T.; Degertekin, C.K.; Yilmaz, B.A.; Toruner, F.B.; Akturk, M.; Arslan, M. Elevated mean platelet volume is associated with gestational diabetes mellitus. Gynecol. Endocrinol. 2014, 30, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Datta, C.; Chowdhury, S.; Mandal, D.; Mondal, N.K.; Ghosh, A. Gestational diabetes mellitus in a tertiary care hospital of kolkata, India: Prevalence, pathogenesis and potential disease biomarkers. Exp. Clin. Endocrinol. Diabetes 2018, 128, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-M.; Li, Y.-Q.; Xie, S.-F.; Ma, L.-P.; Wu, Y.-D.; Nie, D.-N.; Feng, J.-H.; Xu, L.-Z. Study on the variation of platelet function in pregnancy induced hypertension and gestational diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi 2005, 40, 25–28. [Google Scholar] [PubMed]
- Gorar, S.; Abanonu, G.B.; Uysal, A.; Erol, O.; Unal, A.; Cekin, A.H.; Uyar, S. Comparison of thyroid function tests and blood count in pregnant women with versus without gestational diabetes mellitus. J. Obstet. Gynaecol. Res. 2017, 43, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Erikçi, A.A.; Muhçu, M.; Dündar, Ö.; Öztürk, A. Could mean platelet volume be a predictive marker for gestational diabetes mellitus? Hematology 2008, 13, 46–48. [Google Scholar] [CrossRef]
- Gur, E.B.; Karadeniz, M.; Genc, M.; Eskicioglu, F.; Yalcin, M.; Hepyilmaz, I.; Guclu, S. Relationship between mean platelet volume and vitamin D deficiency in gestational diabetes mellitus. Arch. Endocrinol. Metab. 2015, 59, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, N.; Yılmaz, E.; Biri, A.; Taner, Z.; Himmetoglu, O.; Yilmaz, E. The mean platelet volume in gestational diabetes. J. Thromb. Thrombolysis 2006, 22, 51–54. [Google Scholar] [CrossRef]
- Maconi, M.; Cardaropoli, S.; Cenci, A. Platelet parameters in healthy and pathological pregnancy. J. Clin. Lab. Anal. 2012, 26, 41–44. [Google Scholar] [CrossRef]
- Sahbaz, A.; Cicekler, H.; Aynioglu, O.; Isik, H.; Ozmen, U. Comparison of the predictive value of plateletcrit with various other blood parameters in gestational diabetes development. J. Obstet. Gynaecol. 2016, 36, 589–593. [Google Scholar] [CrossRef]
- Erdoğan, S.; Ozdemir, O.; Doğan, H.O.; Sezer, S.; Atalay, C.R.; Meriç, F.; Yilmaz, F.M.; Koca, Y. Liver enzymes, mean platelet volume, and red cell distribution width in gestational diabetes. Turk. J. Med. Sci. 2014, 44, 121–125. [Google Scholar] [CrossRef]
- Lao, T.T.; Ho, L.-F. Gestational diabetes and maternal third-trimester blood count. J. Reprod. Med. 2002, 47, 309–312. [Google Scholar]
- Hirahara, K.; Nakayama, T. CD4+T-cell subsets in inflammatory diseases: Beyond the Th1/Th2 paradigm. Int. Immunol. 2016, 28, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naïve to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:Th2 dichotomy of pregnancy and preterm labour. Mediat. Inflamm. 2012, 2012, 967629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, B.; Tian, Z.; Wei, H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell. Mol. Immunol. 2014, 11, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, A.; Ito, M.; Yoneda, S.; Shiozaki, A.; Hidaka, T.; Saito, S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am. J. Reprod. Immunol. 2010, 63, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Pongcharoen, S.; Niumsup, P.; Sanguansermsri, D.; Supalap, K.; Butkhamchot, P. The effect of Interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am. J. Reprod. Immunol. 2006, 55, 291–300. [Google Scholar] [CrossRef]
- Pongcharoen, S.; Supalap, K. Interleukin-17 increased progesterone secretion by JEG-3 human choriocarcinoma cells. Am. J. Reprod. Immunol. 2009, 61, 261–264. [Google Scholar] [CrossRef]
- Martínez-García, E.A.; Chávez-Robles, B.; Sánchez-Hernández, P.E.; Núñez-Atahualpa, L.; Martín-Máquez, B.T.; Muñoz-Gómez, A.; González-López, L.; Gámez-Nava, J.I.; Salazar-Páramo, M.; Dávalos-Rodríguez, I.; et al. IL-17 increased in the third trimester in healthy women with term labor. Am. J. Reprod. Immunol. 2011, 65, 99–103. [Google Scholar] [CrossRef]
- Plitas, G.; Rudensky, A.Y. Regulatory T cells: Differentiation and function. Cancer Immunol. Res. 2016, 4, 721–725. [Google Scholar] [CrossRef] [Green Version]
- La Rocca, C.; Carbone, F.; Longobardi, S.; Matarese, G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 2014, 162, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Salvany-Celades, M.; Van Der Zwan, A.; Benner, M.; Setrajcic-Dragos, V.; Gomes, H.A.B.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019, 27, 2537–2547.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimzadeh, M.; Norouzian, M.; Arabpour, F.; Naderi, N. Regulatory T-cells and preeclampsia: An overview of literature. Expert Rev. Clin. Immunol. 2016, 12, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Nakashima, A.; Shima, T.; Saito, S. New Paradigm in the role of regulatory T cells during pregnancy. Front. Immunol. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Bevan, M.J. CD8(+) T Cells: Foot soldiers of the immune system. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lissauer, D.; Piper, K.; Goodyear, O.; Kilby, M.D.; Moss, P.A.H. Fetal-specific CD8+Cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 2012, 189, 1072–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.Y. Multi-tasking of helper T cells. Immunology 2010, 130, 166–171. [Google Scholar] [CrossRef]
- Schober, L.; Radnai, D.; Spratte, J.; Kisielewicz, A.; Schmitt, E.; Mahnke, K.; Flühr, H.; Uhlmann, L.; Sohn, C.; Steinborn, A. The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus. Clin. Exp. Immunol. 2014, 177, 76–85. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Liu, B.; Li, Q.; Wang, Z.; Fan, S.; Wang, L. Functional defects of regulatory T cell through interleukin 10 mediated mechanism in the induction of gestational diabetes mellitus. DNA Cell Biol. 2018, 37, 278–285. [Google Scholar] [CrossRef]
- Mahmoud, F.F.; Haines, D.D.; Abul, H.T.; Omu, A.E.; Abu-Donia, M.B. Butyrylcholinesterase activity in gestational diabetes: Correlation with lymphocyte subpopulations in peripheral blood. Am. J. Reprod. Immunol. 2006, 56, 185–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Du, N.; Sun, H.; Chen, L.; Bao, H.; Zhao, Q.; Qu, Q.; Ma, D.; Kwak-Kim, J.; et al. Immune checkpoint molecules on T cell subsets of pregnancies with preeclampsia and gestational diabetes mellitus. J. Reprod. Immunol. 2020, 142, 103208. [Google Scholar] [CrossRef] [PubMed]
- Seck, A.; Hichami, A.; Doucouré, S.; Agne, F.D.; Bassène, H.; Ba, A.; Sokhna, C.; Khan, N.A.; Samb, A. Th1/Th2 dichotomy in obese women with gestational diabetes and their macrosomic babies. J. Diabetes Res. 2018, 2018, 8474617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sifnaios, E.; Mastorakos, G.; Psarra, K.; Panagopoulos, N.-D.; Panoulis, K.; Vitoratos, N.; Rizos, D.; Creatsas, G. Gestational Diabetes and T-cell (Th1/Th2/Th17/Treg) immune profile. In Vivo 2019, 33, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Lapolla, A.; Sanzari, M.; Betterle, C.; Dalfra, M.G.; Masin, M.; Zanchetta, R.; Zancanaro, F.; Capovilla, F.; Toniato, R.; Plebani, M.; et al. Evaluation of T-cell receptor CD3+ gamma delta in gestational diabetes mellitus. Acta Diabetol. 2000, 37, 207–211. [Google Scholar] [CrossRef]
- Lapolla, A.; Dalfrà, M.; Sanzari, M.; Fedele, D.; Betterle, C.; Masin, M.; Zanchetta, R.; Faggian, D.; Masotti, M.; Nucera, V.; et al. Lymphocyte subsets and cytokines in women with gestational diabetes mellitus and their newborn. Cytokine 2005, 31, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Lawand, M.; Déchanet-Merville, J.; Dieu-Nosjean, M.-C. Key Features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front. Immunol. 2017, 8, 761. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B cells, antibodies, and more. Clin. J. Am. Soc. Nephrol. 2016, 11, 137–154. [Google Scholar] [CrossRef]
- Lund, F.E. Cytokine-producing B lymphocytes—key regulators of immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Sengupta, P.; Haque, N. Reproductive immunomodulatory functions of B cells in pregnancy. Int. Rev. Immunol. 2019, 39, 53–66. [Google Scholar] [CrossRef]
- Lima, J.; Martins, C.; Leandro, M.J.; Nunes, G.; Sousa, M.-J.; Branco, J.C.; Borrego, L.-M. Characterization of B cells in healthy pregnant women from late pregnancy to post-partum: A prospective observational study. BMC Pregnancy Childbirth 2016, 16, 139. [Google Scholar] [CrossRef] [Green Version]
- Muzzio, D.O.; Soldati, R.; Ehrhardt, J.; Utpatel, K.; Evert, M.; Zenclussen, A.C.; Zygmunt, M.; Jensen, F. B cell development undergoes profound modifications and adaptations during pregnancy in Mice1. Biol. Reprod. 2014, 91, 115. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, J.; Li, Y.; Gu, H.; Zhao, J.; Sun, Y.; Wang, R.; Zhang, C.; Chen, W.; Weng, J.; et al. B Lymphocytes are predictors of insulin resistance in women with gestational diabetes mellitus. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Luck, H.; Khan, S.; Kim, J.H.; Copeland, J.K.; Revelo, X.S.; Tsai, S.; Chakraborty, M.; Cheng, K.; Chan, Y.T.; Nøhr, M.K.; et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat. Commun. 2019, 10, 3650. [Google Scholar] [CrossRef]
- Deng, C.; Xiang, Y.; Tan, T.; Ren, Z.; Cao, C.; Liu, B.; Huang, G.; Wang, X.; Zhou, Z. The imbalance of B-lymphocyte subsets in subjects with different glucose tolerance: Relationship with metabolic parameter and disease status. J. Diabetes Res. 2017, 2017, 5052812. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanim, H.; Mohanty, P.; Deopurkar, R.; Sia, C.L.; Korzeniewski, K.; Abuaysheh, S.; Chaudhuri, A.; Dandona, P. Acute modulation of toll-like receptors by insulin. Diabetes Care 2008, 31, 1827–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffler, M.; Hrach, T.; Stuerzl, M.; Horch, R.E.; Herndon, D.N.; Jeschke, M.G. Insulin attenuates apoptosis and exerts anti-inflammatory effects in endotoxemic human macrophages. J. Surg. Res. 2007, 143, 398–406. [Google Scholar] [CrossRef]
- Dandona, P.; Ghanim, H.; Green, K.; Sia, C.L.; Abuaysheh, S.; Kuhadiya, N.; Batra, M.; Dhindsa, S.; Chaudhuri, A. Insulin infusion suppresses while glucose infusion induces Toll-like receptors and high-mobility group-B1 protein expression in mononuclear cells of type 1 diabetes patients. Am. J. Physiol. Metab. 2013, 304, E810–E818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Niekerk, G.; Christowitz, C.; Conradie, D.; Engelbrecht, A.M. Insulin as an immunomodulatory hormone. Cytokine Growth Factor Rev. 2020, 52, 34–44. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Metformin in gestational diabetes: An emerging contender. Indian J. Endocrinol. Metab. 2015, 19, 236–244. [Google Scholar] [CrossRef]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res. Care 2018, 6, e000456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Given, J.E.; Loane, M.; Garne, E.; Addor, M.C.; Bakker, M.; Bertaut-Nativel, B.; Gatt, M.; Klungsoyr, K.; Lelong, N.; Morgan, M.; et al. Metformin exposure in first trimester of pregnancy and risk of all or specific congenital anomalies: Exploratory case-control study. BMJ 2018, 361, k2477. [Google Scholar] [CrossRef] [Green Version]
- Schuiveling, M.; Vazirpanah, N.; Radstake, T.; Zimmermann, M.; Broen, J.C.A. Metformin, a new era for an old drug in the treatment of immune mediated disease? Curr. Drug Targets 2018, 19, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Turnquist, H.R.; Raimondi, G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009, 9, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell. Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Kalra, S.; Gupta, Y.; Singla, R.; Kalra, S. Use of oral anti-diabetic agents in pregnancy: A pragmatic approach. N. Am. J. Med. Sci. 2015, 7, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Ma, J.; Tang, J.; Hu, D.; Zhang, W.; Zhao, X. Comparative efficacy and safety of metformin, glyburide, and insulin in treating gestational diabetes mellitus: A meta-analysis. J. Diabetes Res. 2019, 2019, 9804708. [Google Scholar] [CrossRef] [Green Version]
- Koh, G.C.; Maude, R.R.; Schreiber, M.F.; Limmathurotsakul, D.; Wiersinga, W.J.; Wuthiekanun, V.; Lee, S.J.; Mahavanakul, W.; Chaowagul, W.; Chierakul, W.; et al. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin. Infect. Dis. 2011, 52, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-W.; Liu, P.-S.; Pook, K.A.; Wei, L.-N. Glyburide and retinoic acid synergize to promote wound healing by anti-inflammation and RIP140 degradation. Sci. Rep. 2018, 8, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, K.; Fukunaga, K.; Kamikozuru, K.; Kashiwamura, S.; Hida, N.; Ohda, Y.; Takeda, N.; Yoshida, K.; Iimuro, M.; Yokoyama, Y.; et al. Infliximab therapy impacts the peripheral immune system of immunomodulator and corticosteroid naive patients with Crohn’s disease. Gut Liver 2011, 5, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.A.; Mancari, R.; Azim, H.A., Jr.; Colombo, N.; Peccatori, F.A. Are monoclonal antibodies a safe treatment for cancer during pregnancy? Immunotherapy 2013, 5, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Djokanovic, N.; Klieger-Grossmann, C.; Pupco, A.; Koren, G. Safety of infliximab use during pregnancy. Reprod. Toxicol. 2011, 32, 93–97. [Google Scholar] [CrossRef]
- Chaparro, M.; Gisbert, J.P. How safe is infliximab therapy during pregnancy and lactation in inflammatory bowel disease? Expert Opin. Drug Saf. 2014, 13, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.D.; Johnson, D.L.; Xu, R.; Luo, Y.; Lopez-Jimenez, J.; Adam, M.P.; Braddock, S.R.; Robinson, L.K.; Vaux, K.; Jones, K.L.; et al. Birth outcomes in women who have taken adalimumab in pregnancy: A prospective cohort study. PLoS ONE 2019, 14, e0223603. [Google Scholar] [CrossRef] [PubMed]
- McElwain, C.J.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows Into Future Cardiometabolic Health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Shimazaki, S.; Kaneko, Y.; Karasawa, T.; Takahashi, M.; Ohkuchi, A.; Takahashi, H.; Kurosawa, A.; Torii, Y.; Iwata, H.; et al. Palmitic acid activates NLRP3 inflammasome and induces placental inflammation during pregnancy in mice. J. Reprod. Dev. 2020, 66, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Stødle, G.S.; Silva, G.B.; Tangerås, L.H.; Gierman, L.M.; Nervik, I.; Dahlberg, U.E.; Sun, C.; Aune, M.H.; Thomsen, L.C.V.; Bjørge, L.; et al. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin. Exp. Immunol. 2018, 193, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, P. Oxidative stress and macrophage function: A failure to resolve the inflammatory response. Biochem. Soc. Trans. 2007, 35, 284–287. [Google Scholar] [CrossRef]
- Williamson, R.D.; McCarthy, F.P.; Kenny, L.C.; McCarthy, C.M. Activation of a TLR9 mediated innate immune response in preeclampsia. Sci. Rep. 2019, 9, 5920. [Google Scholar] [CrossRef]
- McElwain, C.; McCarthy, C.M. Investigating mitochondrial dysfunction in gestational diabetes mellitus and elucidating if BMI is a causative mediator. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251C, 60–65. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.-W.; Cindrova-Davies, T.; Spiroski, A.-M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental adaptation to early-onset hypoxic pregnancy and mitochondria-targeted antioxidant therapy in a rodent model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cochemé, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ 10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adlam, V.J.; Harrison, J.C.; Porteous, C.M.; James, A.M.; Smith, R.A.J.; Murphy, M.P.; Sammut, I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005, 19, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Kerley, R.N.; McCarthy, C.; Kell, D.B.; Kenny, L.C. The potential therapeutic effects of ergothioneine in pre-eclampsia. Free Radic. Biol. Med. 2018, 117, 145–157. [Google Scholar] [CrossRef]
- Forster, R.; Spézia, F.; Papineau, D.; Sabadie, C.; Erdelmeier, I.; Moutet, M.; Yadan, J.-C. Reproductive safety evaluation of L-ergothioneine. Food Chem. Toxicol. 2015, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.D.; McCarthy, F.P.; Manna, S.; Groarke, E.; Kell, D.B.; Kenny, L.C.; McCarthy, C.M. L-(+)-Ergothioneine significantly improves the clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 2020, 75, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ruholamin, S.; Eshaghian, S.; Allame, Z. Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: A randomized clinical trial. J. Res. Med. Sci. 2014, 19, 970–975. [Google Scholar] [PubMed]
- Stenninger, E.; Flink, R.; Eriksson, B.; Sahlen, C. Long term neurological dysfunction and neonatal hypoglycaemia after diabetic pregnancy. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F174–F179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
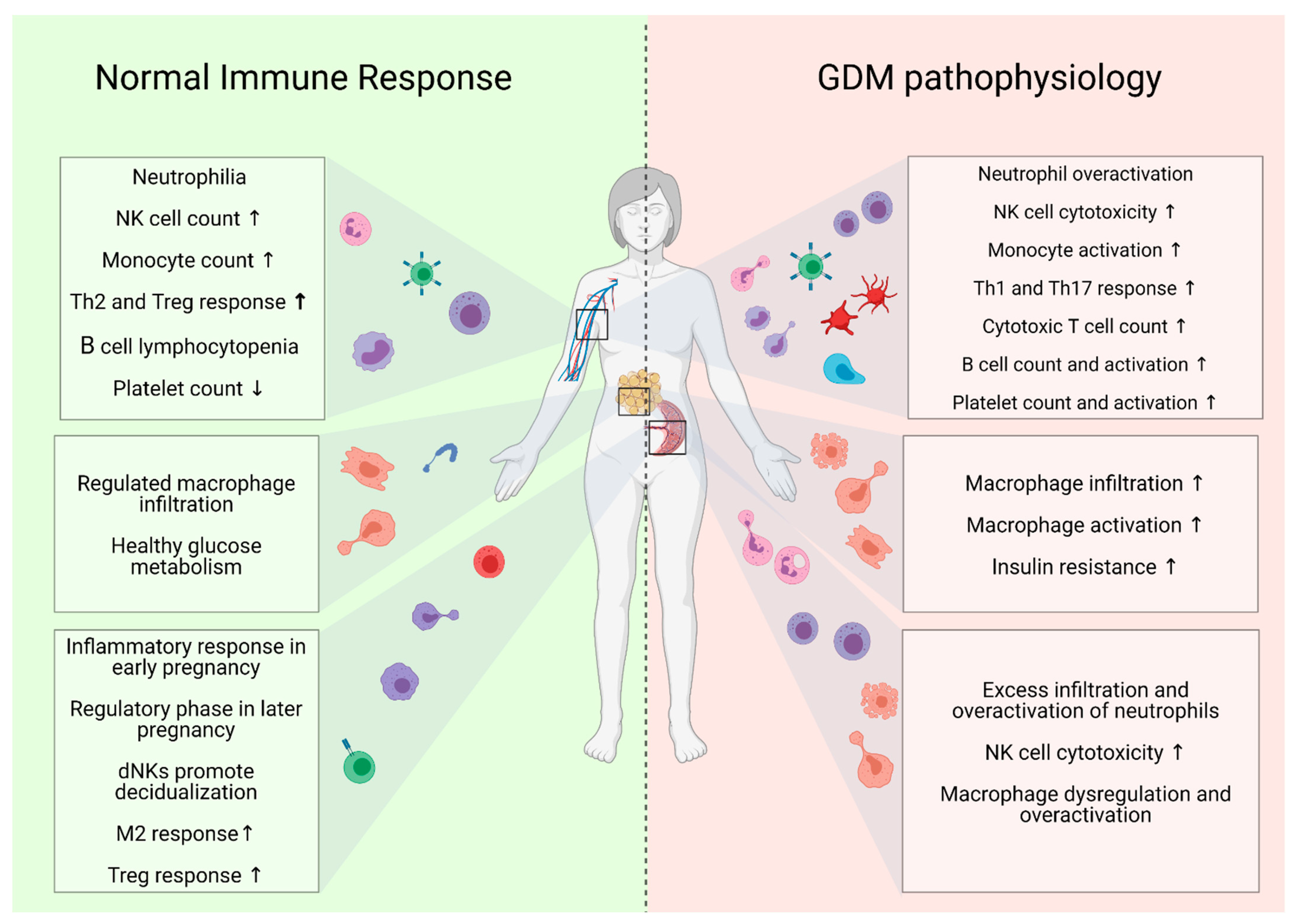
| Immune Cell Population | Functional Role | Studies | Implication in GDM | |
|---|---|---|---|---|
| In circulation | ||||
| Neutrophils | Phagocytosis; cytokine secretion | [26,27,28,29,30,31,32] | Blood count ↑ Activation ↑ | |
| NK cells | Regulates innate immune response; cytokine secretion | [34,45,46] | NKCD56dim cell count ↑ NKCD56bright cell count ↓ Pro-inflammatory cytokine release ↑ | |
| NKT cells | Cytokine production | [50,51] | ↔ | |
| Monocytes | Phagocytosis; antigen-presenting cells | [62,64] | CD14+ cell count ↑ Activation ↑ Intermediate monocyte count ↓ | |
| Dendritic cells | Antigen-presenting cells | [99] | ↔ | |
| Platelets | Coagulation; vasoconstriction | [27,29,30,111,112,113,114,115,116,117,118,119,120,121] | Blood count ↑ Activation ↑ | |
| T cells | T helper cells | Inflammation; defence against IC bacterial pathogens | [11] | Th1 cell count ↑ Th17 cell count ↑ |
| Regulatory T cells | Regulates immune response | [11,45,141,142,143,144,145,146] | Mixed findings on Treg count and functionality | |
| γδ T cells | Inflammation and cytotoxicity | [99,147,148] | γδ T cell count ↑ | |
| Cytotoxic T cells | Cytotoxicity | [51,148] | CD8+ T cell count ↑ | |
| Naïve/memory phenotype | [50,153] | Naïve T cell count ↓ Memory T cell count ↑ | ||
| B cells | Antibody secretion | [155] | Blood count ↑ Activation ↑ | |
| Placental tissue | ||||
| Neutrophils | Phagocytosis; cytokine secretion | [32] | Infiltration ↑ Activation ↑ | |
| Decidual NK cells | Promoting decidual vascularization | [34] | DNKCD56dim cell count ↑ | |
| Macrophages | Homeostasis of placenta environment and host defence against infections | [79,84,85,86,87] | Activation ↑ Mixed findings on M1/M2 polarity | |
| Adipose tissue (visceral and subcutaneous) | ||||
| Macrophages | Lipid and energy metabolism; adipocyte mitochondrial function | [79,91] | Infiltration ↑ Activation ↑ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McElwain, C.J.; McCarthy, F.P.; McCarthy, C.M. Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far. Int. J. Mol. Sci. 2021, 22, 4261. https://doi.org/10.3390/ijms22084261
McElwain CJ, McCarthy FP, McCarthy CM. Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far. International Journal of Molecular Sciences. 2021; 22(8):4261. https://doi.org/10.3390/ijms22084261
Chicago/Turabian StyleMcElwain, Colm J., Fergus P. McCarthy, and Cathal M. McCarthy. 2021. "Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far" International Journal of Molecular Sciences 22, no. 8: 4261. https://doi.org/10.3390/ijms22084261
APA StyleMcElwain, C. J., McCarthy, F. P., & McCarthy, C. M. (2021). Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far. International Journal of Molecular Sciences, 22(8), 4261. https://doi.org/10.3390/ijms22084261






