Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review
Abstract
:1. Introduction
2. TRPV1: Family and Subfamily
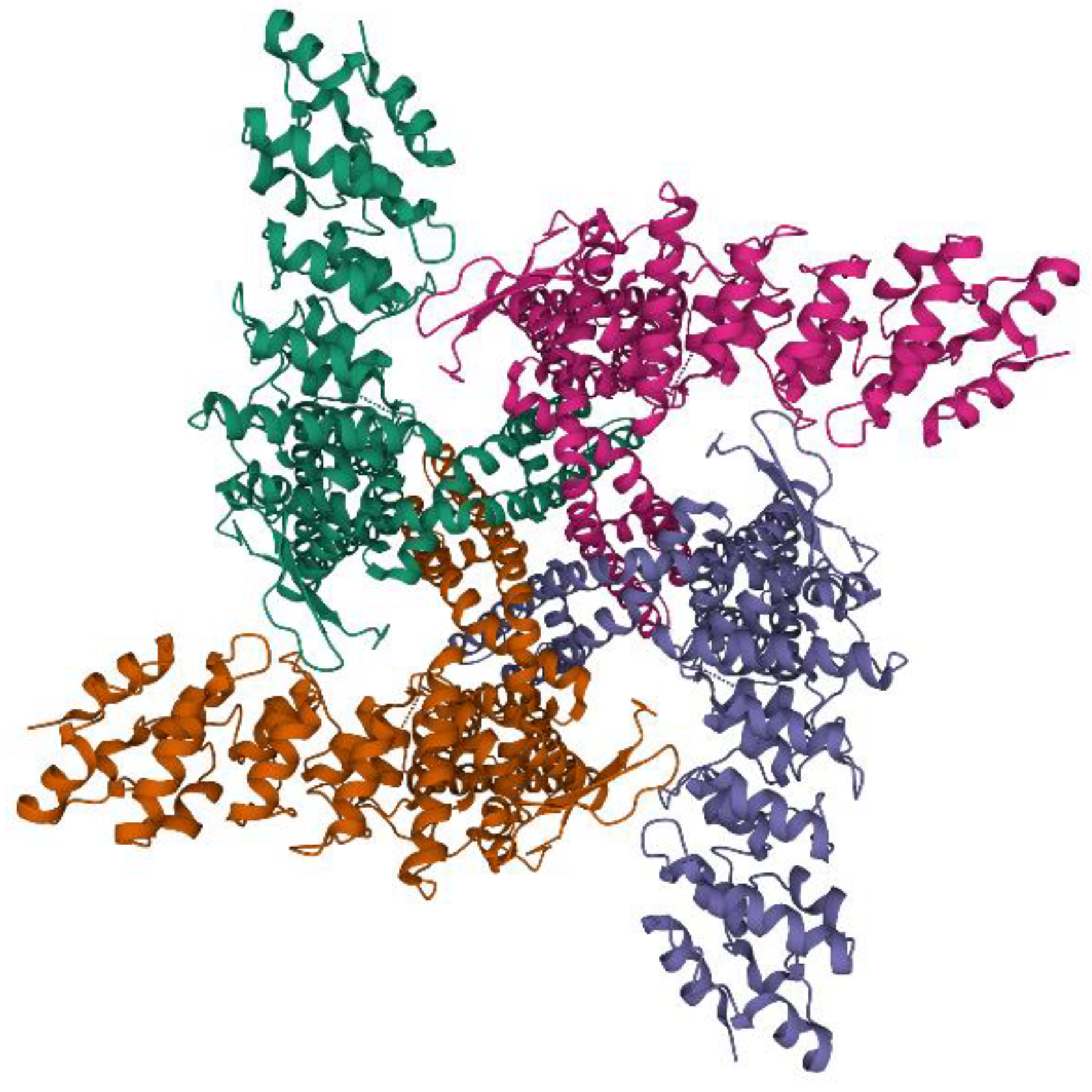
3. TRPV1: Protein Structure
4. TRPV1: Function, Modulators, and Mechanisms of Regulation
5. Role of TRPV1 on Spermatozoa
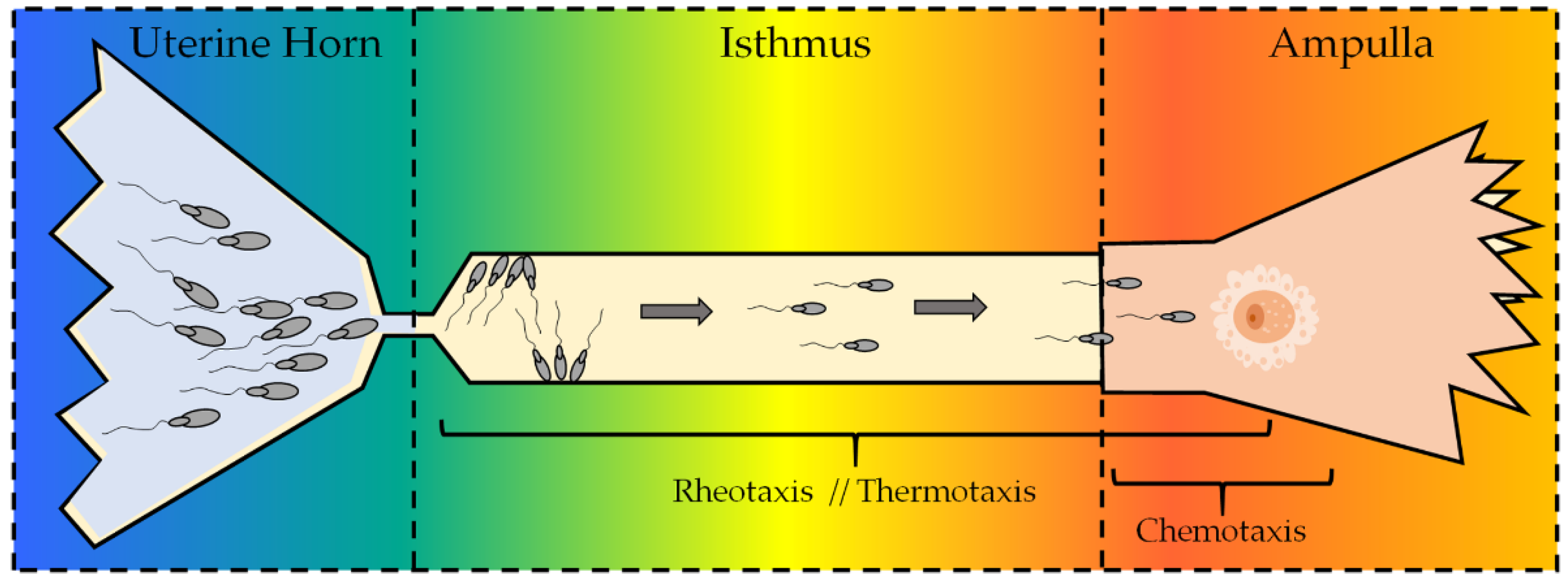
5.1. Sperm Chemotaxis, TRPV1, and the Endocannabinoid System (ECS)
5.2. Thermotaxis and TRPV1: Role in Human Spermatozoa
5.3. Sperm Rheotaxis
6. TRPV1 as a Pharmacological Target and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef] [PubMed]
- Jara-Oseguera, A.; Simon, S.; Rosenbaum, T. TRPV1: On the Road to Pain Relief. Curr. Mol. Pharmacol. 2010, 1, 255–269. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.M.; Urban, L.; Nagy, I. TRPV1 Function in Health and Disease. Curr. Pharm. Biotechnol. 2010, 12, 130–144. [Google Scholar] [CrossRef]
- Geron, M.; Hazan, A.; Priel, A. Animal toxins providing insights into TRPV1 activation mechanism. Toxins 2017, 9, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Ardon, F.; Markello, R.D.; Hu, L.; Deutsch, Z.I.; Tung, C.-K.; Wu, M.; Suarez, S.S. Dynamics of Bovine Sperm Interaction with Epithelium Differ Between Oviductal Isthmus and Ampulla. Biol. Reprod. 2016, 95, 90. [Google Scholar] [CrossRef]
- Ramal-Sanchez, M.; Bernabo, N.; Tsikis, G.; Blache, M.C.; Labas, V.; Druart, X.; Mermillod, P.; Saint-Dizier, M. Progesterone induces sperm release from oviductal epithelial cells by modifying sperm proteomics, lipidomics and membrane fluidity. Mol. Cell. Endocrinol. 2020, 504. [Google Scholar] [CrossRef]
- De Toni, L.; Garolla, A.; Menegazzo, M.; Magagna, S.; Di Nisio, A.; Šabović, I.; Rocca, M.S.; Scattolini, V.; Filippi, A.; Foresta, C. Heat sensing receptor TRPV1 is a mediator of thermotaxis in human spermatozoa. PLoS ONE 2016, 11, e0167622. [Google Scholar] [CrossRef] [Green Version]
- Szallasi, A.; Conte, B.; Goso, C.; Blumberg, P.M.; Manzini, S. Characterization of a peripheral vanilloid (capsaicin) receptor in the urinary bladder of the rat. Life Sci. 1993, 52. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.; Wang, Z.; Mergler, S.; Wolosin, J.M.; Reinach, P.S. Functional TRPV1 expression in human corneal fibroblasts. Exp. Eye Res. 2013, 107, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef]
- Weber, L.V.; Al-Refae, K.; Wölk, G.; Bonatz, G.; Altmüller, J.; Becker, C.; Gisselmann, G.; Hatt, H. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer Targets Ther. 2016, 8, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Dörr, J.; Fecher-Trost, C. TRP channels in female reproductive organs and placenta. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 704, pp. 909–928. [Google Scholar]
- Maccarrone, M.; Barboni, B.; Paradisi, A.; Bernabò, N.; Gasperi, V.; Pistilli, M.G.; Fezza, F.; Lucidi, P.; Mattioli, M. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J. Cell Sci. 2005, 118, 4393–4404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta-Cepas, J.; Serra, F.; Bork, P. ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. Mol. Biol. Evol. 2016, 33, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. Fasttree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the Mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef]
- Majhi, R.K.; Kumar, A.; Giri, S.; Goswami, C. Differential expression and localization of thermosensitive Transient Receptor Potential Vanilloid (TRPV) channels in the mature sperm of white pekin duck (Anas platyrhynchos). bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.X. TRP Channels; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781439818619. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, A.K.; Swain, D.K.; Singh, V.; Yadav, S.; Saxena, A. Role of transient receptor potential channels in regulating spermatozoa functions: A mini-review. Vet. World 2018, 11, 1618–1623. [Google Scholar] [CrossRef] [Green Version]
- Moiseenkova-Bell, V.Y.; Stanciu, L.A.; Serysheva, I.I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef] [Green Version]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The Ankyrin Repeats of TRPV1 Bind Multiple Ligands and Modulate Channel Sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflugers Arch. Eur. J. Physiol. 2005, 451, 143–150. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Gordon-Shaag, A.; Munari, M.; Gordon, S.E. Ca2+/Calmodulin Modulates TRPV1 Activation by Capsaicin. J. Gen. Physiol. 2004, 123, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Yang, F.; Vu, S.; Zheng, J. Exploring functional roles of TRPV1 intracellular domains with unstructured peptide-insertion screening. Sci. Rep. 2016, 6, 33827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RCSB PDB: Homepage. Available online: https://www.rcsb.org/ (accessed on 26 January 2021).
- Sehnal, D.; Rose, A.S.; Koča, J.; Burley, S.K.; Velankar, S. Mol*: Towards a common library and tools for web molecular graphics. MolVA. In Proceedings of the Conference of Visualization, EuroVis, Brno, Czech Republic, 4–8 June 2018. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity—Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Christie, S.; Wittert, G.A.; Li, H.; Page, A.J. Involvement of TRPV1 Channels in Energy Homeostasis. Front. Endocrinol. 2018, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- López-Romero, A.E.; Hernández-Araiza, I.; Torres-Quiroz, F.; Tovar-Y-Romo, L.B.; Islas, L.D.; Rosenbaum, T. TRP ion channels: Proteins with conformational flexibility. Channels 2019, 13, 207–226. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wen, H. Heat activation mechanism of TRPV1: New insights from molecular dynamics simulation. Temperature 2019, 6, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Caterina, M.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Kang, S.M.; Lee, S.R.; Kong, K.H.; Lee, J.Y.; Kim, E.J.; Chung, J.H. Inhibitory effects of TRPV1 blocker on UV-induced responses in the hairless mice. Arch. Dermatol. Res. 2011, 303, 727–736. [Google Scholar] [CrossRef]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [Green Version]
- Szolcsányi, J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nam, Y.S.; Lee, S.Y.; Kim, H.C.; Jang, C.G. Effects of capsazepine, a transient receptor potential vanilloid type 1 antagonist, on morphine-induced antinociception, tolerance, and dependence in mice. Br. J. Anaesth. 2010, 105, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Raisinghani, M.; Pabbidi, R.M.; Premkumar, L.S. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J. Physiol. 2005, 567, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Devesa, I.; Changeux, J.P.; Ferrer-Montiel, A. Bradykinin induces TRPV1 exocytotic recruitment in peptidergic nociceptors. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Dessaint, J.; Yu, W.; Krause, J.E.; Yue, L. Yohimbine inhibits firing activities of rat dorsal root ganglion neurons by blocking Na+ channels and vanilloid VR1 receptors. Eur. J. Pharmacol. 2004, 485, 11–20. [Google Scholar] [CrossRef]
- Nicoletti, P.; Trevisani, M.; Manconi, M.; Gatti, R.; De Siena, G.; Zagli, G.; Benemei, S.; Capone, J.A.; Geppetti, P.; Pini, L.A. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia 2008, 28, 9–17. [Google Scholar] [CrossRef]
- Pearce, L.V.; Petukhov, P.A.; Szabo, T.; Kedei, N.; Bizik, F.; Kozikowski, A.P.; Blumberg, P.M. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org. Biomol. Chem. 2004, 2, 2281–2286. [Google Scholar] [CrossRef] [Green Version]
- Payrits, M.; Sághy, É.; Csekő, K.; Pohóczky, K.; Bölcskei, K.; Ernszt, D.; Barabás, K.; Szolcsányi, J.; Ábrahám, I.M.; Helyes, Z.; et al. Estradiol Sensitizes the Transient Receptor Potential Vanilloid 1 Receptor in Pain Responses. Endocrinology 2017, 158, 3249–3258. [Google Scholar] [CrossRef]
- Zheng, X.; Hodgetts, K.J.; Brielmann, H.; Hutchison, A.; Burkamp, F.; Brian Jones, A.; Blurton, P.; Clarkson, R.; Chandrasekhar, J.; Bakthavatchalam, R.; et al. From arylureas to biarylamides to aminoquinazolines: Discovery of a novel, potent TRPV1 antagonist. Bioorganic Med. Chem. Lett. 2006, 16, 5217–5221. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Rentería, M.; Juárez-Contreras, R.; González-Ramírez, R.; Islas, L.D.; Sierra-Ramírez, F.; Llorente, I.; Simon, S.A.; Hiriart, M.; Rosenbaum, T.; Morales-Lázaro, S.L. TRPV1 channels and the progesterone receptor Sig-1R interact to regulate pain. Proc. Natl. Acad. Sci. USA 2018, 115, E1657–E1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roohbakhsh, A.; Shamsizadeh, A. Opioids and TRPV1 Receptors. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 3, pp. 433–442. ISBN 9780128006771. [Google Scholar]
- Kanezaki, M.; Ebihara, S.; Gui, P.; Ebihara, T.; Kohzuki, M. Effect of cigarette smoking on cough reflex induced by TRPV1 and TRPA1 stimulations. Respir. Med. 2012, 106, 406–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caires, R.; Luis, E.; Taberner, F.J.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Balazs, E.A.; Gomis, A.; Belmonte, C.; De La Peña, E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat. Commun. 2015, 6, 8095. [Google Scholar] [CrossRef] [Green Version]
- Van Buren, J.J.; Bhat, S.; Rotello, R.; Pauza, M.E.; Premkumar, L.S. Sensitization and translocation of TRPVI by insulin and IGF-I. Mol. Pain 2005, 1, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, D.G.; Cassar, S.C.; Masters, J.N.; Esbenshade, T.; Hancock, A.A. Use of a fluorescent imaging plate reader-based calcium assay to assess pharmacological differences between the human and rat vanilloid receptor. J. Biomol. Screen. 2002, 7, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, J.; Supronsinchai, W.; Andreou, A.P.; Summ, O.; Akerman, S.; Goadsby, P.J. Olvanil acts on transient receptor potential vanilloid channel 1 and cannabinoid receptors to modulate neuronal transmission in the trigeminovascular system. Pain 2012, 153, 2226–2232. [Google Scholar] [CrossRef]
- Maurer, K.; Binzen, U.; Mörz, H.; Bugert, P.; Schedel, A.; Treede, R.D.; Greffrath, W. Acetylsalicylic acid enhances tachyphylaxis of repetitive capsaicin responses in TRPV1-GFP expressing HEK293 cells. Neurosci. Lett. 2014, 563, 101–106. [Google Scholar] [CrossRef]
- Park, C.K.; Kim, K.; Jung, S.J.; Kim, M.J.; Ahn, D.K.; Hong, S.D.; Kim, J.S.; Oh, S.B. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain 2009, 144, 84–94. [Google Scholar] [CrossRef]
- André, E.; Campi, B.; Trevisani, M.; Ferreira, J.; Malheiros, Â.; Yunes, R.A.; Calixto, J.B.; Geppetti, P. Pharmacological characterisation of the plant sesquiterpenes polygodial and drimanial as vanilloid receptor agonists. Biochem. Pharmacol. 2006, 71, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, M.; Walter, A.; Lee, B.; DeLaat, A.; Trigueros, R.R.; Happel, K.; Sepesy, R.; Nguyen, B.; Manwill, P.K.; Rakotondraibe, L.H.; et al. Stop the crop: Insights into the insecticidal mode of action of cinnamodial against mosquitoes. Pestic. Biochem. Physiol. 2021, 171, 104743. [Google Scholar] [CrossRef] [PubMed]
- Bessac, B.F.; Jordt, S.E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology 2008, 23, 360–370. [Google Scholar] [CrossRef]
- Starkus, J.; Jansen, C.; Shimoda, L.M.N.; Stokes, A.J.; Small-Howard, A.L.; Turner, H. Diverse TRPV1 responses to cannabinoids. Channels 2019, 13, 172–191. [Google Scholar] [CrossRef] [Green Version]
- Vriens, J.; Nilius, B.; Vennekens, R. Herbal Compounds and Toxins Modulating TRP Channels. Curr. Neuropharmacol. 2008, 6, 79–96. [Google Scholar] [PubMed] [Green Version]
- Kim, Y.S.; Hong, C.; Lee, S.W.; Nam, J.H.; Kim, B.J. Effects of ginger and its pungent constituents on transient receptor potential channels. Int. J. Mol. Med. 2016, 38, 1905–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, E.; Reeve, A.; Bevan, S.; McIntyre, P. Identification of Species-specific Determinants of the Action of the Antagonist Capsazepine and the Agonist PPAHV on TRPV1. J. Biol. Chem. 2004, 279, 17165–17172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, S.; Zheng, Y.; Meng, M.; Guo, Z.; Cao, N.; Ma, X.; Du, Z.; Li, J.; Duan, Y.; Du, G. Gingerol Reverses the Cancer-Promoting Effect of Capsaicin by Increased TRPV1 Level in a Urethane-Induced Lung Carcinogenic Model. J. Agric. Food Chem. 2016, 64, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Tamir, R.; Klionsky, L.; Norman, M.H.; Louis, J.C.; Wild, K.D.; Treanor, J.J.S. Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol. Pharmacol. 2005, 68, 1524–1533. [Google Scholar] [CrossRef] [Green Version]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol. 2018, 223, e13038. [Google Scholar] [CrossRef] [Green Version]
- Kym, P.R.; Kort, M.E.; Hutchins, C.W. Analgesic potential of TRPV1 antagonists. Biochem. Pharmacol. 2009, 78, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Brederson, J.D.; Kym, P.R.; Szallasi, A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013, 716, 61–76. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Priel, A.; Zhou, S.; King, D.; Siemens, J.; Julius, D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 2010, 141, 834–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamers, A.J.P.; Ahluwalia, A. Arachidonic acid metabolites induce TRPV1-mediated inflammation. FASEB J. 2017, 31. [Google Scholar] [CrossRef]
- Nersesyan, Y.; Demirkhanyan, L.; Cabezas-Bratesco, D.; Oakes, V.; Kusuda, R.; Dawson, T.; Sun, X.; Cao, C.; Cohen, A.M.; Chelluboina, B.; et al. Oxytocin Modulates Nociception as an Agonist of Pain-Sensing TRPV1. Cell Rep. 2017, 21, 1681–1691. [Google Scholar] [CrossRef] [Green Version]
- Puntambekar, P.; Van Buren, J.; Raisinghani, M.; Premkumar, L.S.; Ramkumar, V. Direct Interaction of Adenosine with the TRPV1 Channel Protein. J. Neurosci. 2004, 24, 3663–3671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Shyue, S.K.; Lee, T.S. Excess nitric oxide activates TRPV1-Ca2+-calpain signaling and promotes PEST-dependent degradation of liver X receptor α. Int. J. Biol. Sci. 2016, 12, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Koroleva, K.; Mustafina, A.; Yakovlev, A.; Hermann, A.; Giniatullin, R.; Sitdikova, G. Receptor Mechanisms Mediating the Pro-Nociceptive Action of Hydrogen Sulfide in Rat Trigeminal Neurons and Meningeal Afferents. Front. Cell. Neurosci. 2017, 11, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto-Posadas, A.; Picazo-Juárez, G.; Llorente, I.; Jara-Oseguera, A.; Morales-Lázaro, S.; Escalante-Alcalde, D.; Islas, L.D.; Rosenbaum, T. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat. Chem. Biol. 2012, 8, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Jung, S.J.; Zhu, M.H.; Park, C.K.; Kim, Y.H.; Oh, S.B.; Lee, C.J. Direct activation of Transient Receptor Potential Vanilloid 1(TRPV1) by Diacylglycerol (DAG). Mol. Pain 2008, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 is activated by both acidic and basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, B.R.; Lee, C.H.; Jarvis, M.F.; El Kouhen, R.; Moreland, R.B.; Faltynek, C.R.; Puttfarcken, P.S. Modulation of human TRPV1 receptor activity by extracellular protons and host cell expression system. Eur. J. Pharmacol. 2006, 537, 20–30. [Google Scholar] [CrossRef]
- Long, W.; Fatehi, M.; Soni, S.; Panigrahi, R.; Philippaert, K.; Yu, Y.; Kelly, R.; Boonen, B.; Barr, A.; Golec, D.; et al. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J. Physiol. 2020, 598, 4321–4338. [Google Scholar] [CrossRef]
- Connor, M.; Vaughan, C.W.; Vandenberg, R.J. N-Acyl amino acids and N-acyl neurotransmitter conjugates: Neuromodulators and probes for new drug targets. Br. J. Pharmacol. 2010, 160, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, Q.; Li, S.; Chen, M.; Jin, B.; Wang, M. TRPV1, Targeted by miR-338-3p, Induces Neuropathic Pain by Interacting with NECAB2. J. Mol. Neurosci. 2021, 71, 55–65. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Zhang, N.; Jin, W.; Ren, Y.; Chen, C. Establishment of preliminary regulatory network of TRPV1 and related cytokines. Saudi J. Biol. Sci. 2017, 24, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Raboune, S.; Stuart, J.M.; Leishman, E.; Takacs, S.M.; Rhodes, B.; Basnet, A.; Jameyfield, E.; McHugh, D.; Widlanski, T.; Bradshaw, H.B. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Hanack, C.; Moroni, M.; Lima, W.C.; Wende, H.; Kirchner, M.; Adelfinger, L.; Schrenk-Siemens, K.; Tappe-Theodor, A.; Wetzel, C.; Kuich, P.H.; et al. GABA blocks pathological but not acute TRPV1 pain signals. Cell 2015, 160, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Hernandez, A.; Charlet, A. Oxytocin, GABA, and TRPV1, the Analgesic Triad? Front. Mol. Neurosci. 2018, 11, 398. [Google Scholar] [CrossRef] [Green Version]
- Movahed, P.; Jönsson, B.A.G.; Birnir, B.; Wingstrand, J.A.; Jørgensen, T.D.; Ermund, A.; Sterner, O.; Zygmunt, P.M.; Högestätt, E.D. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J. Biol. Chem. 2005, 280, 38496–38504. [Google Scholar] [CrossRef] [Green Version]
- Van der Stelt, M.; Trevisani, M.; Vellani, V.; De Petrocellis, L.; Schiano Moriello, A.; Campi, B.; McNaughton, P.; Geppetti, P.; Di Marzo, V. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J. 2005, 24, 3026–3037. [Google Scholar] [CrossRef]
- Ahern, G.P. Activation of TRPV1 by the satiety factor oleoylethanolamide. J. Biol. Chem. 2003, 278, 30429–30434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karwad, M.A.; Macpherson, T.; Wang, B.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARα. FASEB J. 2017, 31, 469–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Lázaro, S.L.; Llorente, I.; Sierra-Ramírez, F.; López-Romero, A.E.; Ortíz-Rentería, M.; Serrano-Flores, B.; Simon, S.A.; Islas, L.D.; Rosenbaum, T. Inhibition of TRPV1 channels by a naturally occurring omega-9 fatty acid reduces pain and itch. Nat. Commun. 2016, 7, 13092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, Y.; Cáceres, J.; Sepúlveda, R.V.; Arriagada, D.; Olivares, P.; Díaz-Franulic, I.; Stehberg, J.; González-Nilo, F. Novel TRPV1 Channel Agonists With Faster and More Potent Analgesic Properties Than Capsaicin. Front. Pharmacol. 2020, 11, 1040. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.M.; Newstead, S.; Swartz, K.J.; Sansom, M.S.P. Capsaicin interaction with TRPV1 channels in a lipid bilayer: Molecular dynamics simulation. Biophys. J. 2015, 108, 1425–1434. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Hwang, S.W.; Kwak, J.; Lee, S.Y.; Kang, C.J.; Kim, W.B.; Kim, D.; Oh, U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999, 19, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.; Cohen, B.E.; Chuang, H.H. A single TRPV1 amino acid controls species sensitivity to capsaicin. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kasimova, M.A.; Yazici, A.T.; Yudin, Y.; Granata, D.; Klein, M.L.; Rohacs, T.; Carnevale, V. A hypothetical molecular mechanism for TRPV1 activation that invokes rotation of an S6 asparagine. J. Gen. Physiol. 2018, 150, 1554–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbaum, T.; Simon, S.A. TRPV1 Receptors and Signal Transduction; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2007; ISBN 0849340489. [Google Scholar]
- Botto, L.; Bernabò, N.; Palestini, P.; Barboni, B. Bicarbonate Induces Membrane Reorganization and CBR1 and TRPV1 Endocannabinoid Receptor Migration in Lipid Microdomains in Capacitating Boar Spermatozoa. J. Membr. Biol. 2010, 238, 33–41. [Google Scholar] [CrossRef]
- Majhi, R.K.; Kumar, A.; Yadav, M.; Swain, N.; Kumari, S.; Saha, A.; Pradhan, A.; Goswami, L.; Saha, S.; Samanta, L.; et al. Thermosensitive ion channel TRPV1 is endogenously expressed in the sperm of a fresh water teleost fish (Labeo rohita) and regulates sperm motility. Channels 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Gervasi, M.G.; Osycka-Salut, C.; Caballero, J.; Vazquez-Levin, M.; Pereyra, E.; Billi, S.; Franchi, A.; Perez-Martinez, S. Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation. PLoS ONE 2011, 6, e16993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francavilla, F.; Battista, N.; Barbonetti, A.; Vassallo, M.R.C.; Rapino, C.; Antonangelo, C.; Pasquariello, N.; Catanzaro, G.; Barboni, B.; Maccarrone, M. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology 2009, 150, 4692–4700. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Ye, H.; Gao, W.; Pu, X.; Yang, Z. Distribution profiles of transient receptor potential melastatin-and vanilloid-related channels in rat spermatogenic cells and sperm. Mol. Biol. Rep. 2010, 37, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Majhi, R.K.; Swain, N.; Giri, S.C.; Kar, S.; Samanta, L.; Goswami, C. TRPV4 is endogenously expressed in vertebrate spermatozoa and regulates intracellular calcium in human sperm. Biochem. Biophys. Res. Commun. 2016, 473, 781–788. [Google Scholar] [CrossRef]
- Grimaldi, P.; Orlando, P.; Di Siena, S.; Lolicato, F.; Petrosino, S.; Bisogno, T.; Geremia, R.; De Petrocellis, L.; Di Marzo, V. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 11131–11136. [Google Scholar] [CrossRef] [Green Version]
- Lamy, J.; Liere, P.; Pianos, A.; Aprahamian, F.; Mermillod, P.; Saint-Dizier, M. Steroid hormones in bovine oviductal fluid during the estrous cycle. Theriogenology 2016, 86, 1409–1420. [Google Scholar] [CrossRef]
- Ballester, L.; Romero-Aguirregomezcorta, J.; Soriano-Úbeda, C.; Matás, C.; Romar, R.; Coy, P. Timing of oviductal fluid collection, steroid concentrations, and sperm preservation method affect porcine in vitro fertilization efficiency. Fertil. Steril. 2014, 102, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the early embryonic microenvironment: Physiology and regulation of oviductal secretions. Int. J. Mol. Sci. 2020, 21, 223. [Google Scholar] [CrossRef] [Green Version]
- Gervasi, M.G.; Marczylo, T.H.; Lam, P.M.; Rana, S.; Franchi, A.M.; Konje, J.C.; Perez-Martinez, S. Anandamide Levels Fluctuate in the Bovine Oviduct during the Oestrous Cycle. PLoS ONE 2013, 8, e72521. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.E.M.; Rapino, C.; Di Tommaso, M.; Pucci, M.; Battista, N.; Paro, R.; Simon, L.; Lutton, D.; Maccarrone, M. Differences in the Endocannabinoid System of Sperm from Fertile and Infertile Men. PLoS ONE 2012, 7, e47704. [Google Scholar] [CrossRef] [Green Version]
- Catanzaro, G.; Battista, N.; Rossi, G.; Di Tommaso, M.; Pucci, M.; Pirazzi, V.; Cecconi, S.; Maccarrone, M. Effect of capacitation on the endocannabinoid system of mouse sperm. Mol. Cell. Endocrinol. 2011, 343, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safarinejad, M.R.; Hosseini, S.Y.; Dadkhah, F.; Asgari, M.A. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: A comparison between fertile and infertile men. Clin. Nutr. 2010, 29, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Castellini, C.; Lee, J.C.Y.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Zhang, B.R.; Hettinger, A.M.; Goad, D.W.; Malayer, J.R.; Geisert, R.D. Detection of bradykinin and bradykinin-β2 receptors in the porcine endometrium during the estrous cycle and early pregnancy. Biol. Reprod. 2002, 66, 574–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef] [Green Version]
- Ning, N.; Zhu, J.; Du, Y.; Gao, X.; Liu, C.; Li, J. Dysregulation of hydrogen sulphide metabolism impairs oviductal transport of embryos. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kadlec, M.; Ros-Santaella, J.L.; Pintus, E. The roles of no and h2s in sperm biology: Recent advances and new perspectives. Int. J. Mol. Sci. 2020, 21, 2174. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Martinez, H.; Tienthai, P.; Atikuzzaman, M.; Vicente-Carrillo, A.; Rubér, M.; Alvarez-Rodriguez, M. The ubiquitous hyaluronan: Functionally implicated in the oviduct? Theriogenology 2016, 86, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Fouladi-Nashta, A.A.; Raheem, K.A.; Marei, W.F.; Ghafari, F.; Hartshorne, G.M. Regulation and roles of the hyaluronan system in mammalian reproduction. Reproduction 2017, 153, R43–R58. [Google Scholar] [CrossRef] [Green Version]
- Leisegang, K.; Bouic, P.J.D.; Menkveld, R.; Henkel, R.R. Obesity is associated with increased seminal insulin and leptin alongside reduced fertility parameters in a controlled male cohort. Reprod. Biol. Endocrinol. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Korzekwa, A.J.; Bah, M.M.; Kurzynowski, A.; Lukasik, K.; Groblewska, A.; Skarzynski, D.J. Leukotrienes modulate secretion of progesterone and prostaglandins during the estrous cycle and early pregnancy in cattle: An in vivo study. Reproduction 2010, 140, 767–776. [Google Scholar] [CrossRef] [Green Version]
- De Mateo, S.; Sassone-Corsi, P. Regulation of spermatogenesis by small non-coding RNAs: Role of the germ granule. Semin. Cell Dev. Biol. 2014, 29, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Fereshteh, Z.; Schmidt, S.A.; Al-Dossary, A.A.; Accerbi, M.; Arighi, C.; Cowart, J.; Song, J.L.; Green, P.J.; Choi, K.; Yoo, S.; et al. Murine Oviductosomes (OVS) microRNA profiling during the estrous cycle: Delivery of OVS-borne microRNAs to sperm where miR-34c-5p localizes at the centrosome. Sci. Rep. 2018, 8, 16094. [Google Scholar] [CrossRef]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic profiling reveals correlations between spermiogram parameters and the metabolites present in human spermatozoa and seminal plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellini, C.; Mattioli, S.; Dal Bosco, A.; Collodel, G.; Pistilli, A.; Stabile, A.M.; MacChioni, L.; Mancuso, F.; Luca, G.; Rende, M. In vitro effect of nerve growth factor on the main traits of rabbit sperm. Reprod. Biol. Endocrinol. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Tribulo, P.; Bogle, O.; Mapletoft, R.J.; Adams, G.P. Bioactivity of ovulation inducing factor (or nerve growth factor) in bovine seminal plasma and its effects on ovarian function in cattle. Theriogenology 2015, 83, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Yamamoto, Y.; Kageyama, S.; Hirayama, H.; Kimura, K.; Okuda, K. Regulation of bovine oviductal NO synthesis by follicular steroids and prostaglandins. Reproduction 2016, 151, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Agirregoitia, E.; Valdivia, A.; Carracedo, A.; Casis, L.; Gil, J.; Subiran, N.; Ochoa, C.; Irazusta, J. Expression and Localization of δ-, κ-, and μ-Opioid Receptors in Human Spermatozoa and Implications for Sperm Motility. J. Clin. Endocrinol. Metab. 2006, 91, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, T.; Rashed, L.A.; Osman, I.; Marawan, M. Seminal plasma oxytocin and oxidative stress levels in infertile men with varicocele. Andrologia 2015, 47, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, R.D.; Rahmani, E.; Rahsepar, M.; Firouzabadi, M.M. Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch. Gynecol. Obstet. 2014, 289, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Anifandis, G.M.; Dafopoulos, K.; Messini, C.I.; Chalvatzas, N.; Liakos, N.; Pournaras, S.; Messinis, I.E. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod. Biol. Endocrinol. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Jueraitetibaike, K.; Ding, Z.; Wang, D.D.; Peng, L.P.; Jing, J.; Chen, L.; Ge, X.; Qiu, X.H.; Yao, B. The effect of Vitamin D on sperm motility and the underlying mechanism. Asian J. Androl. 2019, 21, 400–407. [Google Scholar] [CrossRef]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Ion Channels in Sperm Physiology and Male Fertility and Infertility. J. Androl. 2012, 33, 777–788. [Google Scholar] [CrossRef]
- Gadella, B.M. Reproductive tract modifications of the boar sperm surface. Mol. Reprod. Dev. 2017, 84, 822–831. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.S. The Oviductal Sperm Reservoir in Mammals: Mechanisms of Formation. Biol. Reprod. 1998, 58, 1105–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgadaki, K.; Khoury, N.; Spandidos, D.A.; Zoumpourlis, V. The molecular basis of fertilization (Review). Int. J. Mol. Med. 2016, 38, 979–986. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.J. Review: The epic journey of sperm through the female reproductive tract. Animal 2018, 12, s110–s120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki, K.; Clapham, D.E. Rheotaxis guides mammalian sperm. Curr. Biol. 2013, 23, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hino, T.; Yanagimachi, R. Active peristaltic movements and fluid production of the mouse oviduct: Their roles in fluid and sperm transport and fertilization. Biol. Reprod. 2019, 101, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Eisenbach, M.; Giojalas, L.C. Sperm guidance in mammals—An unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006, 7, 276–285. [Google Scholar] [CrossRef]
- Caterina, M.J.; Park, U. Chapter 4 TRPV1: A Polymodal Sensor in the Nociceptor Terminal. Curr. Top. Membr. 2006, 57, 113–150. [Google Scholar]
- Teves, M.E.; Barbano, F.; Guidobaldi, H.A.; Sanchez, R.; Miska, W.; Giojalas, L.C. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil. Steril. 2006, 86, 745–749. [Google Scholar] [CrossRef]
- Oren-Benaroya, R.; Orvieto, R.; Gakamsky, A.; Pinchasov, M.; Eisenbach, M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum. Reprod. 2008, 23, 2339–2345. [Google Scholar] [CrossRef] [Green Version]
- Eisenbach, M. Mammalian sperm chemotaxis and its association with capacitation. Dev. Genet. 1999, 25, 87–94. [Google Scholar] [CrossRef]
- Berendsen, J.T.W.; Kruit, S.A.; Atak, N.; Willink, E.; Segerink, L.I. Flow-Free Microfluidic Device for Quantifying Chemotaxis in Spermatozoa. Anal. Chem. 2020, 92, 3302–3306. [Google Scholar] [CrossRef]
- Dominguez, E.M.; Moreno-Irusta, A.; Bragulat, A.F.; Castex, H.R.; Losinno, L.; Giojalas, L.C. Equine Spermatozoa at Optimum Physiological State Are Selected by Chemotaxis Toward Progesterone. J. Equine Vet. Sci. 2018, 66, 21. [Google Scholar] [CrossRef]
- Sun, F.; Bahat, A.; Gakamsky, A.; Girsh, E.; Katz, N.; Giojalas, L.C.; Tur-Kaspa, I.; Eisenbach, M. Human sperm chemotaxis: Both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum. Reprod. 2005, 20, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Guidobaldi, H.A.; Teves, M.E.; Uñates, D.R.; Anastasía, A.; Giojalas, L.C. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS ONE 2008, 3, e3040. [Google Scholar] [CrossRef] [PubMed]
- Guidobaldi, H.A.; Hirohashi, N.; Cubilla, M.; Buffone, M.G.; Giojalas, L.C. An intact acrosome is required for the chemotactic response to progesterone in mouse spermatozoa. Mol. Reprod. Dev. 2017, 84, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, U.B.; Solzin, J.; Hildebrand, E.; Brown, J.E.; Helbig, A.; Hagen, V.; Beyermann, M.; Pampaloni, F.; Weyand, I. The signal flow and motor response controling chemotaxis of sea urchin sperm. Nat. Cell Biol. 2003, 5, 109–117. [Google Scholar] [CrossRef]
- Yoshida, M.; Murata, M.; Inaba, K.; Morisawa, M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc. Natl. Acad. Sci. USA 2002, 99, 14831–14836. [Google Scholar] [CrossRef] [Green Version]
- Levitan, I.; Christian, A.E.; Tulenko, T.N.; Rothblat, G.H. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 2000, 115, 405–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitan, I.; Fang, Y.; Rosenhouse-Dantsker, A.; Romanenko, V. Cholesterol and ion channels. Subcell. Biochem. 2010, 51, 509–549. [Google Scholar] [CrossRef] [Green Version]
- Picazo-Juárez, G.; Romero-Suárez, S.; Nieto-Posadas, A.S.; Llorente, I.; Jara-Oseguera, A.S.; Briggs, M.; McIntosh, T.J.; Simon, S.A.; Ladrón-de-Guevara, E.; Islas, L.D.; et al. Identification of a binding motif in the S5 helix that confers cholesterol sensitivity to the TRPV1 ion channel. J. Biol. Chem. 2011, 286, 24966–24976. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Huang, W.; Wu, D.; Priestley, J.V. TRPV1, but not P2X3, requires cholesterol for its function and membrane expression in rat nociceptors. Eur. J. Neurosci. 2006, 24, 1–6. [Google Scholar] [CrossRef]
- Storti, B.; Di Rienzo, C.; Cardarelli, F.; Bizzarri, R.; Beltram, F. Unveiling TRPV1 Spatio-Temporal Organization in Live Cell Membranes. PLoS ONE 2015, 10, e0116900. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, N.; Pistilli, M.G.; Mattioli, M.; Barboni, B. Role of TRPV1 channels in boar spermatozoa acquisition of fertilizing ability. Mol. Cell. Endocrinol. 2010, 323, 224–231. [Google Scholar] [CrossRef]
- Schmid, P.C.; Paria, B.C.; Krebsbach, R.J.; Schmid, H.H.O.; Dey, S.K. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 1997, 94, 4188–4192. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Paria, B.C.; Chakraborty, I.; Dey, S.K. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc. Natl. Acad. Sci. USA 1995, 92, 4332–4336. [Google Scholar] [CrossRef] [Green Version]
- Gentilini, D.; Besana, A.; Vigano, P.; Dalino, P.; Vignali, M.; Melandri, M.; Busacca, M.; Di Blasio, A.M. Endocannabinoid system regulates migration of endometrial stromal cells via cannabinoid receptor 1 through the activation of PI3K and ERK1/2 pathways. Fertil. Steril. 2010, 93, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta -Rev. Biomembr. 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Leahy, T.; Gadella, B.M. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J. Androl. 2015, 17, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Bernabò, N.; Palestini, P.; Chiarini, M.; Maccarrone, M.; Mattioli, M.; Barboni, B. Endocannabinoid-binding CB1 and TRPV1 receptors as modulators of sperm capacitation. Commun. Integr. Biol. 2012, 5, 68–70. [Google Scholar] [CrossRef]
- Bernabò, N.; Berardinelli, P.; Mauro, A.; Russo, V.; Lucidi, P.; Mattioli, M.; Barboni, B.; Knoll, A.; Javaux, E.; Hewitt, D.; et al. The role of actin in capacitation-related signaling: An in silico and in vitro study. BMC Syst. Biol. 2011, 5, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervasi, M.G.; Osycka-Salut, C.; Sanchez, T.; Alonso, C.A.I.; Llados, C.; Castellano, L.; Franchi, A.M.; Villalõn, M.; Perez-Martinez, S. Sperm Release from the Oviductal Epithelium Depends on Ca2+ Influx Upon Activation of CB1 and TRPV1 by Anandamide. J. Cell. Biochem. 2016, 117, 320–333. [Google Scholar] [CrossRef]
- Guerrero, A.; Nishigaki, T.; Carneiro, J.; Tatsu, Y.; Wood, C.D.; Darszon, A. Tuning sperm chemotaxis by calcium burst timing. Dev. Biol. 2010, 344, 52–65. [Google Scholar] [CrossRef] [Green Version]
- MicroRNA Signature Targeting Transient Receptor Potential Channels in the Prognosis and Therapy of Cancer. J. Cell. Immunol. 2020, 2. [CrossRef]
- Zhuang, X.; Li, Z.; Lin, H.; Gu, L.; Lin, Q.; Lu, Z.; Tzeng, C.M. Integrated miRNA and mRNA expression profiling to identify mRNA targets of dysregulated miRNAs in non-obstructive azoospermia. Sci. Rep. 2015, 5, 7922. [Google Scholar] [CrossRef]
- Heidary, Z.; Zaki-Dizaji, M.; Saliminejad, K.; Khorram Khorshid, H.R. MicroRNA profiling in spermatozoa of men with unexplained asthenozoospermia. Andrologia 2019, 51, e13284. [Google Scholar] [CrossRef]
- Khawar, M.B.; Mehmood, R.; Roohi, N. Micrornas: Recent insights towards their role in male infertility and reproductive cancers. Bosn. J. Basic Med. Sci. 2019, 19, 31–42. [Google Scholar] [CrossRef]
- Eisenbach, M. Sperm chemotaxis. Rev. Reprod. 1999, 4, 56–66. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Vilensky, A.; Nathan, H. Temperature Changes in the Different Parts of the Rabbit’s Oviduct. Int. J. Gynecol. Obstet. 1972, 10, 52–56. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; Nichol, R. A preovulatory temperature gradient between the isthmus and ampulla of pig oviducts during the phase of sperm storage. J. Reprod. Fertil. 1986, 77, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahat, A.; Caplan, S.R.; Eisenbach, M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS ONE 2012, 7, e41915. [Google Scholar] [CrossRef] [Green Version]
- Brauchi, S.; Orta, G.; Salazar, M.; Rosenmann, E.; Latorre, R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci. 2006, 26, 4835–4840. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cerezales, S.; Bermejo-Álvarez, P. TRPV1: A channel for mammalian sperm thermotaxis? Transl. Cancer Res. 2017, 6. [Google Scholar] [CrossRef]
- Curtis, M.P.; Kirkman-Brown, J.C.; Connolly, T.J.; Gaffney, E.A. Modelling a tethered mammalian sperm cell undergoing hyperactivation. J. Theor. Biol. 2012, 309, 1–10. [Google Scholar] [CrossRef]
- Simons, J.; Olson, S.; Cortez, R.; Fauci, L. The dynamics of sperm detachment from epithelium in a coupled fluid-biochemical model of hyperactivated motility. J. Theor. Biol. 2014, 354, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Chien, K.H.; Yarmishyn, A.A.; Buddhakosai, W.; Wu, W.J.; Lin, T.C.; Chiou, S.H.; Chen, J.T.; Peng, C.H.; Hwang, D.K.; et al. Modulation of osmotic stress-induced TRPV1 expression rescues human iPSC-derived retinal ganglion cells through PKA. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Meriano, J.; Ru, C.; Xie, S.; Luo, J.; Sun, Y. Human sperm rheotaxis: A passive physical process. Sci. Rep. 2016, 6, 23553. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Kenny, S.J.; Mannowetz, N.; Mansell, S.A.; Wojcik, M.; Mendoza, S.; Zucker, R.S.; Xu, K.; Lishko, P.V. Asymmetrically Positioned Flagellar Control Units Regulate Human Sperm Rotation. Cell Rep. 2018, 24, 2606–2613. [Google Scholar] [CrossRef] [Green Version]
- Schiffer, C.; Rieger, S.; Brenker, C.; Young, S.; Hamzeh, H.; Wachten, D.; Tüttelmann, F.; Röpke, A.; Kaupp, U.B.; Wang, T.; et al. Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca2+ signaling. EMBO J. 2020, 39. [Google Scholar] [CrossRef]
- Yonghak, P.; Miyata, S.; Kurganov, E. TRPV1 is crucial for thermal homeostasis in the mouse by heat loss behaviors under warm ambient temperature. Sci. Rep. 2020, 10, 8799. [Google Scholar] [CrossRef]
- Bernabò, N.; Ordinelli, A.; Di Agostino, R.; Mattioli, M.; Barboni, B. Network analyses of sperm-egg recognition and binding: Ready to rethink fertility mechanisms? Omi. A J. Integr. Biol. 2014, 18, 740–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-León, E.; Osycka-Salut, C.; Signorelli, J.; Kong, M.; Morales, P.; Pérez-Martínez, S.; Díaz, E.S. Fibronectin modulates the endocannabinoid system through the cAMP/PKA pathway during human sperm capacitation. Mol. Reprod. Dev. 2019, 86, 224–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Animal Group (Species or Strain) | Year of Publication | Reference |
|---|---|---|
| Bovine (Bos taurus) Swine (Sus scrofa) Rodent (Swiss CD-1 and Sprague-Dawley) Fish (Labeo rohita) Duck (Anas platyrhynchos) Human (Homo sapiens) | 2011 2005 2010, 2009 2013 2020 2016 | [98] [14] [100,102] [97] [18] [8] |
| TRPV1 Modulator | Female Reproductive Tract | Seminal Plasma/Spermatozoa | Animal Group * | Reference |
|---|---|---|---|---|
| 17-β-estradiol | X | Bovine, swine, rabbit, equine | [103,104,105] | |
| Anandamide (AEA) | X | X | Bovine, human, swine, murine | [14,106,107,108] |
| Arachidonic acid (AA) ** | X | Human | [109,110] | |
| Bradykinin | X | Swine | [111] | |
| Docosahexaenoic acid (DHA) ** | X | Human | [109,110,112] | |
| Eicosapentanoic acid (EPA) ** | X | Human | [109,110] | |
| H2S | X | X | Human | [113,114,115] |
| Hyaluronan | X | All mammals | [116,117] | |
| Insulin | X | Human | [118] | |
| Leukotriene B4 | X | Bovine | [119] | |
| Linoleic acid (LA) ** | X | Human | [109,110] | |
| miRNAs | X | X | Murine, bovine, human | [120,121,122] |
| N-acyl amino acids | X | X | Human | [123] |
| Nerve growth factors | X | Rabbit, bovine | [124,125] | |
| NO | X | X | Bovine, human, swine, mouse, equine | [113,115,126] |
| Oleoylethanolamine (OEA) | X | Bovine | [106] | |
| Opioids | X | Human | [127] | |
| Oxytocin | X | Human | [128] | |
| Palmitoylethanolamine (PEA) | X | Bovine | [106] | |
| pH variations | X | Human | [129] | |
| Progesterone | X | Bovine, swine, rabbit, equine | [103,104,105] | |
| Prostaglandins | X | Bovine | [126] | |
| Vitamin D | X | X | Human | [130,131,132] |
| α-linolenic acid (ALA) ** | X | Human | [109,110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramal-Sanchez, M.; Bernabò, N.; Valbonetti, L.; Cimini, C.; Taraschi, A.; Capacchietti, G.; Machado-Simoes, J.; Barboni, B. Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review. Int. J. Mol. Sci. 2021, 22, 4306. https://doi.org/10.3390/ijms22094306
Ramal-Sanchez M, Bernabò N, Valbonetti L, Cimini C, Taraschi A, Capacchietti G, Machado-Simoes J, Barboni B. Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review. International Journal of Molecular Sciences. 2021; 22(9):4306. https://doi.org/10.3390/ijms22094306
Chicago/Turabian StyleRamal-Sanchez, Marina, Nicola Bernabò, Luca Valbonetti, Costanza Cimini, Angela Taraschi, Giulia Capacchietti, Juliana Machado-Simoes, and Barbara Barboni. 2021. "Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review" International Journal of Molecular Sciences 22, no. 9: 4306. https://doi.org/10.3390/ijms22094306
APA StyleRamal-Sanchez, M., Bernabò, N., Valbonetti, L., Cimini, C., Taraschi, A., Capacchietti, G., Machado-Simoes, J., & Barboni, B. (2021). Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review. International Journal of Molecular Sciences, 22(9), 4306. https://doi.org/10.3390/ijms22094306








