Influence of Trace Elements on Neurodegenerative Diseases of The Eye—The Glaucoma Model
Abstract
:1. Introduction
2. Trace Elements in Glaucoma
2.1. Iron
2.2. Copper
2.3. Calcium
2.4. Magnesium
2.5. Molybdenum
2.6. Zinc
2.7. Selenium
2.8. Sodium and Potassium
2.9. Manganese
2.10. Heavy Metals
3. Role of Endothelins
4. Genetic and Genomic Studies
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longo, A.; Avitabile, T.; Uva, M.G.; Bonfiglio, V.; Russo, A.; Toro, M.D.; Faro, S.; Reibaldi, M. Morphology of the optic nerve head in glaucomatous eyes with visual field defects in superior or inferior hemifield. Eur. J. Ophthalmol. 2018, 28, 175–181. [Google Scholar] [CrossRef]
- Chou, T.H.; Romano, G.L.; Amato, R.; Porciatti, V. Nicotinamide-rich diet in dba/2j mice preserves retinal ganglion cell metabolic function as assessed by perg adaptation to flicker. Nutrients 2020, 12, 1910. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussel, I.I.; Aref, A.A. Dietary factors and the risk of glaucoma: A review. Ther. Adv. Chronic. Dis. 2014, 5, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedorowicz, M.; Choragiewicz, T.; Turski, W.A.; Kocki, T.; Nowakowska, D.; Wertejuk, K.; Kaminska, A.; Avitabile, T.; Welniak-Kaminska, M.; Grieb, P.; et al. Tryptophan pathway abnormalities in a murine model of hereditary glaucoma. Int. J. Mol. Sci. 2021, 22, 1039. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, M.; Welniak-Kaminska, M.; Swiatkiewicz, M.; Orzel, J.; Choragiewicz, T.; Toro, M.D.; Rejdak, R.; Bogorodzki, P.; Grieb, P. Changes of ocular dimensions as a marker of disease progression in a murine model of pigmentary glaucoma. Front. Pharmacol. 2020, 11, 573238. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Cheung, L.T.Y.; Manthey, A.L.; Lai, J.S.M.; Chiu, K. Targeted delivery of mitochondrial calcium channel regulators: The future of glaucoma treatment? Front. Neurosci. 2017, 11, 648. [Google Scholar] [CrossRef]
- Toro, M.D.; Brezin, A.P.; Burdon, M.; Cummings, A.B.; Evren Kemer, O.; Malyugin, B.E.; Prieto, I.; Teus, M.A.; Tognetto, D.; Tornblom, R.; et al. Early impact of covid-19 outbreak on eye care: Insights from eurocovcat group. Eur. J. Ophthalmol. 2021, 31, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Posarelli, C.; Ortenzio, P.; Ferreras, A.; Toro, M.D.; Passani, A.; Loiudice, P.; Oddone, F.; Casini, G.; Figus, M. Twenty-four-hour contact lens sensor monitoring of aqueous humor dynamics in surgically or medically treated glaucoma patients. J. Ophthalmol. 2019, 2019, 9890831. [Google Scholar] [CrossRef] [Green Version]
- Posarelli, C.; Toro, M.D.; Rejdak, R.; Zarnowski, T.; Pozarowska, D.; Longo, A.; Miccoli, M.; Nardi, M.; Figus, M. Safety and efficacy of second ahmed valve implant in refractory glaucoma. J. Clin. Med. 2020, 9, 2039. [Google Scholar] [CrossRef] [PubMed]
- Ekici, F.; Korkmaz, S.; Karaca, E.E.; Sul, S.; Tufan, H.A.; Aydin, B.; Dilekoz, E. The role of magnesium in the pathogenesis and treatment of glaucoma. Int. Sch. Res. Notices 2014, 2014, 745439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crish, S.D.; Calkins, D.J. Neurodegeneration in glaucoma: Progression and calcium-dependent intracellular mechanisms. Neuroscience 2011, 176, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedorowicz, M.; Choragiewicz, T.; Thaler, S.; Schuettauf, F.; Nowakowska, D.; Wojtunik, K.; Reibaldi, M.; Avitabile, T.; Kocki, T.; Turski, W.A.; et al. Tryptophan and kynurenine pathway metabolites in animal models of retinal and optic nerve damage: Different dynamics of changes. Front. Physiol. 2019, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Sourkes, T.L. Influence of specific nutrients on catecholamine synthesis and metabolism. Pharmacol Rev. 1972, 24, 349–359. [Google Scholar]
- Paoletti, P.; Vergnano, A.M.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Calcium control of neurotransmitter release. Cold Spring Harb Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef]
- Corona, C.; Pensalfini, A.; Frazzini, V.; Sensi, S.L. New therapeutic targets in alzheimer’s disease: Brain deregulation of calcium and zinc. Cell Death Dis. 2011, 2, e176. [Google Scholar] [CrossRef] [Green Version]
- Hohberger, B.; Chaudhri, M.A.; Michalke, B.; Lucio, M.; Nowomiejska, K.; Schlotzer-Schrehardt, U.; Grieb, P.; Rejdak, R.; Junemann, A.G.M. Levels of aqueous humor trace elements in patients with open-angle glaucoma. J. Trace Elem. Med. Biol. 2018, 45, 150–155. [Google Scholar] [CrossRef]
- Farkas, R.H.; Chowers, I.; Hackam, A.S.; Kageyama, M.; Nickells, R.W.; Otteson, D.C.; Duh, E.J.; Wang, C.; Valenta, D.F.; Gunatilaka, T.L.; et al. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1410–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gye, H.J.; Kim, J.M.; Yoo, C.; Shim, S.H.; Won, Y.S.; Sung, K.C.; Lee, M.Y.; Park, K.H. Relationship between high serum ferritin level and glaucoma in a south korean population: The kangbuk samsung health study. Br. J. Ophthalmol 2016, 100, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Shimazawa, M.; Sasaoka, M.; Yamada, C.; Iwakura, Y.; Sakai, T.; Maeda, Y.; Yamaguchi, T.; Sukamoto, T.; Hashimoto, M. Selective effects of lomerizine, a novel diphenylmethylpiperazine ca2+ channel blocker, on cerebral blood flow in rats and dogs. Clin. Exp. Pharmacol. Physiol. 1999, 26, 870–876. [Google Scholar] [CrossRef]
- Al-Dabbagh, N.; Al-Shahrani, H.; Al-Dohayan, N.; Mustafa, M.; Arfin, M.; Al-Asmari, A.K. The sparc-related modular calcium binding protein 2 (smoc2) gene polymorphism in primary glaucoma: A case-control study. Clin. Ophthalmol 2017, 11, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol 2005, 18, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Head, K.A. Natural therapies for ocular disorders, part two: Cataracts and glaucoma. Altern. Med. Rev. 2001, 6, 141–166. [Google Scholar] [PubMed]
- Houde, M.; Desbiens, L.; D’Orleans-Juste, P. Endothelin-1: Biosynthesis, signaling and vasoreactivity. Adv. Pharmacol. 2016, 77, 143–175. [Google Scholar]
- Hurst, S.; Hoek, J.; Sheu, S.S. Mitochondrial ca(2+) and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2017, 49, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Muhammad, Z.; Shah, M.T.; Bashir, S.; Khan, T.; Khan, M.D. Relationship between the concentration of copper and iron in the aqueous humour and intraocular pressure in rabbits treated with topical steroids. Clin. Exp. Ophthalmol. 2002, 30, 28–35. [Google Scholar] [PubMed]
- Ishikawa, K.; Funayama, T.; Ohtake, Y.; Kimura, I.; Ideta, H.; Nakamoto, K.; Yasuda, N.; Fukuchi, T.; Fujimaki, T.; Murakami, A.; et al. Association between glaucoma and gene polymorphism of endothelin type a receptor. Mol. Vis. 2005, 11, 431–437. [Google Scholar]
- Hara, H.; Toriu, N.; Shimazawa, M. Clinical potential of lomerizine, a Ca2+ channel blocker as an anti-glaucoma drug: Effects on ocular circulation and retinal neuronal damage. Cardiovasc. Drug Rev. 2004, 22, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Kitazawa, Y.; Shirai, H.; Go, F.J. The effect of ca2(+) -antagonist on visual field in low-tension glaucoma. Graefes. Arch. Clin. Exp. Ophthalmol. 1989, 227, 408–412. [Google Scholar] [CrossRef]
- Brown, N.A.; Bron, A.J.; Harding, J.J.; Dewar, H.M. Nutrition supplements and the eye. Eye 1998, 12 (Pt 1), 127–133. [Google Scholar] [CrossRef]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Trace Elem. Med. Biol. 2014, 28, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, G.; Krishnamoorthy, R.; Clark, A.F.; Wordinger, R.J.; Yorio, T. Human optic nerve head astrocytes as a target for endothelin-1. Invest. Ophthalmol. Vis. Sci. 2002, 43, 2704–2713. [Google Scholar] [PubMed]
- DeToma, A.S.; Dengler-Crish, C.M.; Deb, A.; Braymer, J.J.; Penner-Hahn, J.E.; van der Schyf, C.J.; Lim, M.H.; Crish, S.D. Abnormal metal levels in the primary visual pathway of the dba/2j mouse model of glaucoma. Biometals 2014, 27, 1291–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahn, B.H.; Paterson, P.G.; Gottschall-Pass, K.T.; Zhang, Z. Zinc and the eye. J. Am. Coll. Nutr. 2001, 20, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Newsome, D.; Miceli, M.; Tate, D.; Alcock, N.; Oliver, P. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration, but superoxide dismutase activity increases. J. Trace Elem. Exp. Med. 1996, 8, 193–199. [Google Scholar] [CrossRef]
- Noske, W.; Hensen, J.; Wiederholt, M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefes. Arch. Clin. Exp. Ophthalmol. 1997, 235, 551–552. [Google Scholar] [CrossRef]
- Osborne, N.N.; Nunez-Alvarez, C.; Joglar, B.; Del Olmo-Aguado, S. Glaucoma: Focus on mitochondria in relation to pathogenesis and neuroprotection. Eur. J. Pharmacol. 2016, 787, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.R.; Kurup, P.A. Inhibition of membrane na+-k+ atpase activity: A common pathway in central nervous system disorders. J. Assoc. Physicians India 2002, 50, 400–406. [Google Scholar]
- Laganovska, G.; Martinsons, A.; Pitrans, B.; Widner, B.; Fuchs, D. Kynurenine and neopterin in the aqueous humor of the anterior chamber of the eye and in serum of cataract patients. Adv. Exp. Med. Biol. 2003, 527, 367–374. [Google Scholar] [PubMed]
- Lee, S.H.; Kang, E.M.; Kim, G.A.; Kwak, S.W.; Kim, J.M.; Bae, H.W.; Seong, G.J.; Kim, C.Y. Three toxic heavy metals in open-angle glaucoma with low-teen and high-teen intraocular pressure: A cross-sectional study from south korea. PLoS ONE 2016, 11, e0164983. [Google Scholar] [CrossRef] [PubMed]
- Lenartowicz, M.; Krzeptowski, W.; Lipinski, P.; Grzmil, P.; Starzynski, R.; Pierzchala, O.; Moller, L.B. Mottled mice and non-mammalian models of menkes disease. Front. Mol. Neurosci. 2015, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Jankovic, J.; Le, W. Iron chelation and neuroprotection in neurodegenerative diseases. J. Neural. Transm. 2011, 118, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Lillico, A.J.E.; Reid, M.E. Selenium Supplementation and Risk of Glaucoma in the npc Trial; University of Arizona: Tucson, Arizona, 2002. [Google Scholar]
- Junemann, A.G.M.; Michalke, B.; Lucio, M.; Chaudhri, A.; Schlotzer-Schrehardt, U.; Rejdak, R.; Rekas, M.; Hohberger, B. Aqueous humor selenium level and open-angle glaucoma. J. Trace Elem. Med. Biol. 2018, 50, 67–72. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Neuroprotection by sodium channel blockade with phenytoin in an experimental model of glaucoma. Invest. Ophthalmol. Vis. Sci. 2005, 46, 4164–4169. [Google Scholar] [CrossRef] [Green Version]
- Ramdas, W.D. The relation between dietary intake and glaucoma: A systematic review. Acta Ophthalmol. 2018, 96, 550–556. [Google Scholar] [CrossRef]
- Ribas, V.T.; Koch, J.C.; Michel, U.; Bahr, M.; Lingor, P. Attenuation of axonal degeneration by calcium channel inhibitors improves retinal ganglion cell survival and regeneration after optic nerve crush. Mol. Neurobiol. 2017, 54, 72–86. [Google Scholar] [CrossRef]
- Chowdhury, U.R.; Bahler, C.K.; Holman, B.H.; Dosa, P.I.; Fautsch, M.P. Ocular hypotensive effects of the atp-sensitive potassium channel opener cromakalim in human and murine experimental model systems. PLoS ONE 2015, 10, e0141783. [Google Scholar]
- Chowdhury, U.R.; Viker, K.B.; Stoltz, K.L.; Holman, B.H.; Fautsch, M.P.; Dosa, P.I. Analogs of the atp-sensitive potassium (katp) channel opener cromakalim with in vivo ocular hypotensive activity. J. Med. Chem. 2016, 59, 6221–6231. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, U.R.; Bahler, C.K.; Holman, B.H.; Fautsch, M.P. Atp-sensitive potassium (katp) channel openers diazoxide and nicorandil lower intraocular pressure by activating the erk1/2 signaling pathway. PLoS ONE 2017, 12, e0179345. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, U.R.; Dosa, P.I.; Fautsch, M.P. Atp sensitive potassium channel openers: A new class of ocular hypotensive agents. Exp. Eye Res. 2019, 178, 225. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Ohashi, M.; Naito, A.; Fukaya, Y.; Suzuki, Y.; Araie, M. Neuroprotective effect of the novel na+/ca2+ channel blocker ns-7 on rat retinal ganglion cells. Jpn. J. Ophthalmol. 2005, 49, 371–376. [Google Scholar] [CrossRef]
- Sakamoto, K.; Yonoki, Y.; Kuwagata, M.; Saito, M.; Nakahara, T.; Ishii, K. Histological protection against ischemia-reperfusion injury by early ischemic preconditioning in rat retina. Brain Res. 2004, 1015, 154–160. [Google Scholar] [CrossRef]
- Salvatore, S.; Vingolo, E.M. Endothelin-1 role in human eye: A review. J. Ophthalmol. 2010, 2010, 354645. [Google Scholar] [CrossRef]
- Sample, P.A.; Weinreb, R.N.; Boynton, R.M. Acquired dyschromatopsia in glaucoma. Surv. Ophthalmol. 1986, 31, 54–64. [Google Scholar] [CrossRef]
- Savigni, D.L.; O’Hare Doig, R.L.; Szymanski, C.R.; Bartlett, C.A.; Lozic, I.; Smith, N.M.; Fitzgerald, M. Three ca2+ channel inhibitors in combination limit chronic secondary degeneration following neurotrauma. Neuropharmacology 2013, 75, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwalfenberg, G.K.; Genuis, S.J. The importance of magnesium in clinical healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef] [PubMed]
- Sheck, L.; Davies, J.; Wilson, G. Selenium and ocular health in new zealand. N. Z. Med. J. 2010, 123, 85–94. [Google Scholar] [PubMed]
- Shoshani, Y.Z.; Harris, A.; Shoja, M.M.; Rusia, D.; Siesky, B.; Arieli, Y.; Wirostko, B. Endothelin and its suspected role in the pathogenesis and possible treatment of glaucoma. Curr. Eye Res. 2012, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siegner, S.W.; Netland, P.A.; Schroeder, A.; Erickson, K.A. Effect of calcium channel blockers alone and in combination with antiglaucoma medications on intraocular pressure in the primate eye. J. Glaucoma. 2000, 9, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Silverstone, B.; Berson, D.; Eilat, U.; Kuperman, O. Copper metabolism changes in pigmentary retinopathies and high myopia. Metab. Pediatr. Ophthalmol. 1981, 5, 49–53. [Google Scholar] [PubMed]
- Silverstone, B.Z. Effects of zinc and copper metabolism in highly myopic patients. Ciba Found. Symp. 1990, 155, 210–217, discussion 217-221. [Google Scholar] [PubMed]
- Skatchkov, S.N.; Rojas, L.; Eaton, M.J.; Orkand, R.K.; Biedermann, B.; Bringmann, A.; Pannicke, T.; Veh, R.W.; Reichenbach, A. Functional expression of kir 6.1/sur1-k(atp) channels in frog retinal muller glial cells. Glia 2002, 38, 256–267. [Google Scholar] [CrossRef]
- Demirci, F.Y.; Chang, M.H.; Mah, T.S.; Romero, M.F.; Gorin, M.B. Proximal renal tubular acidosis and ocular pathology: A novel missense mutation in the gene (slc4a4) for sodium bicarbonate cotransporter protein (nbce1). Mol. Vis. 2006, 12, 324–330. [Google Scholar]
- Tykocki, N.R.; Watts, S.W. The interdependence of endothelin-1 and calcium: A review. Clin. Sci. 2010, 119, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, O.M.; Can Demirdogen, B.; Mumcuoglu, T.; Aykut, O. Evaluation of essential and toxic trace elements in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Biol. Trace Elem. Res. 2013, 153, 28–34. [Google Scholar] [CrossRef]
- Akyol, N.; Deger, O.; Keha, E.E.; Kilic, S. Aqueous humour and serum zinc and copper concentrations of patients with glaucoma and cataract. Br. J. Ophthalmol. 1990, 74, 661–662. [Google Scholar] [CrossRef] [Green Version]
- Vinetskaia, M.I.; Iomdina, E.N. Study of lacrimal fluid trace elements in several eye diseases. Vestn. Oftalmol. 1994, 110, 24–26. [Google Scholar]
- Bucolo, C.; Drago, F. Carbon monoxide and the eye: Implications for glaucoma therapy. Pharmacol. Ther. 2011, 130, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Youdim, M.B. Iron and neurodegeneration: Prospects for neuroprotection chapter 4. In Neurodegeneration and Neuroprotection in Parkinson’s Disease Neuroscience Perspectives; Academic Press: Cambridge, MA, USA, 1996; p. 224. [Google Scholar]
- Lipinski, P.; Starzynski, R.R.; Stys, A.; Gajowiak, A.; Staron, R. Heme metabolism as an integral part of iron homeostasis. Postepy Hig Med Dosw (Online) 2014, 68, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Thaler, S.; Fiedorowicz, M.; Rejdak, R.; Choragiewicz, T.J.; Sulejczak, D.; Stopa, P.; Zarnowski, T.; Zrenner, E.; Grieb, P.; Schuettauf, F. Neuroprotective effects of tempol on retinal ganglion cells in a partial optic nerve crush rat model with and without iron load. Exp. Eye Res. 2010, 90, 254–260. [Google Scholar] [CrossRef]
- Lin, S.C.; Wang, S.Y.; Yoo, C.; Singh, K.; Lin, S.C. Association between serum ferritin and glaucoma in the south korean population. JAMA Ophthalmol. 2014, 132, 1414–1420. [Google Scholar] [CrossRef] [Green Version]
- Lenartowicz, M.; Grzmil, P.; Shoukier, M.; Starzynski, R.; Marciniak, M.; Lipinski, P. Mutation in the cpc motif-containing 6th transmembrane domain affects intracellular localization, trafficking and copper transport efficiency of atp7a protein in mosaic mutant mice--an animal model of menkes disease. Metallomics 2012, 4, 197–204. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Y.; Yu, C.; Huang, R.; Du, Q.; Zhao, J.; Sun, W.; Wang, W. Enhanced fenton-like catalytic activity and stability of g-c3n4 nanosheet-wrapped copper phosphide with strong anti-interference ability: Kinetics and mechanistic study. J. Colloid Interface Sci. 2021, 595, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Ucgun, N.I.; Kilic, N.; Gursel, E.; Sepici-Dincel, A. Pseudoexfoliation syndrome and trace elements. Ann. N. Y. Acad. Sci. 2007, 1100, 207–212. [Google Scholar] [CrossRef]
- Braet, K.; Cabooter, L.; Paemeleire, K.; Leybaert, L. Calcium signal communication in the central nervous system. Biol. Cell 2004, 96, 79–91. [Google Scholar] [CrossRef]
- Vosler, P.S.; Brennan, C.S.; Chen, J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008, 38, 78–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netland, P.A.; Chaturvedi, N.; Dreyer, E.B. Calcium channel blockers in the management of low-tension and open-angle glaucoma. Am. J. Ophthalmol. 1993, 115, 608–613. [Google Scholar] [CrossRef]
- Wang, S.Y.; Singh, K.; Lin, S.C. The association between glaucoma prevalence and supplementation with the oxidants calcium and iron. Invest. Ophthalmol. Vis. Sci. 2012, 53, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium ions in neuronal degeneration. IUBMB Life 2008, 60, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Araie, M.; Mayama, C. Use of calcium channel blockers for glaucoma. Prog. Retin. Eye Res. 2011, 30, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Ganekal, S.; Dorairaj, S.; Jhanji, V.; Kudlu, K. Effect of topical calcium channel blockers on intraocular pressure in steroid-induced glaucoma. J. Curr. Glaucoma. Pract. 2014, 8, 15–19. [Google Scholar] [PubMed] [Green Version]
- Yu, D.Y.; Su, E.N.; Cringle, S.J.; Alder, V.A.; Yu, P.K.; DeSantis, L. Systemic and ocular vascular roles of the antiglaucoma agents beta-adrenergic antagonists and Ca2+ entry blockers. Surv. Ophthalmol. 1999, 43 (Suppl. 1), S214–S222. [Google Scholar] [CrossRef]
- Piltz, J.R.; Bose, S.; Lanchoney, D. The effect of nimodipine, a centrally active calcium antagonist, on visual function and mascular blood flow in patients with normal-tension glaucoma and control subjects. J. Glaucoma. 1998, 7, 336–342. [Google Scholar] [CrossRef]
- Yamamoto, T.; Niwa, Y.; Kawakami, H.; Kitazawa, Y. The effect of nilvadipine, a calcium-channel blocker, on the hemodynamics of retrobulbar vessels in normal-tension glaucoma. J. Glaucoma. 1998, 7, 301–305. [Google Scholar] [CrossRef]
- Osborne, N.N.; Wood, J.P.; Chidlow, G.; Casson, R.; DeSantis, L.; Schmidt, K.G. Effectiveness of levobetaxolol and timolol at blunting retinal ischaemia is related to their calcium and sodium blocking activities: Relevance to glaucoma. Brain Res. Bull. 2004, 62, 525–528. [Google Scholar] [CrossRef]
- Wang, R.F.; Gagliuso, D.J.; Podos, S.M. Effect of flunarizine, a calcium channel blocker, on intraocular pressure and aqueous humor dynamics in monkeys. J. Glaucoma. 2008, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Melena, J.; Stanton, D.; Osborne, N.N. Comparative effects of antiglaucoma drugs on voltage-dependent calcium channels. Graefes. Arch. Clin. Exp. Ophthalmol. 2001, 239, 522–530. [Google Scholar] [CrossRef]
- Hong, S.J.; Wu, K.Y.; Wang, H.Z.; Fong, J.C. Effects of commercial antiglaucoma drugs to glutamate-induced [ca2+)]i increase in cultured neuroblastoma cells. J. Ocul. Pharmacol. Ther. 2003, 19, 205–215. [Google Scholar] [CrossRef]
- Liu, S.; Araujo, S.V.; Spaeth, G.L.; Katz, L.J.; Smith, M. Lack of effect of calcium channel blockers on open-angle glaucoma. J. Glaucoma. 1996, 5, 187–190. [Google Scholar] [CrossRef]
- Mayama, C. Calcium channels and their blockers in intraocular pressure and glaucoma. Eur. J. Pharmacol. 2014, 739, 96–105. [Google Scholar] [CrossRef]
- DeWitt, C.R.; Waksman, J.C. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol. Rev. 2004, 23, 223–238. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, L.; Agarwal, P. Pathogenetic role of magnesium deficiency in ophthalmic diseases. Biometals 2014, 27, 5–18. [Google Scholar] [CrossRef] [PubMed]
- George, G.A.; Heaton, F.W. Changes in cellular composition during magnesium deficiency. Biochem. J. 1975, 152, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gathwala, G. Neuronal protection with magnesium. Indian J. Pediatr. 2001, 68, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.Z.; Gasser, P.; Flammer, J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Ophthalmologica 1995, 209, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B.; Onol, M.; Hondur, A.; Kaya, M.G.; Ozdemir, H.; Cengel, A.; Hasanreisoglu, B. The effect of oral magnesium therapy on visual field and ocular blood flow in normotensive glaucoma. Eur. J. Ophthalmol. 2010, 20, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Mlyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-Depend. nf-kappab Signal. Inflammopharmacol. 2017, 25, 11–24. [Google Scholar]
- Formigari, A.; Santon, A.; Irato, P. Efficacy of zinc treatment against iron-induced toxicity in rat hepatoma cell line h4-ii-e-c3. Liver Int. 2007, 27, 120–127. [Google Scholar] [CrossRef]
- Karcioglu, Z.A. Zinc in the eye. Surv. Ophthalmol. 1982, 27, 114–122. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N. Zinc in the retina. Prog. Neurobiol. 2001, 64, 219–249. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N. The localization of free zinc varies in rat photoreceptors during light and dark adaptation. Exp. Eye Res. 1999, 69, 459–461. [Google Scholar] [CrossRef] [PubMed]
- King, A.J. Should we be considering selenium in glaucoma? Br. J. Ophthalmol. 2009, 93, 1132–1133. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, R.L.; Stamer, W.D.; Herrygers, L.A.; Levine, J.M.; Noecker, R.J. Relationship between glaucoma and selenium levels in plasma and aqueous humour. Br. J. Ophthalmol. 2009, 93, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Ayaz, L.; Tamer, L. Selenium and pseudoexfoliation syndrome. Am. J. Ophthalmol. 2011, 151, 272–276.e271. [Google Scholar] [CrossRef] [PubMed]
- Conley, S.M.; McKay, B.S.; Gandolfi, A.J.; Stamer, W.D. Alterations in human trabecular meshwork cell homeostasis by selenium. Exp. Eye Res. 2006, 82, 637–647. [Google Scholar] [CrossRef]
- Winder, A.F.; Siddiqui, A.A.; Donovan, H.C. Ocular hypertension and systemic responses to the water-drinking test. Br. J. Ophthalmol. 1978, 62, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.S.; Wang, J.; Liu, W.M.; Zhu, Y.H. Potassium ion channels in retinal ganglion cells (review). Mol. Med. Rep. 2013, 8, 311–319. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.E.; Brandt, J.D.; Curry, F.R. Na-k-cl cotransport regulates intracellular volume and monolayer permeability of trabecular meshwork cells. Am. J. Physiol. 1995, 268, C1067–C1074. [Google Scholar] [CrossRef] [PubMed]
- Alberty, R.A. Effect of ph and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J. Biol. Chem. 1968, 243, 1337–1343. [Google Scholar] [CrossRef]
- Dong, C.J.; Hare, W.A. Contribution to ischemic injury of rat optic nerves by intracellular sodium overload. Doc. Ophthalmol. 2005, 110, 15–23. [Google Scholar] [CrossRef]
- Katz, A.; Tal, D.M.; Heller, D.; Habeck, M.; Ben Zeev, E.; Rabah, B.; Bar Kana, Y.; Marcovich, A.L.; Karlish, S.J. Digoxin derivatives with selectivity for the alpha2beta3 isoform of na,k-atpase potently reduce intraocular pressure. Proc. Natl. Acad. Sci. USA 2015, 112, 13723–13728. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kashii, S.; Yasuyoshi, H.; Zhang, S.; Honda, Y.; Akaike, A. Mitochondrial atp-sensitive potassium channel: A novel site for neuroprotection. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2750–2756. [Google Scholar] [CrossRef] [Green Version]
- Crowston, J.G.; Weinreb, R.N. Glaucoma medication and aqueous humor dynamics. Curr. Opin. Ophthalmol. 2005, 16, 94–100. [Google Scholar] [CrossRef]
- Marquis, R.E.; Whitson, J.T. Management of glaucoma: Focus on pharmacological therapy. Drugs. Aging 2005, 22, 1–21. [Google Scholar] [CrossRef]
- Choi, A.; Choi, J.S.; Yoon, Y.J.; Kim, K.A.; Joo, C.K. Kr-31378, a potassium-channel opener, induces the protection of retinal ganglion cells in rat retinal ischemic models. J. Pharmacol. Sci. 2009, 109, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Bahler, C.K.; Howell, K.G.; Hann, C.R.; Fautsch, M.P.; Johnson, D.H. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am. J. Ophthalmol. 2008, 145, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putney, L.K.; Brandt, J.D.; O’Donnell, M.E. Effects of dexamethasone on sodium-potassium-chloride cotransport in trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1997, 38, 1229–1240. [Google Scholar] [PubMed]
- Wolonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postepy Hig. Med. Dosw 2016, 70, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Singh, K.; Lin, S.C. Association between body levels of trace metals and glaucoma prevalence. JAMA Ophthalmol. 2015, 133, 1144–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamall, I.S.; Roque, H. Cadmium-induced alterations in ocular trace elements. Influence of dietary selenium and copper. Biol. Trace Elem. Res. 1989, 23, 55–63. [Google Scholar] [CrossRef]
- Yuki, K.; Dogru, M.; Imamura, Y.; Kimura, I.; Ohtake, Y.; Tsubota, K. Lead accumulation as possible risk factor for primary open-angle glaucoma. Biol. Trace Elem. Res. 2009, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Siddiqui, M.K. Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta 2007, 383, 57–64. [Google Scholar] [CrossRef]
- Wu, K.Y.; Wang, H.Z.; Hong, S.J. Inhibition of endothelin-1 and kcl-induced increase of [ca2+]i by antiglaucoma drugs in cultured a7r5 vascular smooth-muscle cells. J. Ocul. Pharmacol. Ther. 2004, 20, 201–209. [Google Scholar] [CrossRef]
- Marasciulo, F.L.; Montagnani, M.; Potenza, M.A. Endothelin-1: The yin and yang on vascular function. Curr. Med. Chem. 2006, 13, 1655–1665. [Google Scholar] [CrossRef]
- Orgul, S.; Cioffi, G.A.; Wilson, D.J.; Bacon, D.R.; Van Buskirk, E.M. An endothelin-1 induced model of optic nerve ischemia in the rabbit. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1860–1869. [Google Scholar]
- Nakano, D.; Pollock, D. New concepts in endothelin control of sodium balance. Clin. Exp. Pharmacol. Physiol. 2012, 39, 104–110. [Google Scholar] [CrossRef]
- Bkaily, G.; Nader, M.; Avedanian, L.; Choufani, S.; Jacques, D.; D’Orleans-Juste, P.; Gobeil, F.; Chemtob, S.; Al-Khoury, J. G-protein-coupled receptors, channels, and na+-h+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. Can. J. Physiol. Pharmacol. 2006, 84, 431–441. [Google Scholar] [CrossRef]
- Okafor, M.C.; Delamere, N.A. The inhibitory influence of endothelin on active sodium-potassium transport in porcine lens. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1018–1023. [Google Scholar]
- Mozaffarieh, M.; Flammer, J. Is there more to glaucoma treatment than lowering iop? Surv. Ophthalmol. 2007, 52, S174–S179. [Google Scholar] [CrossRef] [PubMed]
- Dettmann, E.S.; Luscher, T.F.; Flammer, J.; Haefliger, I.O. Modulation of endothelin-1-induced contractions by magnesium/calcium in porcine ciliary arteries. Graefes. Arch. Clin. Exp. Ophthalmol. 1998, 236, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Toro, M.D.; Rejdak, R.; Zaluska, W.; Gagliano, C.; Sikora, P. Ophthalmic evaluation of diagnosed cases of eye cystinosis: A tertiary care center’s experience. Diagnostics 2020, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Wiest, M.R.J.; Toro, M.D.; Nowak, A.; Baur, J.; Fasler, K.; Hamann, T.; Al-Sheikh, M.; Zweifel, S.A. Globotrioasylsphingosine levels and optical coherence tomography angiography in fabry disease patients. J. Clin. Med. 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Koman-Wierdak, E.; Nowomiejska, K.; Brzozowska, A.; Nowakowska, D.; Toro, M.D.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Rejdak, R. Kinetic and static perimetry after 16 years and additional oct-a analysis in eyes with long-lasting optic disc drusen. PLoS ONE 2021, 16, e0247399. [Google Scholar] [CrossRef]
- Haas, M. The na-k-cl cotransporters. Am. J. Physiol. 1994, 267, C869–C885. [Google Scholar] [CrossRef]
- Haas, M.; Forbush, B., 3rd. The na-k-cl cotransporters. J. Bioenerg. Biomembr. 1998, 30, 161–172. [Google Scholar] [CrossRef]
- Putney, L.K.; Brandt, J.D.; O’Donnell, M.E. Na-k-cl cotransport in normal and glaucomatous human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1999, 40, 425–434. [Google Scholar]
- Janssen, S.F.; Gorgels, T.G.; van der Spek, P.J.; Jansonius, N.M.; Bergen, A.A. In silico analysis of the molecular machinery underlying aqueous humor production: Potential implications for glaucoma. J. Clin. Bioinforma. 2013, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Bok, D.; Schibler, M.J.; Pushkin, A.; Sassani, P.; Abuladze, N.; Naser, Z.; Kurtz, I. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pnbc1 and knbc1 in the rat eye. Am. J. Physiol. Renal. Physiol. 2001, 281, F920–F935. [Google Scholar] [CrossRef]
- Vibat, C.R.; Holland, M.J.; Kang, J.J.; Putney, L.K.; O’Donnell, M.E. Quantitation of na+-k+-2cl- cotransport splice variants in human tissues using kinetic polymerase chain reaction. Anal. Biochem. 2001, 298, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Inatomi, J.; Sekine, T.; Cha, S.H.; Kanai, Y.; Kunimi, M.; Tsukamoto, K.; Satoh, H.; Shimadzu, M.; Tozawa, F.; et al. Mutations in slc4a4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat. Genet. 1999, 23, 264–266. [Google Scholar] [CrossRef]
- Dinour, D.; Chang, M.H.; Satoh, J.; Smith, B.L.; Angle, N.; Knecht, A.; Serban, I.; Holtzman, E.J.; Romero, M.F. A novel missense mutation in the sodium bicarbonate cotransporter (nbce1/slc4a4) causes proximal tubular acidosis and glaucoma through ion transport defects. J. Biol. Chem. 2004, 279, 52238–52246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Van Paesschen, W.; Stalmans, I.; Horita, S.; Yamada, H.; Bergmans, B.A.; Legius, E.; Riant, F.; De Jonghe, P.; Li, Y.; et al. Defective membrane expression of the na(+)-hco(3)(-) cotransporter nbce1 is associated with familial migraine. Proc. Natl. Acad. Sci. USA 2010, 107, 15963–15968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, T.; Inatomi, J.; Sekine, T.; Seki, G.; Shimadzu, M.; Tozawa, F.; Takeshima, Y.; Takumi, T.; Takahashi, T.; Yoshikawa, N.; et al. Novel nonsense mutation in the na+/hco3- cotransporter gene (slc4a4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J. Am. Soc. Nephrol. 2001, 12, 713–718. [Google Scholar] [PubMed]
- Minton, A.Z.; Phatak, N.R.; Stankowska, D.L.; He, S.; Ma, H.Y.; Mueller, B.H.; Jiang, M.; Luedtke, R.; Yang, S.; Brownlee, C.; et al. Endothelin b receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE 2012, 7, e43199. [Google Scholar]
- McGrady, N.R.; Minton, A.Z.; Stankowska, D.L.; He, S.; Jefferies, H.B.; Krishnamoorthy, R.R. Upregulation of the endothelin a (eta) receptor and its association with neurodegeneration in a rodent model of glaucoma. BMC Neurosci. 2017, 18, 27. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Park, Y.H.; Yorio, T.; Krishnamoorthy, R.R. Endothelin-mediated changes in gene expression in isolated purified rat retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6144–6161. [Google Scholar] [CrossRef] [Green Version]
- Mi, X.S.; Zhang, X.; Feng, Q.; Lo, A.C.; Chung, S.K.; So, K.F. Progressive retinal degeneration in transgenic mice with overexpression of endothelin-1 in vascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 2012, 53, 4842–4851. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, J.Y.; Kim, D.M.; Ko, H.S.; Kim, S.Y.; Yoo, T.; Hwang, S.S.; Park, S.S. Investigations on the association between normal tension glaucoma and single nucleotide polymorphisms of the endothelin-1 and endothelin receptor genes. Mol. Vis. 2006, 12, 1016–1021. [Google Scholar]
- Kosior-Jarecka, E.; Wrobel-Dudzinska, D.; Lukasik, U.; Aung, T.; Khor, C.C.; Kocki, J.; Zarnowski, T. Plasma endothelin-1 and single nucleotide polymorphisms of endothelin-1 and endothelin type a receptor genes as risk factors for normal tension glaucoma. Mol. Vis. 2016, 22, 1256–1266. [Google Scholar] [PubMed]
- Liu, W.; Liu, Y.; Qin, X.J.; Schmidt, S.; Hauser, M.A.; Allingham, R.R. Aqp1 and slc4a10 as candidate genes for primary open-angle glaucoma. Mol. Vis. 2010, 16, 93–97. [Google Scholar] [PubMed]
- Golubnitschaja, O.; Yeghiazaryan, K.; Liu, R.; Monkemann, H.; Leppert, D.; Schild, H.; Haefliger, I.O.; Flammer, J. Increased expression of matrix metalloproteinases in mononuclear blood cells of normal-tension glaucoma patients. J. Glaucoma. 2004, 13, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Souzeau, E.; Sharma, S.; Landers, J.; Mills, R.; Goldberg, I.; Healey, P.R.; Graham, S.; Hewitt, A.W.; Mackey, D.A.; et al. Whole exome sequencing implicates eye development, the unfolded protein response and plasma membrane homeostasis in primary open-angle glaucoma. PLoS ONE 2017, 12, e0172427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Singh, K.; Lin, S.C. Glaucoma prevalence and the intake of iron and calcium in a population-based study. Curr. Eye Res. 2013, 38, 1049–1056. [Google Scholar] [CrossRef]

| Elements | Authors/Year | Main Results |
|---|---|---|
| Iron and copper | Farkas R.H., et al., 2014 [21] | Comparison of glaucomatous with control monkey retinas demonstrated increased mRNA expression of iron-regulating proteins. The increased levels of iron-regulating proteins in glaucoma are beneficial, because of their ability to limit iron-related oxidation. |
| Gye H.J. et al., 2016 [22] | Serum ferritin has become the preferred marker for assessing iron-related oxidative stress. High serum ferritin levels were independently associated with greater risk for glaucoma. | |
| Hara H. et al., 1999 [23] | Lomerizine increased cerebral blood flow in animal models, with no significant adverse effects. This suggests that this drug may be clinically useful in conditions associated with circulatory disturbances (such as migraine, normal-tension glaucoma, vertigo and stroke). | |
| Calcium | Al-Dabbagh N. et al., 2017 [24] | Mutation of the SPARC-related modular calcium-binding protein 2 (SMOC2) gene may be a risk factor for glaucoma, probably secondary to modification of the biological function of the Ca receptor proteins in ocular tissues. |
| Hartikainen H. et al., 2005 [25] | Calpain, a ubiquitous calcium-sensitive protease, is known to play a role in the neurodegenerative diseases such as cerebral ischemia, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and others | |
| Head K.A. et al., 2001 [26] | Altered calcium homeostasis makes neurons more vulnerable to oxidative stress | |
| Hohberger B. et al., 2018 [20] | Plasma membrane calcium channel inhibitors were found to arrest acute axonal degeneration and improve regeneration after damage to the optic nerve | |
| Houde M. et al., 2016 [27] | In vivo studies showed the neuroprotective effects of calcium channel blockers. Moreover, beta-adrenoceptors show calcium channel blocking activity, which may be responsible for the neuroprotective effects. | |
| Hurst S. et al., 2017 [28] | Animals topically treated with calcium channel blocking agents showed a significant reduction of intraocular pressure in steroid induced glaucoma. | |
| Iqbal Z. et al., 2002 [29] | Treatment with calcium channel antagonist (nimodipine) significantly improved the visual field and color vision in glaucoma patients. | |
| Ishikawa K. et al., 2005 [30] | Oral nilvadipine increased the blood flow in distal retrobulbar arteries in normal-tension glaucoma. | |
| Hara et al., 1999, 2004 [23,31] | The Ca2+ channel blocker lomerizine increases ocular circulation and protects neuronal cells in animal models. It may be useful as a therapeutic drug against retinal diseases that involve a disturbance of the ocular circulation (such as glaucoma and retinal vascular occlusive diseases). | |
| Kitazawa Y. et al., 1989 [32] | Patients with low-tension glaucoma treated with the Ca2+ antagonist nifedipine with for 6 months. Six patients showed a constant improvement of visual field. | |
| Selenium | Phelps Brown N.A. et al., 1998 [33] | Excessive selenium supplementation may increase the glaucoma incidence |
| Prasad A.S. et al., 2014 [34] | High plasma selenium concentration and middle concentration of aqueous humour selenium was significantly associated with glaucoma. | |
| Prasanna G. et al., 2002 [35] | The mean selenium levels in aqueous humor and in serum of patients with PEX syndrome were lower than in the control group. These results may support the role of impairment in antioxidant defense system in the pathogenesis of PEX syndrome. | |
| Quigley H.A. et al., 2006 [3] | Selenium supplementation (200 mg/daily) was linked to the development of glaucoma. The risk was even higher in those who continued selenium supplementation after the trial. | |
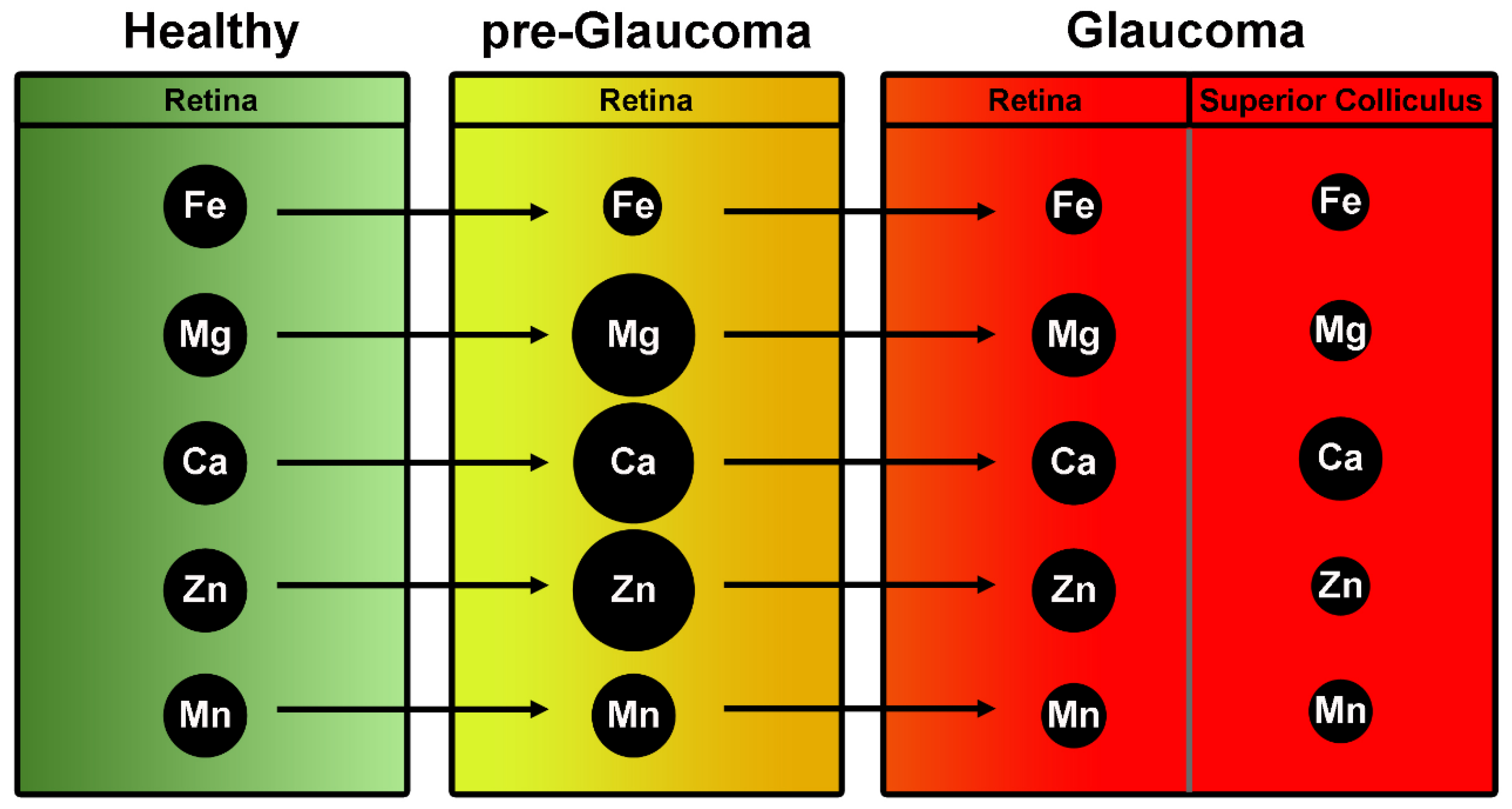
| Zinc | DeToma A.S. et al., 2014 [36] | Retinas of pre-glaucomatous mice had greater Mg, Ca, and Zn concentrations than those of glaucomatous and greater Mg and Ca than controls |
| Grahn B.H. et al., 2001 [37] | Zn supplementation seems beneficial for the patients with diabetes. Zn as an antioxidant attenuates ROS effect, therefore it might protect retina from ROS damage, thereby being protective against DR | |
| Newsome D.A. et al., 1995 [38] | The concentrations of zinc are reduced in human eyes with signs of age-related macular degeneration (AMD) suggesting that zinc deficiency may lead to oxidative stress and retinal damage | |
| Noske W. et al., 1997 [39] | Zinc is essential in the in the eye functioning. In retina and retinal pigment epithelium zinc interacts with taurine and vitamin A and modify photoreceptor plasma membranes, regulate the light-rhodopsin reaction, modulate synaptic transmission and serve as an antioxidant. | |
| Osborne N.N. et al., 2016 [40] | Zinc could be involved in light induced retinal injury; however, the mechanisms of retinal light damage in the pathology of glaucoma remain unknown. | |
| Magnesium | Ekici F. et al., 2014 [13] | Mg may protect retinal ganglion cells from oxidative injury by combined effects on voltage-dependent calcium channels, glutathione synthesis, lipid peroxidation, and maintaining the regulation of many metabolic enzymatic reactions |
| Kumar A.R. et al., 2002 [41] | Mg is important for maintaining the structural and functional integrity of several vital ocular tissues | |
| Laganovska G. et al., 2003 [42] | Mg deficiency has been shown to cause defective neurotransmitter transport mechanism, mitochondrial dysfunction, defective Golgi body function and protein processing dysfunction, neuronal degeneration and apoptosis | |
| Lee S.H. et al., 2016 [43] | Mg plays a crucial role in Na+ and K+ transport in cells. It is an important cofactor of Na+/K+-ATPase | |
| Lenartowicz M. et al., 2015 [44] | Mg increases blood supply to the optic nerve by dilating the optic blood vessels | |
| Li X. et al., 2011 [45] | Administration of Mg twice a day for 1 month had beneficial effects on visual field | |
| Lillico A. et al., 2002 [46] | Treatment with 300 mg of Mg citrate for 1 month did not change the ocular blood flow but caused some improvement in visual field | |
| Molybdenum | DeToma A.S. et al., 2014 [36] | Increased and decreased concentrations of Molybdenum have been observed to affect the illness |
| Sodium ions/Potassium cations | Jünemann A.G.M. et al., 2018 [47] | Studies on the isolated optic nerve showed that Sodium reduced the influx of sodium and would-be effective neuroprotectants |
| Hains B.C. et al., 2005 [48] | Phenytoin (a sodium channel blocker) resulted in neuroprotection of RGCs and optic nerve axons in an experimental animal model of glaucoma | |
| Ramdas W.D. et al., 2018 [49] | The early studies showed the differences in plasma concentration between normal subjects and glaucoma patients | |
| Ribas V.T. et al., 2015 [50] | The death of RGCs is a major cause of eye neuropathies. Potassium (K+) channels play key roles in modulating the electrical properties of RG cells | |
| Roy Chowdhury U. et al., 2015 [51] | The presence of Na/K/Cl co-transport activity in trabecular meshwork (TM) cells is able to test the hypothesis that modulation of Na/K co-transport alters intracellular volume and, consequently, permeability of the TM cell membranes. | |
| Roy Chowdhury U. et al., 2016 [52] | Sodium channel blockade with phenytoin would result in neuroprotection of RGCs | |
| Roy Chowdhury U. et al., 2017 [53] | Orally delivered phenytoin was effective in protecting neurons, NS-7(4-(4-fluorophenyl)-2-methyl-6-(5-piperidinopentyloxy) pyrimidine hydrochloride a novel Na+/Ca2+ channel blocker, can protect the rat retina | |
| Roy Chowdhury U. et al., 2019 [54] | The role of intracellular Na+ overload in ischemic injury of acutely isolated rat optic nerves by evaluating electrically potentials (CAPs) from the optic nerves | |
| Saito S. et al., 2005 [55] | Cromakalim is a hypotensive agent acting via activation of Kir6.2 containing KATP channels and its effect is additive in combination with the commonly used anti-glaucoma drug latanoprost | |
| Sakamoto K. et al., 2004 [56] | Derivatives of the KATP channel were evaluated to control intraocular pressure lowering eye capabilities | |
| Salvatore S. et al., 2010 [57] | A new class of glaucoma therapeutics, opening the KATP channels, may have an effect on the trabecular meshwork and intraocular pressure regulation | |
| Sample P.A. et al., 1986 [58] | KATP channel openers—diazoxide and nicorandill—lower intraocular pressure by specifically activating the Erk1/2 pathway in ocular cells | |
| Savigni D.L. et al., 2013 [59] | Application of digoxin, a selective Na+/K+-ATPase inhibitor, for the α2β3 isoform of the enzyme, efficiently reduces the pharmacologically induced and basal intraocular pressure in rabbits | |
| Schwalfenberg G.K. et al., 2017 [60] | ATP-dependent potassium channels (KATP channels) in the mitochondrial or plasma membranes may provide protection against retinal ischemia | |
| Sheck L., Davies J. et al., 2010 [61] | ATP-dependent potassium channels (KATP channels) in the mitochondrial or plasma membranes may provide protection against retinal ischemia | |
| Shoshani Y.Z. et al., 2012 [62] | In Müller glial cells, KATP channels regulate retinal current and play a key role in retinal protection against ischemic conditions, e.g., ischemic insult | |
| Siegner S.W. et al., 2000 [63] | MaxiK channels and KATP channels were found in the eye trabecular meshwork cells | |
| Silverstone B. et al., 1981 [64] | Opening of potassium channels may play a protective role by increasing the uveal outflow | |
| Silverstone B.Z. et al., 1990 [65] | KR-31378 ((2S,3S,4R)-N’’-cyano-N(6-amino-3,4dihydro-3-hydroxy-2-methyl-2-dimethoxymethyl-2Hbenzopyran-4-yl)-N’-benzyl-guanidine) as a potent KATP-channel opener, on reducing intraocular pressure and its protective effect on RGCs | |
| Skatchkov S.N. et al., 2002 [66] | Cromakalim showed a reduction in pressure (by 30–40%) and outflow facility (by 50–80%) | |
| Sourkes T.L. et al., 1972 [16] | Effects of dexamethasone treatment on Na/K/Cl co-transport activity and co-transporter protein expression in trabecular meshwork (TM) cells | |
| DeToma A.S. et al., 2014 [36] | Mn concentration was significantly increased in patients with pseudoexfoliation (PEX) syndrome | |
| Manganese | Südhof T.C. et al., 2012 [18] | Mn level was negatively associated with glaucoma diagnosis in a population-based study of South Korean 2680 individuals |
| Demirci F.Y. et al., 2006 [67] | Study on the association between levels of three heavy metals and the occurrence of open-angle glaucoma (OAG) with low and high intraocular pressure | |
| Heavy metals [mercury (Hg), lead (Pb), cadmium (Cd), chromium (Cr), nickel (Ni), bismuth (Bi), semi-metals] | Südhof T.C. et al., 2012 [18] | Blood heavy metals level were negatively associated with glaucoma diagnosis in a population-based study on South Korean 2680 individuals |
| Tham Y.C. et al., 2014 [8] | The accumulation of Hm may be an unrecognized risk factor of non-pressure-dependent glaucomatous optic neuropathy | |
| Tykocki N.R. et al., 2010 [68] | Lead is known to cause tissue damage by oxidative stress, lipid peroxidation, and DNA damage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, A.; Romano, G.L.; Rejdak, R.; Zweifel, S.; Fiedorowicz, M.; Rejdak, M.; Bajka, A.; Amato, R.; Bucolo, C.; Avitabile, T.; et al. Influence of Trace Elements on Neurodegenerative Diseases of The Eye—The Glaucoma Model. Int. J. Mol. Sci. 2021, 22, 4323. https://doi.org/10.3390/ijms22094323
Kamińska A, Romano GL, Rejdak R, Zweifel S, Fiedorowicz M, Rejdak M, Bajka A, Amato R, Bucolo C, Avitabile T, et al. Influence of Trace Elements on Neurodegenerative Diseases of The Eye—The Glaucoma Model. International Journal of Molecular Sciences. 2021; 22(9):4323. https://doi.org/10.3390/ijms22094323
Chicago/Turabian StyleKamińska, Agnieszka, Giovanni Luca Romano, Robert Rejdak, Sandrine Zweifel, Michal Fiedorowicz, Magdalena Rejdak, Anahita Bajka, Rosario Amato, Claudio Bucolo, Teresio Avitabile, and et al. 2021. "Influence of Trace Elements on Neurodegenerative Diseases of The Eye—The Glaucoma Model" International Journal of Molecular Sciences 22, no. 9: 4323. https://doi.org/10.3390/ijms22094323
APA StyleKamińska, A., Romano, G. L., Rejdak, R., Zweifel, S., Fiedorowicz, M., Rejdak, M., Bajka, A., Amato, R., Bucolo, C., Avitabile, T., Drago, F., & Toro, M. D. (2021). Influence of Trace Elements on Neurodegenerative Diseases of The Eye—The Glaucoma Model. International Journal of Molecular Sciences, 22(9), 4323. https://doi.org/10.3390/ijms22094323











