Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases
Abstract
:1. Introduction
2. Results
2.1. The Level of Expression of Only a Few Oncogenes and Tumor Suppressor Genes Is Associated with Patient Survival
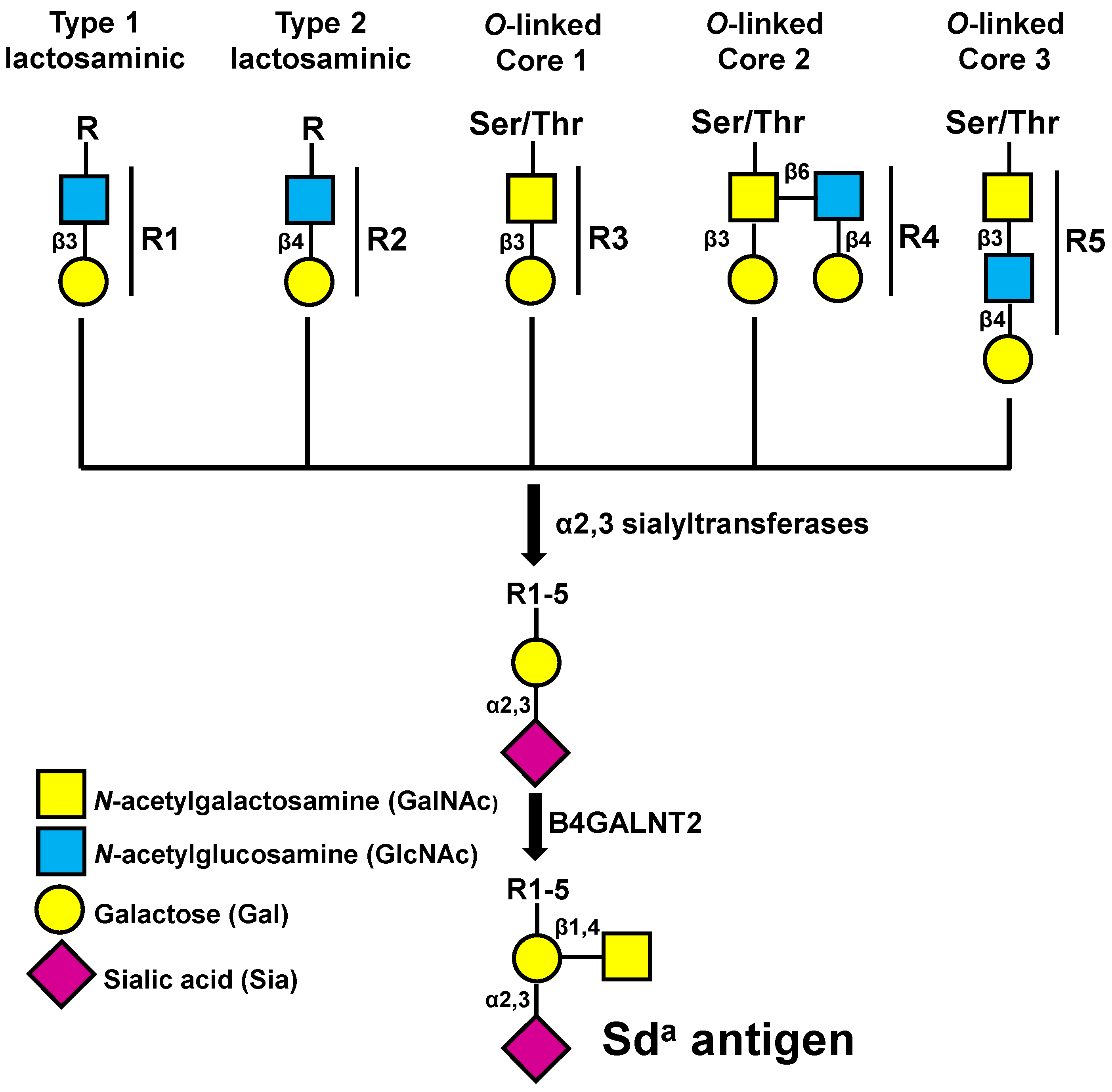
2.2. Among Glycosyltransferases B4GALNT2 Has a Very Good Prognostic Value in CRC
2.3. Comparison of High and Low B4GALNT2 Expressers in the COADRED Cohort
2.4. Several Glycogenes Are Differentially Modulated in HBE and LBE
2.5. The Role of Methylation in B4GALNT2 Expression
2.6. The Role of miRNAs in B4GALNT2 Expression
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COADREAD | colon adenocarcinoma rectal adenocarcinoma |
| CRC | colorectal cancer |
| HBE | higher B4GALNT2 expressers |
| LBE | lower B4GALNT2 expressers |
| lncRNA | long noncoding RNA |
| NMR | normalized miRNA score |
| PD-L1 | programmed death ligand-1 |
| sLex | Sialyl LewisX |
| TCGA | The Cancer Genome Atlas |
References
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 2012, 17, 670–699. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta 2014, 1840, 2752–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Malagolini, N.; Chiricolo, M.; Trinchera, M.; Harduin-Lepers, A. The expanding roles of the Sda/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT. Biochim. Biophys. Acta 2014, 1840, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Serafini-Cessi, F.; Dall’Olio, F. Guinea-pig kidney β-N-acetylgalactosaminyltransferase towards Tamm-Horsfall glycoprotein. Requirement of sialic acid in the acceptor for transferase activity. Biochem. J. 1983, 215, 483–489. [Google Scholar] [CrossRef]
- Lo Presti, L.; Cabuy, E.; Chiricolo, M.; Dall’Olio, F. Molecular Cloning of the Human β1,4 N-Acetylgalactosaminyltransferase Responsible for the Biosynthesis of the Sda Histo-Blood Group Antigen: The Sequence Predicts a Very Long Cytoplasmic Domain. J. Biochem. 2003, 134, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Montiel, M.D.; Krzewinski-Recchi, M.A.; Delannoy, P.; Harduin-Lepers, A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Acα2-3Galβ-R β1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: Evidence for an unusual extended cytoplasmic domain. Biochem. J. 2003, 373, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.L.; Lowe, J.B. Molecular cloning of a murine N-acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte-specific CT oligosaccharide differentiation antigen. J. Biol. Chem. 1994, 269, 15162–15171. [Google Scholar] [CrossRef]
- Stenfelt, L.; Hellberg, A.; Moller, M.; Thornton, N.; Larson, G.; Olsson, M.L. Missense mutations in the C-terminal portion of the B4GALNT2-encoded glycosyltransferase underlying the Sd(a-) phenotype. Biochem. Biophys. Rep. 2019, 19, 100659. [Google Scholar] [CrossRef]
- Malagolini, N.; Dall’Olio, F.; Di Stefano, G.; Minni, F.; Marrano, D.; Serafini-Cessi, F. Expression of UDP-GalNAc:NeuAc α2,3Gal β-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989, 49, 6466–6470. [Google Scholar]
- Malagolini, N.; Santini, D.; Chiricolo, M.; Dall’Olio, F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology 2007, 17, 688–697. [Google Scholar] [CrossRef]
- Pucci, M.; Gomes Ferreira, I.; Orlandani, M.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. High Expression of the Sda Synthase B4GALNT2 Associates with Good Prognosis and Attenuates Stemness in Colon Cancer. Cells 2020, 9, 948. [Google Scholar] [CrossRef] [Green Version]
- Staubach, F.; Kunzel, S.; Baines, A.C.; Yee, A.; McGee, B.M.; Backhed, F.; Baines, J.F.; Johnsen, J.M. Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J. 2012, 6, 1345–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groux-Degroote, S.; Schulz, C.; Cogez, V.; Noel, M.; Portier, L.; Vicogne, D.; Solorzano, C.; Dall’Olio, F.; Steenackers, A.; Mortuaire, M.; et al. The extended cytoplasmic tail of the human B4GALNT2 is critical for its Golgi targeting and post-Golgi sorting. FEBS J. 2018, 285, 3442–3463. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.R.; Hsieh, C.Y.; Twu, Y.C.; Yu, L.C. Expression of the human Sda β-1,4-N-acetylgalactosaminyltransferase II gene is dependent on the promoter methylation status. Glycobiology 2008, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.I.; Toyota, M.; Kawashima, R.; Hagiwara, T.; Suzuki, H.; Imai, K.; Shinomura, Y.; Tokino, T.; Kannagi, R.; Dohi, T. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology 2008, 135, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.I.; Kawashima, R.; Fukunaga, R.; Hirai, K.; Toyama-Sorimachi, N.; Tokuhara, M.; Shimizu, T.; Dohi, T. Introduction of Sda carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005, 65, 6220–6227. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.I.; Adachi, Y.; Curiel, D.T.; Kawashima, R.; Kannagi, R.; Nishimoto, N.; Dohi, T. Therapeutic adenoviral gene transfer of a glycosyltransferase for prevention of peritoneal dissemination and metastasis of gastric cancer. Cancer Gene Ther. 2014, 21, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, M.; Gomes, F.I.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. Int. J. Mol. Sci. 2020, 21, 6558. [Google Scholar] [CrossRef]
- Jung, B.; Staudacher, J.J.; Beauchamp, D. Transforming Growth Factor beta Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology 2017, 152. [Google Scholar] [CrossRef] [Green Version]
- Cheasley, D.; Pereira, L.; Sampurno, S.; Sieber, O.; Jorissen, R.; Xu, H.; Germann, M.; Yuqian, Y.; Ramsay, R.G.; Malaterre, J. Defective Myb Function Ablates Cyclin E1 Expression and Perturbs Intestinal Carcinogenesis. Mol. Cancer Res. 2015, 13, 1185–1196. [Google Scholar] [CrossRef] [Green Version]
- Noda, M.; Okayama, H.; Tachibana, K.; Sakamoto, W.; Saito, K.; Thar Min, A.K.; Ashizawa, M.; Nakajima, T.; Aoto, K.; Momma, T.; et al. Glycosyltransferase gene expression identifies a poor prognostic colorectal cancer subtype associated with mismatch repair deficiency and incomplete glycan synthesis. Clin. Cancer Res. 2018, 24, 4468–4481. [Google Scholar] [CrossRef] [Green Version]
- Shimodaira, K.; Nakayama, J.; Nakamura, N.; Hasebe, O.; Katsuyama, T.; Fukuda, M. Carcinoma-associated expression of core 2 β1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: Role of O-glycans in tumor progression. Cancer Res. 1997, 57, 5201–5206. [Google Scholar] [PubMed]
- An, G.; Wei, B.; Xia, B.; McDaniel, J.M.; Ju, T.; Cummings, R.D.; Braun, J.; Xia, L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 2007, 204, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, W.; Boardman, L.A.; Wang, L. Loss of ZG16 is associated with molecular and clinicopathological phenotypes of colorectal cancer. BMC. Cancer 2018, 18, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.; Ding, Y.; Liu, E.; Li, W.; Wang, L. ZG16 regulates PD-L1 expression and promotes local immunity in colon cancer. Transl. Oncol. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Ren, C.; Wang, L.; Zhao, Y.; Wang, S. Knockdown of ST6Gal-I inhibits the growth and invasion of osteosarcoma MG-63 cells. Biomed. Pharmacother. 2015, 72. [Google Scholar] [CrossRef] [PubMed]
- Mito, A.; Nakano, Y.; Saitoh, T.; Gouraud, S.S.S.; Yamaguchi, Y.; Sato, T.; Sasaki, N.; Kojima-Aikawa, K. Lectin ZG16p inhibits proliferation of human colorectal cancer cells via its carbohydrate-binding sites. Glycobiology 2018, 28, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jiang, C.; Xu, C.; Wang, D.; Shen, Y.; Liu, Y.; Gu, L. Identification and development of a novel invasion-related gene signature for prognosis prediction in colon adenocarcinoma. Cancer Cell Int. 2021, 21, 101. [Google Scholar] [CrossRef]
- Kawashima, K.; Maeda, K.; Saigo, C.; Kito, Y.; Yoshida, K.; Takeuchi, T. Adiponectin and Intelectin-1: Important Adipokine Players in Obesity-Related Colorectal Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 866. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Bing, Z.; Wu, J.; Zhang, J.; Zhou, W.; Ni, M.; Meng, Z.; Liu, S.; Tian, J.; Zhang, X.; et al. Integrative Gene Expression Profiling Analysis to Investigate Potential Prognostic Biomarkers for Colorectal Cancer. Med. Sci. Monit. 2020, 26, e918906. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zeng, J.; Shi, N.; Chen, L.; Wang, L. Application of weighted gene coexpression network analysis to explore the potential diagnostic biomarkers for colorectal cancer. Mol. Med. Rep. 2020, 21, 2533–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.S.; Somvanshi, S.; Patel, E.; Chen, T.W.; Singh, V.P.; Zorman, B.; Patil, S.L.; Pan, Y.; Chatterjee, S.S.; Sood, A.K.; et al. Pan-Cancer Analysis of lncRNA Regulation Supports Their Targeting of Cancer Genes in Each Tumor Context. Cell Rep. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.; Wuhrer, M.; Rombouts, Y. Glycosylation characteristics of colorectal cancer. Adv. Cancer Res. 2015, 126, 203–256. [Google Scholar]
- Capon, C.; Maes, E.; Michalski, J.C.; Leffler, H.; Kim, Y.S. Sda-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem. J 2001, 358, 657–664. [Google Scholar] [CrossRef]
- Salvini, R.; Bardoni, A.; Valli, M.; Trinchera, M. β1,3-Galactosyltransferase β3Gal-T5 acts on the GlcNAcβ1-->3Galβ1-->4GlcNAcβ1-->R sugar chains of carcinoembryonic antigen and other N-linked glycoproteins and is down-regulated in colon adenocarcinomas. J Biol. Chem. 2001, 276, 3564–3573. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.; Lee, E.U.; McEntee, K.; Lai, P.H.; Paulson, J.C. Primary structure of β-galactoside α 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J. Biol. Chem. 1987, 262, 17735–17743. [Google Scholar] [CrossRef]
- Takashima, S.; Tsuji, S.; Tsujimoto, M. Characterization of the Second Type of Human β-Galactoside α2,6-Sialyltransferase (ST6Gal II), Which Sialylates Galβ1,4GlcNAc Structures on Oligosaccharides Preferentially. Genomic Analysis of Human Sialyltransferase Genes. J Biol. Chem. 2002, 277, 45719–45728. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olio, F.; Malagolini, N.; Di Stefano, G.; Minni, F.; Marrano, D.; Serafini-Cessi, F. Increased CMP-NeuAc:Galβ1,4GlcNAc-R α 2,6 sialyltransferase activity in human colorectal cancer tissues. Int. J. Cancer 1989, 44, 434–439. [Google Scholar]
- Lise, M.; Belluco, C.; Perera, S.P.; Patel, R.; Thomas, P.; Ganguly, A. Clinical correlations of α2,6-sialyltransferase expression in colorectal cancer patients. Hybridoma 2000, 19, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Venturi, G.; Gomes, F.I.; Pucci, M.; Ferracin, M.; Malagolini, N.; Chiricolo, M.; Dall’Olio, F. Impact of sialyltransferase ST6GAL1 overexpression on different colon cancer cell types. Glycobiology 2019, 29, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Mito, A.; Kumazawa-Inoue, K.; Kojima-Aikawa, K. ZG16p, an Animal Homologue of Plant beta-Prism Fold Lectins: Purification Methods of Natural and Recombinant ZG16p and Inhibition Assay of Cancer Cell Growth Using ZG16p. Methods Mol. Biol. 2020, 2132, 339–347. [Google Scholar] [CrossRef]
- Pattison, A.M.; Merlino, D.J.; Blomain, E.S.; Waldman, S.A. Guanylyl cyclase C signaling axis and colon cancer prevention. World J. Gastroenterol. 2016, 22, 8070–8077. [Google Scholar] [CrossRef] [PubMed]
- Low, E.N.D.; Mokhtar, N.M.; Wong, Z.; Raja Ali, R.A. Colonic Mucosal Transcriptomic Changes in Patients with Long-Duration Ulcerative Colitis Revealed Colitis-Associated Cancer Pathways. J. Crohns. Colitis. 2019, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, M.R.; Costello, J.F. Cancer: Oncogene brought into the loop. Nature 2016, 529, 34–35. [Google Scholar] [CrossRef] [Green Version]




| Gene | Ratio | Gene Role | Reference | |
|---|---|---|---|---|
| CLCA1 | 203 | Involved in mucus secretion and as a tumor suppressor and suppresses CRC malignancy | 28974231 | |
| ZG16 | 151 | Animal lectin; inhibits growth and increases immune surveillance of CRC | [26,27,28,29] | |
| ITLN1 | 62 | Lectin-recognizing microbial carbohydrates; protective in CRC | [30,31,32] | |
| CLCA4 | 51 | Involved in mediating chloride conductance; downregulated genes in CRC | 32027181 | |
| SPINK4 | 48 | Serine peptidase inhibitor; its downregulation is associated with poor survival in CRC | 31888570 | |
| CA1 | 45 | Carbonic anhydrase; predictive biomarker in CRC | 32031891 | |
| MAGEA1 | 37 | Involved in transcriptional regulation; acts as an oncogene in some cancers | 30509089 | |
| PYY | 33 | Inhibitis intestinal mobility; decreased expression is associated with CRC | 11825654 | |
| GUCA2B | 32 | Regulator of intestinal fluid transport; tumor suppressor in CRC | 29788743 | |
| CA4 | 27 | Stimulates the ion transporter activity of SLC4A4; Predictive biomarker in CRC | 32031891 | |
| MS4A12 | 25 | Involved in signal transduction; promotes malignant progression in CRC | 18451174 | |
| BEST2 | 23 | Anion channel; methylation marker for early detection and prognosis of CRC | [33] | |
| HEPACAM2 | 23 | Required for centrosome maturation; associated with good prognosis | 29659199 | |
| TMIGD1 | 22 | Controls cell–cell adhesion and proliferation; tumor suppressor in CRC | 33129760 | |
| CLDN8 | 16 | Claudin 8; component of tight junctions; downregulated in CRC | 21479352 | |
| B3GNT6 | 14 | Synthesizes core 3 O-linked chains; downregulation associated with malignancy in CRC | 28745318 | |
| KIF19 | 13 | Microtubule-dependent motor protein; higher expression associated with longer survival | 28901309 | |
| CSAG2 | 13 | Chondrosarcoma-asociated gene 2/3 protein; necessary for tumorigenesis | 32761762 | |
| FCGBP | 12 | Maintains the mucosal structure; high expression is associated with better prognosis | 31268166 | |
| CDKN2BAS | 12 | CDKN2B antisense RNA 1; promotes progression of ovarian cancer | 32572907 | |
| REG1B | 11 | Regenerating islet-derived protein 1-β; its silencing inhibits CRC growth | 25768000 | |
| IGJ | 11 | Joining chain of multimeric IgA and IgM; downregulated in CRC | 31749922 | |
| LEFTY2 | 10 | Member of the TGF-β superfamily; negative regulator of endometrial cell proliferation | 27497669 | |
| FUT5 | 10 | Fucosyltransferase 5; promotes the development of CRC | 28771224 | |
| MUC2 | 10 | Secreted mucus-forming mucin; suppresses CRC migration and metastasis | 28725043 | |
| PLIN1 | −4 | Modulator of adipocyte lipid metabolism; inhibits breast cancer cell proliferation | 27359054 | |
| PCP4 | −4 | Functions as a modulator of calcium-binding by calmodulin; antiapoptotic peptide | 25153723 | |
| IGF2 | −4 | Possess growth-promoting activity; overexpression is associated with poor prognosis | 24080445 | |
| SLC14A1 | −4 | Urea channel; cancer stem cell marker | 29329541 | |
| FREM1 | −5 | Extracellular matrix protein; associated with better prognosis in bladder cancer | 33058542 | |
| CASQ2 | −5 | Calsequestrin; high expression associated with poor survival in bladder cancer | 31991631 | |
| CPLX2 | −6 | Involved in exocytosis; associated with poor prognosis in lung tumors | 3912489 | |
| ADIPOQ | −6 | Adiponectin; anti-inflammatory adipokine; lower expression in CRC | 27061803 | |
| WIF1 | −7 | Inhibits WNT actvities; hypermethylation is associated with a favorable clinical outcome | 31830937 | |
| CHRNB2 | −9 | Cholinergic receptor nicotinic beta 2 subunit; downregulated in gastric cancer | 30175534 | |
| AP3B2 | −10 | Involved in protein sorting; low expression is associated with long-term survival in rectal cancer | 29050227 | |
| TSIX | −11 | XIST antisense RNA; dysregulates cancer pathways in multiple tumor contexts | [34] |
| Functional Class | Gene | Ratio | Role |
|---|---|---|---|
| First steps of O-linked biosynthesis | GALNT8 | 9.5 | Addition of the first GalNAc residue of the O-linked chains |
| B3GNT6 | 14.4 | Synthesis of Core 3 O-glycans by attaching GlcNAc to GalNAc | |
| ST6GALNAC1 | 5.4 | Synthesis of sialyl Tn by attaching Sia to GalNAc | |
| ST6GALNAC2 | 2.1 | Synthesis of sialyl T by attaching Sia to GalNAc of T antigen | |
| Ganglioside biosynthesis | ST6GALNAC6 | 2.5 | Synthesis of higher gangliosides |
| Proteoglycan biosynthesis | B3GNT7 | 6.3 | Keratan sulfate biosynthesis |
| Biosynthesis of sialyl Lewis antigens | B3GALT5 | 4.0 | Synthesis of type 1 chains |
| ST3GAL4 | 4.0 | Sialylation of type 2 chains | |
| FUT5 | 10.8 | Fucosylation of type 2 chains | |
| Biosynthesis of Sia6 LacNAc structures | ST6GAL1 | −2.0 | α2,6 sialylation of glycoproteins |
| ST6GAL2 | −2.7 | α2,6 sialylation of soluble substrates | |
| Galactose recognition | LGALS4 | 2.0 | Galectin 4, expressed in the gut, underexpressed in CRC |
| LGALS9B | 2.2 | Highly similar to Galectin 9 | |
| O-glycoproteins | MUC1 | 2.0 | Membrane bound mucin with multiple functions |
| MUC2 | 10.4 | Secreted mucus forming mucin | |
| MUC4 | 5.2 | Membrane and secreted mucin | |
| MUC5B | 2.6 | Gel-forming mucin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, M.; Malagolini, N.; Dall’Olio, F. Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases. Int. J. Mol. Sci. 2021, 22, 4331. https://doi.org/10.3390/ijms22094331
Pucci M, Malagolini N, Dall’Olio F. Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases. International Journal of Molecular Sciences. 2021; 22(9):4331. https://doi.org/10.3390/ijms22094331
Chicago/Turabian StylePucci, Michela, Nadia Malagolini, and Fabio Dall’Olio. 2021. "Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases" International Journal of Molecular Sciences 22, no. 9: 4331. https://doi.org/10.3390/ijms22094331
APA StylePucci, M., Malagolini, N., & Dall’Olio, F. (2021). Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases. International Journal of Molecular Sciences, 22(9), 4331. https://doi.org/10.3390/ijms22094331







