Characterization of Long Non-Coding RNAs in Systemic Sclerosis Monocytes: A Potential Role for PSMB8-AS1 in Altered Cytokine Secretion
Abstract
:1. Introduction
2. Results
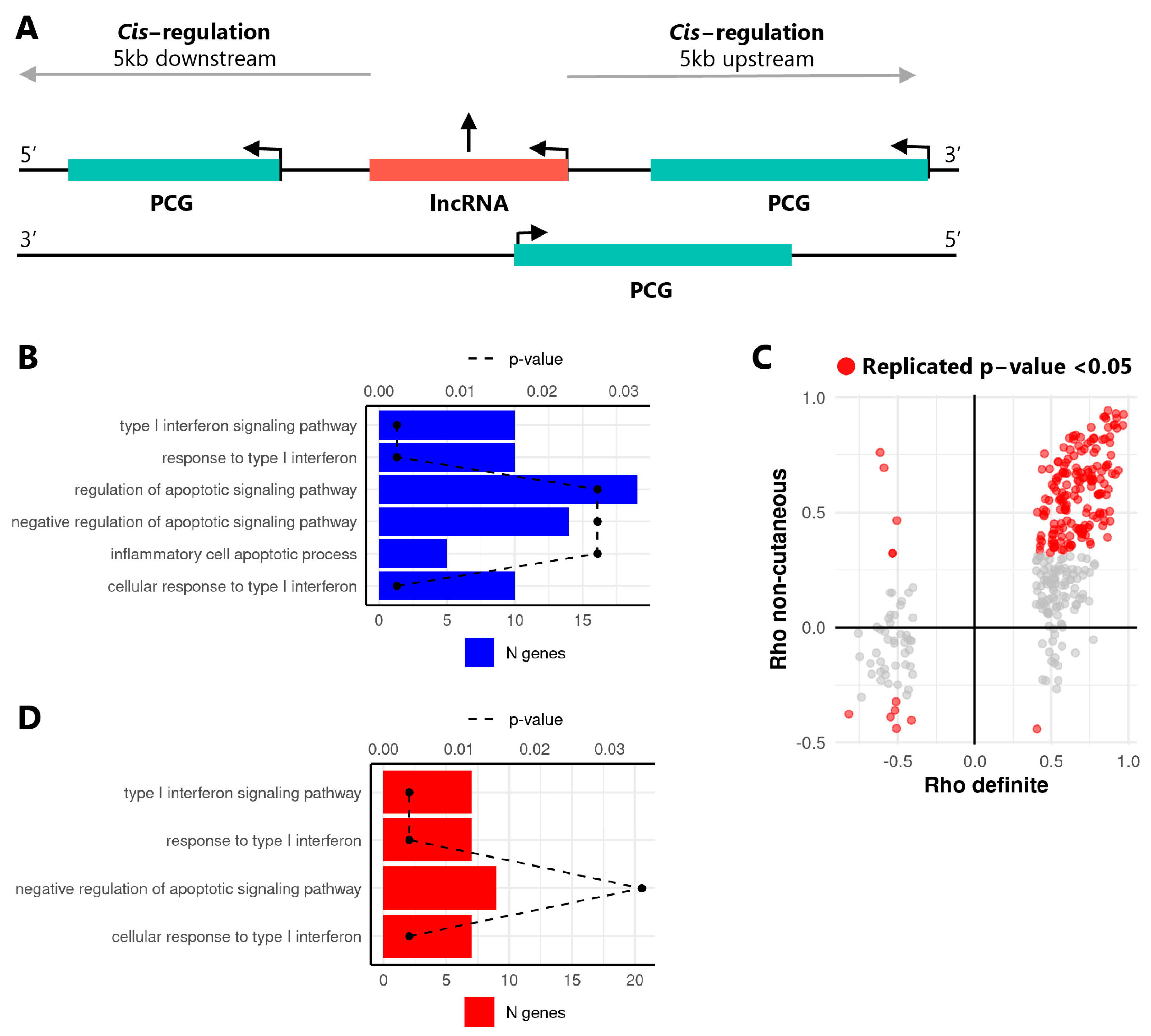
2.1. The Expression of lncRNAs Is Altered in SSc Monocytes and Is Correlated with Neighboring Protein Coding Genes
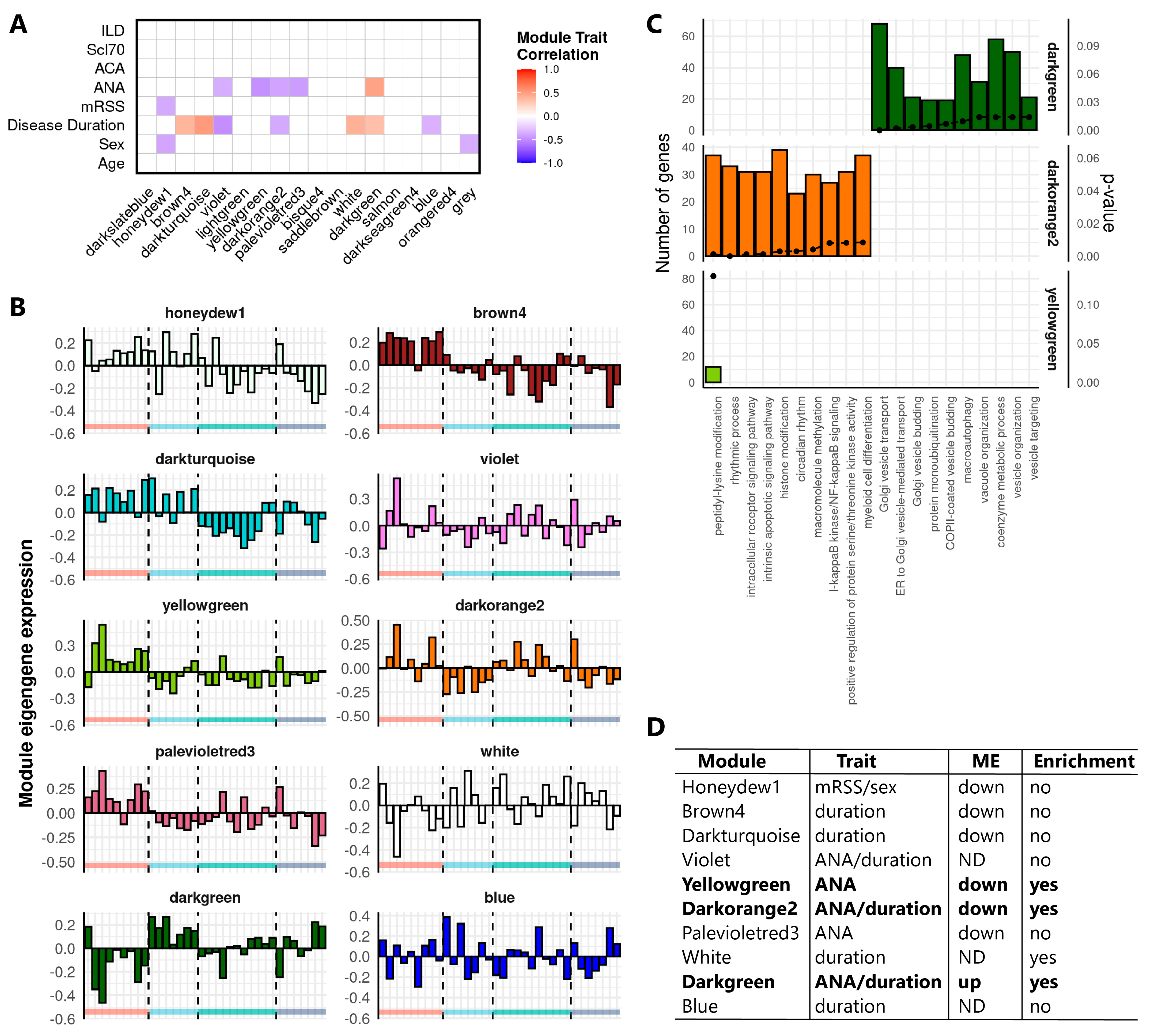
2.2. Weighted Gene Co-Expression Network Analysis Identifies Clusters of Tightly Correlated RNAs Associated to SSc Clinical Features and Relevant Biological Processes
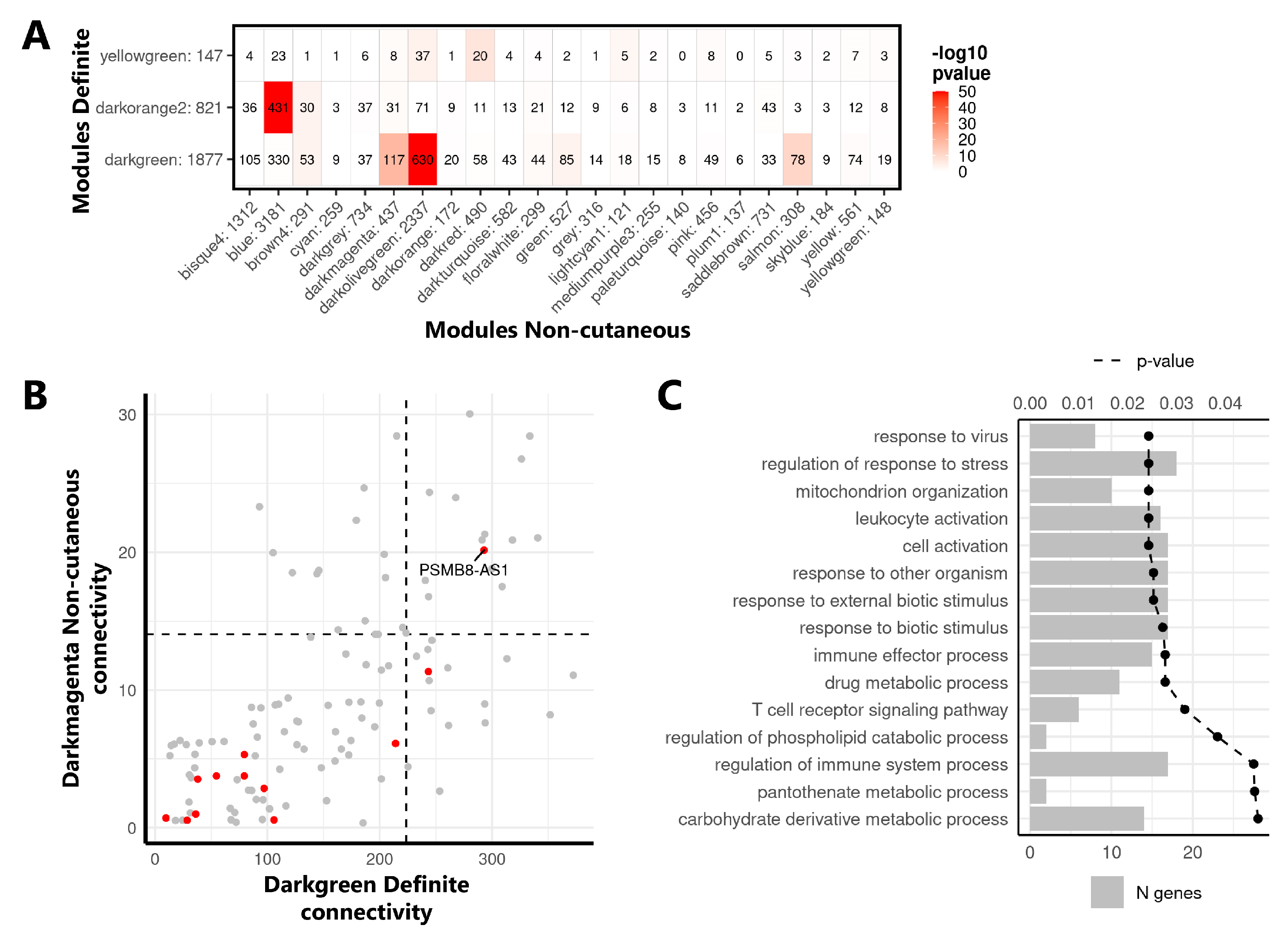
2.3. Identification of the lncRNA PSMB8-AS1 as a Reproducible Hub Gene Relevant for SSc and Monocyte Biology
2.4. Characterization of PSMB8-AS1 Expression in SSc and Healthy Monocytes
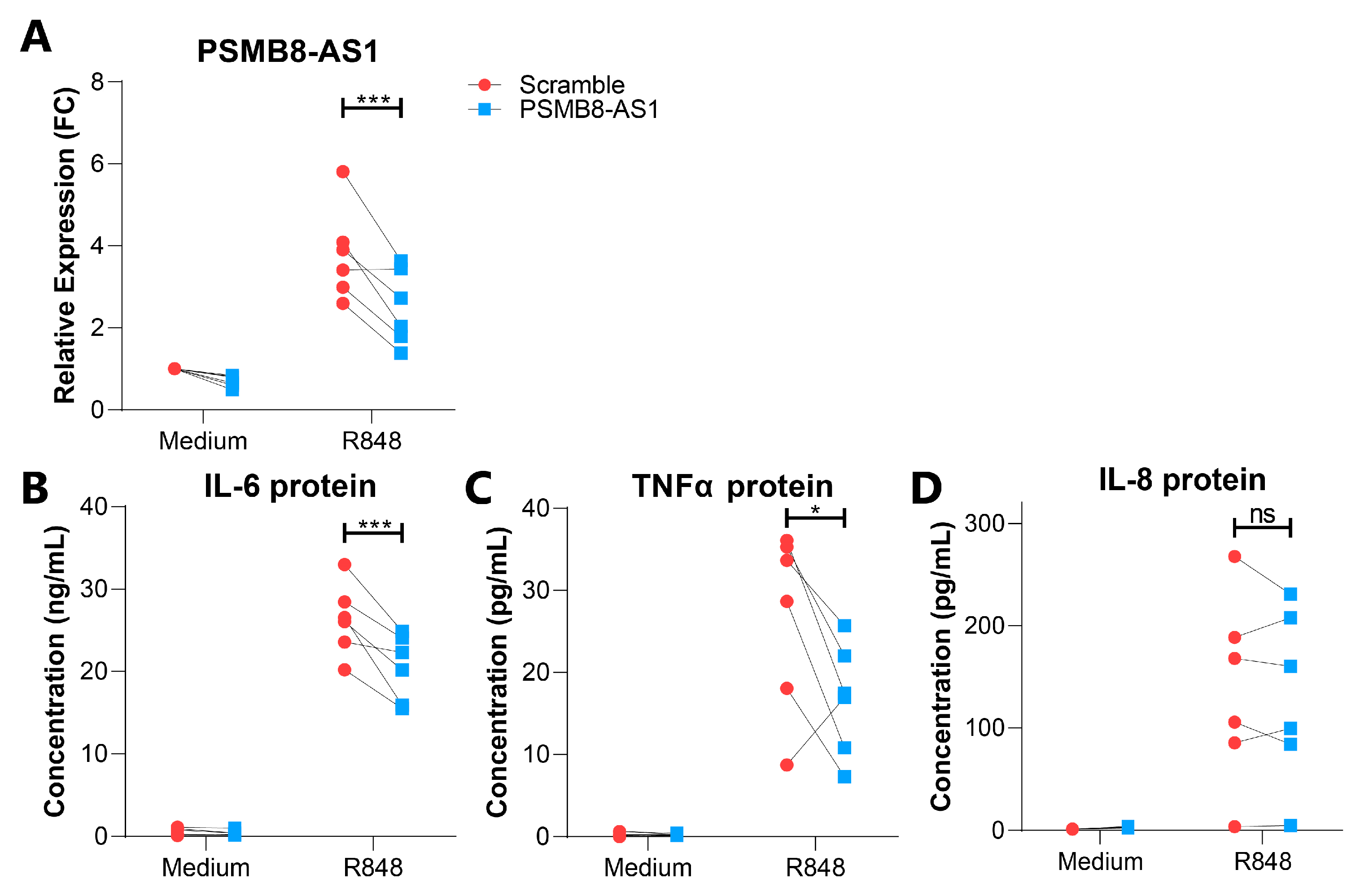
2.5. Characterization of PSMB8-AS1 Function in Monocytes
3. Discussion
4. Materials and Methods
4.1. Patient Demographics
4.2. Purification and Culture of CD14+ Monocytes from Healthy Control Blood and Buffy Coats
4.3. RNA Purification
4.4. RNA-Sequencing Analysis
4.5. In Cis Correlation Analysis
4.6. GO-Term Enrichment Analysis
4.7. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.8. Subcellular Fractionation
4.9. Transfection of CD14+ Monocytes Using siRNA
4.10. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
4.11. FACS Assessment of Monocyte Viability
4.12. Assessment of Cytokine Levels Using ELISA
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Prim. 2015, 1, 15002. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsgen, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [Green Version]
- Leroy, E.C.; Medsger, T.A. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 2001, 28, 1573–1576. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11469464 (accessed on 14 December 2020). [PubMed]
- Belch, J.J.F. Raynaud’s phenomenon: Its relevance to scleroderma. Ann. Rheum. Dis. 1991, 50 (Suppl. 4), 839–845. [Google Scholar] [CrossRef] [Green Version]
- Kahaleh, M.B. Vascular involvement in systemic sclerosis (SSc). Clin. Exp. Rheumatol. 2004, 22, 19–23. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15344592 (accessed on 14 December 2020).
- Epattanaik, D.; Ebrown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, O.; Ishikawa, H. Macrophage infiltration in the skin of patients with systemic sclerosis. J. Rheumatol. 1992, 19, 1202–1206. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1404154 (accessed on 14 December 2020). [PubMed]
- Kräling, B.M.; Maul, G.G.; Jimenez, S.A. Mononuciear cellular infiltrates in clinically involved skin from patients with systemic sclerosis of recent onset predominantly consist of monocytes/macrophages. Pathobiology 1995, 63, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Higashi-Kuwata, N.; Jinnin, M.; Makino, T.; Fukushima, S.; Inoue, Y.; Muchemwa, F.C.; Yonemura, Y.; Komohara, Y.; Takeya, M.; Mitsuya, H.; et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res. Ther. 2010, 12, R128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Kroef, M.; Hoogen, L.L.V.D.; Mertens, J.S.; Blokland, S.L.; Haskett, S.; Devaprasad, A.; Carvalheiro, T.; Chouri, E.; Vazirpanah, N.; Cossu, M.; et al. Cytometry by time of flight identifies distinct signatures in patients with systemic sclerosis, systemic lupus erythematosus and Sjögrens syndrome. Eur. J. Immunol. 2019, 50, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.K.D.; Quinn, K.; Li, Q.; Carroll, R.; Warsinske, H.; Vallania, F.; Chen, S.; Carns, M.A.; Aren, K.; Sun, J.; et al. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: A retrospective, multicenter cohort study. Lancet Respir Med. 2019, 7, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Brkic, Z.; van Bon, L.; Cossu, M.; van Helden-Meeuwsen, C.G.; Vonk, M.C.; Knaapen, H.; Berg, W.V.D.; Dalm, V.A.; van Daele, P.L.; Severino, A.; et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis. 2016, 75, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Ciechomska, M.; Wojtas, B.; Swacha, M.; Olesinska, M.; Benes, V.; Maslinski, W. Global miRNA and mRNA expression profiles identify miRNA-26a-2-3p-dependent repression of IFN signature in systemic sclerosis human monocytes. Eur. J. Immunol. 2020, 50, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.K.; Gulati, M.; Peng, X.; Russell, T.R.; Shaw, A.C.; Rubinowitz, A.N.; Murray, L.A.; Siner, J.M.; Antin-Ozerkis, D.E.; Montgomery, R.R.; et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab. Investig. 2010, 90, 812–823. [Google Scholar] [CrossRef]
- Carvalheiro, T.; Horta, S.; van Roon, J.A.G.; Santiago, M.; Salvador, M.J.; Trindade, H.; Radstake, T.R.D.J.; da Silva, J.A.P.; Paiva, A. Increased frequencies of circulating CXCL10-, CXCL8- and CCL4-producing monocytes and Siglec-3-expressing myeloid dendritic cells in systemic sclerosis patients. Inflamm. Res. 2018, 67, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Carvalheiro, T.; Lopes, A.P.; van der Kroef, M.; Malvar-Fernandez, B.; Rafael-Vidal, C.; Hinrichs, A.C.; Servaas, N.H.; Bonte-Mineur, F.; Kok, M.R.; Beretta, L.; et al. Angiopoietin-2 promotes inflammatory activation in monocytes of systemic sclerosis patients. Int. J. Mol. Sci. 2020, 21, 9544. [Google Scholar] [CrossRef]
- Van der Kroef, M.; Castellucci, M.; Mokry, M.; Cossu, M.; Garonzi, M.; Bossini-Castillo, L.M.; Chouri, E.; Wichers, C.G.K.; Beretta, L.; Trombetta, E.; et al. Histone modifications underlie monocyte dysregulation in patients with systemic sclerosis, underlining the treatment potential of epigenetic targeting. Ann. Rheum. Dis. 2019, 78, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zaba, L.; Satpathy, A.T.; Longmire, M.; Zhang, W.; Li, K.; Granja, J.; Guo, C.; Lin, J.; Li, R.; et al. Chromatin accessibility landscapes of skin cells in systemic sclerosis nominate dendritic cells in disease pathogenesis. Nat. Commun. 2020, 11, 5843. [Google Scholar] [CrossRef]
- Hadjicharalambous, M.R.; Lindsay, M.A. Long Non-Coding RNAs and the Innate Immune Response. Non Coding RNA 2019, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, B.; Servaas, N.H.; Rossato, M.; Tamassia, N.; Cassatella, M.A.; Cossu, M.; Beretta, L.; van der Kroef, M.; Radstake, T.R.D.J.; Bazzoni, F. The long non-coding RNA NRIR drives IFN-response in monocytes: Implication for systemic sclerosis. Front. Immunol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dolcino, M.; Tinazzi, E.; Puccetti, A.; Lunardi, C. In systemic sclerosis, a unique long non coding RNA regulates genes and pathways involved in the three main features of the disease (vasculopathy, fibrosis and autoimmunity) and in carcinogenesis. J. Clin. Med. 2019, 8, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Jinnin, M.; Nakamura, K.; Harada, M.; Kudo, H.; Nakayama, W.; Inoue, K.; Nakashima, T.; Honda, N.; Fukushima, S.; et al. Long non-coding RNA TSIX is upregulated in scleroderma dermal fibroblasts and controls collagen mRNA stabilization. Exp. Dermatol. 2016, 25, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Messemaker, T.C.; Chadli, L.; Cai, G.; Goelela, V.S.; Boonstra, M.; Dorjée, A.L.; Andersen, S.N.; Mikkers, H.M.M.; van ’t Hof, P.; Mei, H.; et al. Antisense long non-coding RNAs are deregulated in skin tissue of patients with systemic sclerosis. J. Investig. Dermatol. 2018, 138, 826–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Elmawla, M.A.; Hassan, M.; Elsabagh, Y.A.; Alnaggar, A.R.L.; Senousy, M.A. Deregulation of long noncoding RNAs ANCR, TINCR, HOTTIP and SPRY4-IT1 in plasma of systemic sclerosis patients: SPRY4-IT1 as a novel biomarker of scleroderma and its subtypes. Cytokine 2020, 133, 155124. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-L. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef]
- Guh, C.-Y.; Hsieh, Y.-H.; Chu, H.-P. Functions and properties of nuclear lncRNAs-from systematically mapping the interactomes of lncRNAs. J. Biomed. Sci. 2020, 27, 44. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1471. [Google Scholar] [CrossRef]
- Font-Cunill, B.; Arnes, L.; Ferrer, J.; Sussel, L.; Beucher, A. Long non-coding RNAs as local regulators of pancreatic islet transcription factor genes. Front. Genet. 2018, 9. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Frasca, L.; Lande, R. Toll-like receptors in mediating pathogenesis in systemic sclerosis. Clin. Exp. Immunol. 2020, 201, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Ciechomska, M.; Huigens, C.A.; Hügle, T.; Stanly, T.; Gessner, A.; Griffiths, B.; Radstake, T.R.D.J.; Hambleton, S.; O’Reilly, S.; van Laar, J.M. Toll-like receptor-mediated, enhanced production of profibrotic TIMP-1 in monocytes from patients with systemic sclerosis: Role of serum factors. Ann. Rheum. Dis. 2013, 72, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Skaug, B.; Assassi, S. Type I interferon dysregulation in Systemic Sclerosis. Cytokine 2020, 132, 154635. [Google Scholar] [CrossRef] [PubMed]
- Lafyatis, R. Transforming growth factor β—At the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Cabili, M.N.; Dunagin, M.C.; McClanahan, P.D.; Biaesch, A.; Padovan-Merhar, O.; Regev, A.; Rinn, J.L.; Raj, A. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Wang, F.; Han, G. LncRNA PSMB8-AS1 acts as ceRNA of miR-22-3p to regulate DDIT4 expression in glioblastoma. Neurosci. Lett. 2020, 728, 134896. [Google Scholar] [CrossRef]
- Shen, G.; Mao, Y.; Su, Z.; Du, J.; Yu, Y.; Xu, F. PSMB8-AS1 activated by ELK1 promotes cell proliferation in glioma via regulating miR-574-5p/RAB10. Biomed. Pharmacother. 2020, 122, 109658. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; He, Z.; Chen, S.; Li, L.; Sun, C. LncRNA PSMB8-AS1 contributes to pancreatic cancer progression via modulating miR-382-3p/STAT1/PD-L1 axis. J. Exp. Clin. Cancer Res. 2020, 39, 179. [Google Scholar] [CrossRef]
- Harada, H.; Taniguchi, T.; Tanaka, N. The role of interferon regulatory factors in the interferon system and cell growth control. Biochimie 1998, 80, 641–650. [Google Scholar] [CrossRef]
- Zhou, Y.; Lutz, P.-E.; Wang, Y.C.; Ragoussis, J.; Turecki, G. Global long non-coding RNA expression in the rostral anterior cingulate cortex of depressed suicides. Transl. Psychiatry 2018, 8, 224. [Google Scholar] [CrossRef]
- Honma, K.; Tsuzuki, S.; Nakagawa, M.; Tagawa, H.; Nakamura, S.; Morishima, Y.; Seto, M. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood 2009, 114, 2467–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, D.; Lv, L.; Yang, L.; Wu, W.; Liu, Y.; Tu, Y.; Xu, D.; Jin, Y.; Nong, X.; He, L. Genome-wide analysis of mRNA and long noncoding RNA profiles in chronic actinic dermatitis. BioMed Res. Int. 2017, 2017, 7479523. [Google Scholar] [CrossRef] [Green Version]
- More, S.; Zhu, Z.; Lin, K.; Huang, C.; Pushparaj, S.; Liang, Y.; Sathiaseelan, R.; Yang, X.; Liu, L. Long non-coding RNA PSMB8-AS1 regulates influenza virus replication. RNA Biol. 2019, 16, 340–353. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Perin, J.C.; Leipzig, J.; Zhang, Z.; Sullivan, K.E. Genome-wide analysis of interferon regulatory factor I binding in primary human monocytes. Gene 2011, 487, 21–28. [Google Scholar] [CrossRef] [Green Version]
- El Hassan, M.A.; Huang, K.; Eswara, M.B.K.; Xu, Z.; Yu, T.; Aubry, A.; Ni, Z.; Livne-Bar, I.; Sangwan, M.; Ahmad, M.; et al. Properties of STAT1 and IRF1 enhancers and the influence of SNPs. BMC Mol. Biol. 2017, 18, 6. [Google Scholar] [CrossRef] [Green Version]
- Aillaud, M.; Schulte, L.N. Emerging roles of long noncoding RNAs in the cytoplasmic milieu. Non Coding RNA 2020, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Santini, T.; Martone, J.; Ballarino, M. Visualization of nuclear and cytoplasmic long noncoding RNAs at single-cell level by RNA-FISH. In Capturing Chromosome Conformation: Methods and Protocols; Bodega, B., Lanzuolo, C., Eds.; Springer: New York, NY, USA, 2020; pp. 251–280. [Google Scholar]
- Zhang, P.; Cao, L.; Zhou, R.; Yang, X.; Wu, M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat. Commun. 2019, 10, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, R.Z.; Stow, J.L. Cytokine secretion in macrophages: SNAREs, rabs, and membrane trafficking. Front. Immunol. 2014, 5, 538. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Pallotta, S.; Frezzolini, A.; Abeni, D.; Barbieri, C.; Sampogna, F.; de Pita, O.; Puddu, P.; Paganelli, R.; Russo, G. Cytokine and chemokine levels in systemic sclerosis: Relationship with cutaneous and internal organ involvement. Clin. Exp. Immunol. 2004, 138, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Gourh, P.; Arnett, F.C.; Assassi, S.; Tan, F.K.; Huang, M.; Diekman, L.; Mayes, M.D.; Reveille, J.D.; Agarwal, S.K. Plasma cytokine profiles in systemic sclerosis: Associations with autoantibody subsets and clinical manifestations. Arthritis Res. Ther. 2009, 11, R147. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Hasegawa, M.; Takehara, K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J. Dermatol. Sci. 2001, 27, 140–146. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, M.; Kikuchi, K.; Takehara, K. Elevated serum tumor necrosis factor-α levels in patients with systemic sclerosis: Association with pulmonary fibrosis. J. Rheumatol. 1997, 24, 663–665. [Google Scholar]
- De Lauretis, A.; Sestini, P.; Pantelidis, P.; Hoyles, R.; Hansell, D.M.; Goh, N.S.L.; Zappala, C.J.; Visca, D.; Maher, T.M.; Denton, C.P.; et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 2013, 40, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Martinez-Gamboa, L.; Meier, S.; Witt, C.; Meisel, C.; Hanitsch, L.G.; Becker, M.O.; Huscher, D.; Burmester, G.R.; Riemekasten, G. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res. Ther. 2009, 11, R111. [Google Scholar] [CrossRef] [Green Version]
- Crestani, B.; Seta, N.; de Bandt, M.; Soler, P.; Rolland, C.; Dehoux, M.; Boutten, A.; Dombret, M.C.; Palazzo, E.; Kahn, M.F. Interleukin 6 secretion by monocytes and alveolar macrophages in systemic sclerosis with lung involvement. Am. J. Respir. Crit. Care Med. 1994, 149, 1260–1265. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, F.E., Jr.; Dupont, M.C. The Hmisc Package; R Package Version 4.4.-0; R Team: Vienna, Austria, 2006. [Google Scholar]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langfelder, P.; Luo, R.; Oldham, M.C.; Horvath, S. Is my network module preserved and reproducible? PLoS Comput. Biol. 2011, 7, e1001057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18546601 (accessed on 14 December 2020). [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]

| Definite Cohort | HC (9) | - | - | ncSSc (7) | lcSSc (11) | dcSSc (7) |
|---|---|---|---|---|---|---|
| Non-Cutaneous Cohort | HC (9) | RP (9) | eaSSc (11) | ncSSc (10) | - | - |
| Replication Cohort | HC (8) | - | eaSSc (5) | ncSSc (6) | lcSSc (10) | dcSSc (6) |
| Age (yr.) | 52 (30–64) | - | - | 45 (26–63) | 59 (45–70) | 58 (34–72) |
| 38 (28–49) | 47(22–70) | 57 (40–77) | 52 (25–70) | - | - | |
| 57 (31–64) | - | 47 (22–61) | 41 (36–55) | 58 (38–69) | 56 (53–72) | |
| Female/Male, n | 5/4 | - | - | 6/1 | 8/3 | 3/4 |
| 9/0 | 9/0 | 11/0 | 10/0 | - | - | |
| 7/1 | - | 4/1 | 4/2 | 8/2 | 4/2 | |
| ANA, n (% pos.) | - | - | - | 6 (86%) | 10 (91%) | 7 (100%) |
| - | 3 (33%) | 10 (91%) | 10 (100%) | - | - | |
| - | - | 4 (80%) | 6 (100%) | 8 * (80%) | 6 (100%) | |
| ACA, n (% pos.) | - | - | - | 3 (43%) | 6 * (55%) | 1 (14%) |
| - | 0 (0%) | 7 (64%) | 8 (80%) | - | - | |
| - | - | 1 (20%) | 1 (17%) | 4 * (40%) | 1 (17%) | |
| Scl70, n (% pos.) | - | - | - | 2 (29%) | 2 * (18%) | 4 (57%) |
| - | 0 (0%) | 2 (18%) | 1 (10%) | - | - | |
| - | - | 1 (20%) | 2 (33%) | 2 * (20%) | 4 (67%) | |
| ILD, n (% pos.) | - | - | - | 1 (14%) | 2 (18%) | 5 (71%) |
| - | 0 (0%) | 0 (0%) | 0 (0%) | - | - | |
| - | - | 1 (20%) | 2 (33%) | 3 * (30%) | 3 (50%) | |
| mRSS | - | - | - | 0 | 6 (0–12) | 14 * (5–36) |
| - | 0 | 0 | 0 | - | - | |
| - | - | 0 | 0 | 4 * (2–14) | 13 (4–23) | |
| Tel., n (%) | - | - | - | 3 * (43%) | 4 (36%) | 4 (57%) |
| - | 0 (0%) | 1 (9%) | 4 (40%) | - | - | |
| - | - | 1 * (20%) | 3 (50%) | 6 * (60%) | 2 * (33%) | |
| NVC early, n (%) | - | - | - | 2 * (29%) | 2 ** (18%) | 1 ***(14%) |
| - | 0 (0%) | 9 (82%) | 5 (50%) | - | - | |
| - | - | 3 * (60%) | 4 (66%) | 3 ** (30%) | 3 (50%) | |
| NVC late/active, | - | - | - | 4 * (57%) | 3 ** (27%) | 2 ***(28%) |
| n (%) | - | 0 (0%) | 0 (0%) | 5 (50%) | - | - |
| - | - | 1 * (20%) | 2 (33%) | 1 ** (10%) | 3 (50%) | |
| Steroids, n (%) | - | - | - | 0 (0%) | 0 (9%) | 2 (28%) |
| - | 0 (0%) | 0 (0%) | 0 (0%) | - | - | |
| - | - | 1 * (20%) | 0 (0%) | 1 * (10%) | 1 (17%) | |
| Immunosup., n (%) | - | - | - | 0 (0%) | 1 (9%) | 3 (43%) |
| - | 0 (0%) | 1 (9%) | 0 (0%) | - | - | |
| - | - | 1 (20%) | 2 (33%) | 1 * (10%) | 4 (66%) |
| Definite Cohort | Non-Cutaneous Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| GO-Term | PCG | lncRNA | BM | R | p | BM | R | p |
| Type I interferon response (GO:0071357, GO:0060337, GO:0034340) | PSMB8 | PSMB8-AS1 | 645.23 | 0.67 | 0.000 | 262.01 | 0.68 | 0.000 |
| OAS1 | RP1-71H24.6 | 46.71 | 0.50 | 0.003 | 17.74 | 0.62 | 0.000 | |
| IRF2 | RP11-326I11.3 | 75.09 | 0.61 | 0.000 | 40.19 | 0.73 | 0.000 | |
| CACTIN | CACTIN-AS1 | 6.68 | 0.60 | 0.000 | 3.25 | 0.62 | 0.000 | |
| IFITM3 | RP11-326C3.11 | 14.01 | 0.58 | 0.000 | 9.65 | 0.68 | 0.000 | |
| IFI6 | RP11-288L9.4 | 16.93 | 0.41 | 0.017 | 7.28 | 0.50 | 0.001 | |
| MX1 | AP001610.5 | 7.46 | 0.59 | 0.000 | 8.20 | 0.72 | 0.000 | |
| PAM16 | RP11-295D4.3 | 30.27 | 0.88 | 0.000 | 40.74 | 0.80 | 0.000 | |
| Negative regulation of apoptotic signaling pathway (GO:2001234) | FAS | RP11-399O19.9 | 12.26 | 0.56 | 0.001 | 15.18 | 0.56 | 0.000 |
| BIRC6 | AL133243.2 | 50.33 | 0.45 | 0.008 | 87.28 | 0.51 | 0.001 | |
| THBS1 | CTD-2033D15.2 | 40.78 | 0.76 | 0.000 | 76.70 | 0.87 | 0.000 | |
| SGMS1 | RP11-521C22.2 | 33.86 | 0.60 | 0.000 | 54.93 | 0.53 | 0.001 | |
| CCAR2 | RP11-582J16.5 | 34.78 | 0.59 | 0.000 | 64.17 | 0.67 | 0.000 | |
| AATF | CTC-268N12.3 | 0.58 | −0.41 | 0.015 | 0.56 | −0.40 | 0.011 | |
| TNAIP3 | RP11-356I2.4 | 66.03 | 0.84 | 0.000 | 96.56 | 0.50 | 0.001 | |
| IFI6 | RP11-288L9.4 | 16.93 | 0.41 | 0.017 | 7.28 | 0.50 | 0.001 | |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PSMB8-AS1 | CTTCTCTGCTCTCCCGTTATG | GTGTGTTACCTCCTTTCCAAG |
| RPL32 | AGGGTTCGTAGAAGATTCAAGG | GGAAACATTGTGAGCGATCTC |
| IL-8 | GCTCTGTGTGAAGGTGCAGT | CCAGACAGAGCTCTCTTCCA |
| PT-IL-8 | ATTGAGAGTGGACCACACTG | ACTACTGTAATCCTAACACCTG |
| PSMB8 | GAGGCGTTGTCAATATGTACC | CCTGGGGGAAATGCTTGTTC |
| MMP2 | AGCGAGTGGATGCCGCCTTTAA | CATTCCAGGCATCTGCGATGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servaas, N.H.; Mariotti, B.; van der Kroef, M.; Wichers, C.G.K.; Pandit, A.; Bazzoni, F.; Radstake, T.R.D.J.; Rossato, M. Characterization of Long Non-Coding RNAs in Systemic Sclerosis Monocytes: A Potential Role for PSMB8-AS1 in Altered Cytokine Secretion. Int. J. Mol. Sci. 2021, 22, 4365. https://doi.org/10.3390/ijms22094365
Servaas NH, Mariotti B, van der Kroef M, Wichers CGK, Pandit A, Bazzoni F, Radstake TRDJ, Rossato M. Characterization of Long Non-Coding RNAs in Systemic Sclerosis Monocytes: A Potential Role for PSMB8-AS1 in Altered Cytokine Secretion. International Journal of Molecular Sciences. 2021; 22(9):4365. https://doi.org/10.3390/ijms22094365
Chicago/Turabian StyleServaas, Nila H., Barbara Mariotti, Maarten van der Kroef, Catharina G. K. Wichers, Aridaman Pandit, Flavia Bazzoni, Timothy R. D. J. Radstake, and Marzia Rossato. 2021. "Characterization of Long Non-Coding RNAs in Systemic Sclerosis Monocytes: A Potential Role for PSMB8-AS1 in Altered Cytokine Secretion" International Journal of Molecular Sciences 22, no. 9: 4365. https://doi.org/10.3390/ijms22094365
APA StyleServaas, N. H., Mariotti, B., van der Kroef, M., Wichers, C. G. K., Pandit, A., Bazzoni, F., Radstake, T. R. D. J., & Rossato, M. (2021). Characterization of Long Non-Coding RNAs in Systemic Sclerosis Monocytes: A Potential Role for PSMB8-AS1 in Altered Cytokine Secretion. International Journal of Molecular Sciences, 22(9), 4365. https://doi.org/10.3390/ijms22094365






