1. Introduction
Despite great advances in prevention and treatment, cancer remains one of the most common causes of death worldwide. In the last years, several different anticancer treatments have been developed. However, their success is still limited, therefore, new strategies are needed. Cell metabolism, especially aerobic glycolysis, represents a promising area of anticancer treatment [
1,
2]. Several studies have suggested that the inhibition of specific aspects of cell metabolism can reduce cancer progression [
3] or improve the results of chemotherapy, with limited negative effects on host metabolism and health [
4].
Cancer cells are characterized by a high rate of glycolytic flux, even in the presence of sufficient oxygen supply, a phenomenon known as the Warburg effect [
5]. This metabolic alteration allows cancer cells to proliferate at an abnormally high rate, even under hypoxic conditions. The increased level of glycolysis provides anabolic substrates to support cell proliferation and migration, as well as the high ATP demand of tumor cells. A rate-limiting step in glucose metabolism is the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate (F1.6P2) by the enzyme phosphofructokinase 1 (PFK1) [
6]. The enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) catalyzes the synthesis of fructose-2,6-bisphosphate (F2.6P2), which is an allosteric activator of PFK1 and the most effective stimulator of glycolysis [
7]. Protein levels of the glycolysis regulator PFKFB3 are overexpressed in a variety of cancers, and expression of this enzyme is strongly correlated with a poor prognosis [
4]. Moreover, PFKFB3 is associated with the process of angiogenesis through the stimulation of vessel sprouting and branching mediated by endothelial cells [
1]. In this way, inhibition of glycolysis provides an attractive target in the field of angiogenesis research.
The activity of PFKFB3 can be blocked by various novel compounds, including chalcones, phenoxyindoles, aminoquinoxalines, and peptides [
8]. The first described inhibitor of PFKFB3, (
E)-3-(pyridin-3-yl)-1-(pyridin-4-yl)prop-2-en-1-one (3PO), exhibits potent antitumor activity [
6], reduces the intracellular concentration of F2.6P2, and suppresses the level of glycolysis in cells. Problems with the low solubility of 3PO led to the development of several analogues, among which (
E)-1-(pyridin-4-yl)-3-(quinolin-2-yl)prop-2-en-1-one (PFK15) [
9] and its trifluoromethyl derivative (
E)-1-(4-pyridinyl)-3-(7-(trifluoromethyl)-2E-quinolinyl)-2-propen-1-one (PFK158) seem to be the most promising [
10]. PFK15 results from the substitution of a quinolinyl group for a pyridine ring in the structure of 3PO and displays higher selectivity than 3PO. PFK15 causes a reduction in glucose uptake and lactate production in different types of cells [
9]. Another glycolytic inhibitor, PFK158, shows even higher efficiency in vitro and in vivo, and was included in the first phase of clinical trials [
11].
In addition to tumor cells, an enhanced preference for aerobic glycolysis is also observed in endothelial cells and cells present in the tumor microenvironment [
12]. Endothelial cells play an important role in angiogenesis, especially in vessel sprouting, which is a highly coordinated process regulated by chemical signals produced by tumor cells in the rapid growth phase. Upon the induction of sprouting by growth factors, quiescent endothelial cells become active, increase their level of glycolysis, and start to proliferate and migrate. This process is known as an angiogenic switch [
13]. These newly formed vessels deliver nutrients and oxygen to tumor tissues, thereby contributing to cancer development.
Cellular metabolism, both within the tumor microenvironment and in the host tissue, is under circadian control [
14]. Circadian clocks maintain cell-autonomous circa 24 h oscillations through the circadian transcriptional/translational feedback loop consisting of transcription factors CLOCK and BMAL 1, which control the transcription of canonical clock genes and hundreds of clock-controlled genes. As a result, metabolism in healthy cells exhibits distinct circadian oscillations, which can be disrupted in cancer cells [
15,
16,
17]. Clinical data show that circadian expression of core clock genes is deregulated in several human cancers [
18], including colorectal cancer [
19,
20]. Circadian clocks control a wide array of metabolic processes, including glycolysis, which exhibits a distinct circadian pattern. Additionally, PFKFB3 shows circadian expression at mRNA and protein level, which is controlled predominantly by transcription factor CLOCK, as shown in tongue cancer cell line [
21]. Moreover, the study showed that inhibition of PFKFB3 at its peak levels by glycolysis inhibitor 3PO resulted in a significant decrease of cell proliferation and lactate production. Therefore, specific targeting of metabolic processes in a time-dependent manner (chronotherapy) can offer a potential advantage in cancer treatment [
22].
Approaches aimed at simultaneously inhibiting cancer cell proliferation and angiogenesis represent an attractive possibility to limit tumor progression. Therefore, in the present study, we focused on the mechanisms underlying the effects of PFK15 on a cancer cell line and on primary endothelial cells. We analyzed signaling pathways that regulate the key processes of cell proliferation, migration, and apoptosis to examine whether both cell types respond to PFKFB3 inhibition in a similar way. In the next step, we explored the consequences of glycolysis inhibition on tumor progression induced by subcutaneous administration of human colorectal carcinoma-derived cells to athymic mice. Treatment with PFK15 was performed at two different times of day to explore whether chronotherapy represents a promising approach in relation to glycolysis inhibition.
3. Discussion
In the present study, we investigated the anticancer effects of glycolysis inhibition through PFK15 and the underlying mechanisms of its action on a human colorectal cancer cell line and human endothelial cells (DLD1 and HUVEC, respectively). Our study for the first time explored the effects of glycolysis inhibitor PFK15 on colorectal adenocarcinoma cell line and compared results with those obtained in primary endothelial cells. We found that PFK15 treatment suppressed proliferation and promoted apoptosis of both cell types in vitro. Our subsequent in vivo study on athymic mice revealed an inhibitory effect of PFK15 on the progression of tumors induced by DLD1 cells after morning but not evening treatment.
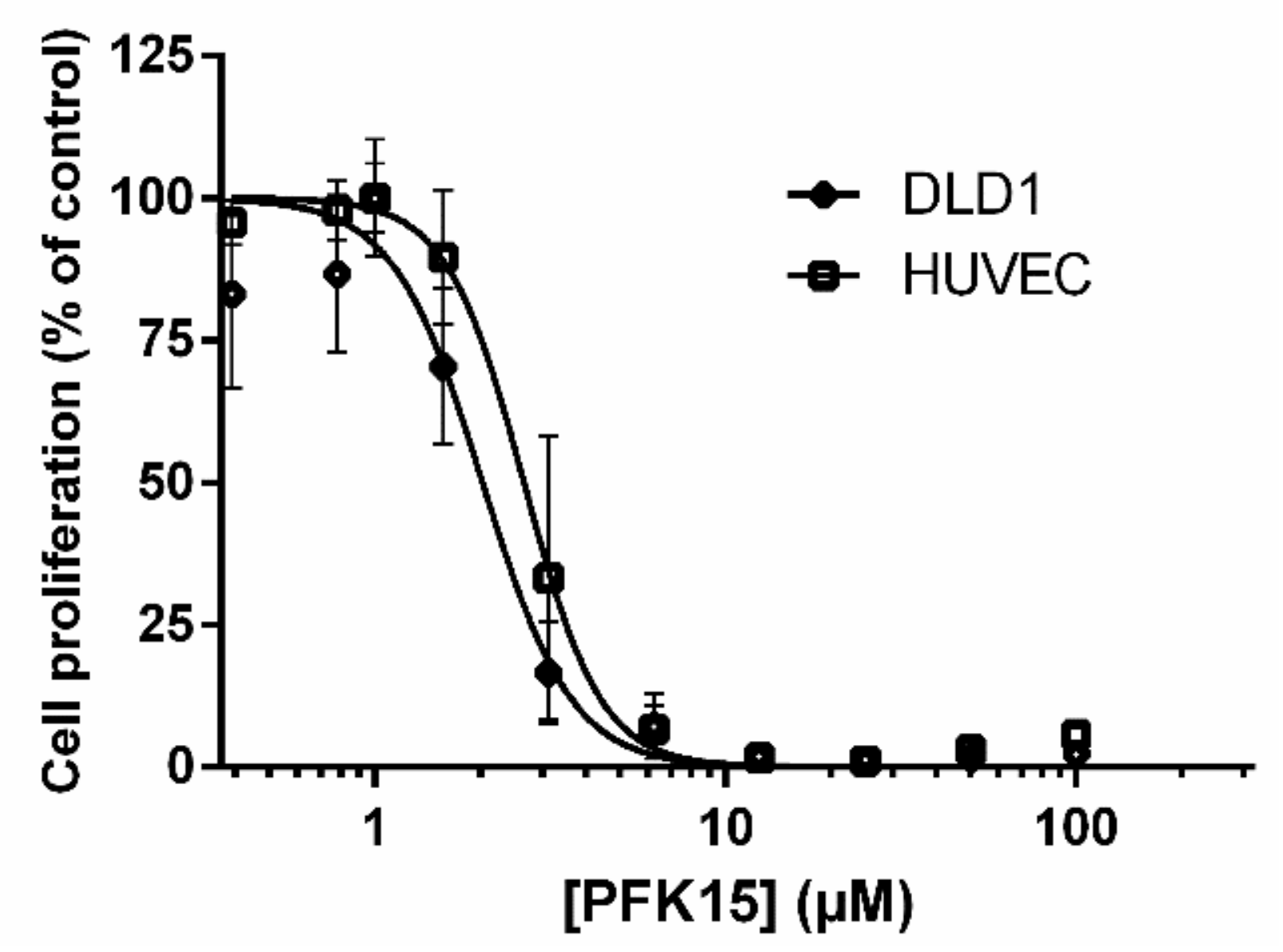
Administration of PFK15 reduced the proliferation of DLD1 and HUVEC cells in a dose-dependent manner. The IC
50 values calculated for both cell types were similar and close to 2 µM. HUVEC and DLD1 cells are of human origin and are characterized by an increased level of glucose metabolism; therefore, the effects of PFK15 could be mediated by similar mechanisms in both cell types. The inhibitory effect of PFK15 is important for cancer treatment because the drug can simultaneously suppress the proliferation of cancer cells and endothelial cells, which are responsible for the formation of new vessels and provide nutrients and oxygen to the proliferating tumor cells [
1]. Previously published data suggest that the effects of PFK15 depend on the cell type, and IC
50 values ranging from 6.6 to 10.6 µM have been reported for the MKN45 and BGC gastric cancer cell lines, respectively [
3]. We confirmed the effect of PFK15 using another colorectal cell line, HCT116 with the IC
50 value 1.34 µM (data not shown). The similar results in HCT116 and DLD1 cells demonstrate that the inhibitory effect of PFK15 is not specific only for DLD1 cells. In HCT116 cells, p53 was found to regulate glycolytic metabolism, and therefore using HCT166 -/- cells may further elucidate p53 function in this process [
23]. In Jurkat leukemia cells, the IC
50 value was lower (2.42 µM) compared with cancer cell line MKN45 and BGC, respectively. The value as low as 0.72 µM was reported in H522 adenocarcinoma cells. Our results show that both cell types tested in the current study are sensitive to glycolysis inhibition and did not differ substantially, even though HUVEC represents normal healthy human cells and DLD1 cells derived from colorectal adenocarcinoma represent a cell line with unlimited cell proliferation. These findings are in line with published data demonstrating decreased glucose uptake and F2.6P2 production in other cell types [
3]. Both lines of evidence suggest a suppressive effect of PFK15 treatment on ATP-consuming processes, such as proliferation, migration, and cell differentiation. Moreover, it is possible that the effects of glycolysis inhibitors can be enhanced by other anticancer drugs or administration in combination with inhibitors, which suppress angiogenesis, cell metabolism, and autophagy [
24]. Indeed, in our recent study, we found that simultaneous treatment of HUVEC with the glycolysis inhibitor 3PO and multikinase inhibitor sunitinib attenuated cell proliferation and migration and potentiated the antiangiogenic action [
25].
To understand the mechanisms underlying the effects of PFK15 on HUVEC and DLD1 cells, we investigated signaling pathways controlling cell proliferation, migration, and apoptosis. PFK15 treatment decreased levels of the protein kinase Akt and reduced its phosphorylation in both cell types. Similar effects have been reported in breast cancer cells treated with the allosteric Akt inhibitor MK-2206 in combination with salinomycin, a potential inhibitor of tumor stem cell growth [
26]. The authors concluded that cancer cells treated with salinomycin were more sensitive to MK-2206 because they displayed a total reduction in Akt. An inhibitory effect on Akt phosphorylation was also observed after PFKFB3 silencing in a breast cancer cell line, confirming the potential of PFKFB3 inhibitors to act as metabolic regulators [
27].
Signaling pathway PI3K/Akt represents the cascade of protein kinases, which regulate cell growth and proliferation and stimulate glycolytic activity [
28]. Akt signaling induces transcription of the glucose transporter GLUT1 and phosphorylation and activation of PFKFB3, thereby increasing the kinase activity of this enzyme. Reduced Akt phosphorylation and subsequent glycolysis activity have been shown to suppress the cell growth and proliferation rate of Chinese hamster ovary cells [
29]. Deregulation of the signaling pathway PI3K/Akt/mTOR has been demonstrated in several types of cancer, such as breast cancer and colorectal cancer [
30]. Because glycolysis is closely related to AMP-activated protein kinase (AMPK), which allows cells to proliferate only under conditions of sufficient energy supply, changes in AMPK activation can be of great importance. AMPK kinase regulates cellular energy homeostasis through the activation of glucose and fatty acid oxidation [
31]. A more pronounced effect on AMPK phosphorylation was observed after 12 h in comparison to two hours of PFK15 treatment in rhabdomyosarcoma cells. These results also demonstrate the time-dependent effects of PFK15 treatment [
32].
Tumor progression reflects a balance between cell proliferation and apoptosis. Therefore, in addition to cell proliferation, we explored the effect of PFK15 on apoptosis in both cell types. Annexin V assay demonstrated a significant proapoptotic effect of PFK15 in both analyzed cell types. Expression of the antiapoptotic Bcl-2 protein decreased with an increasing concentration of PFK15, while expression of the proapoptotic protein Bax did not change significantly. The differential regulation of Bcl-2 and Bax expression resulted in a decreased Bcl-2/Bax ratio, which is a crucial factor favoring increased apoptosis [
33]. The apoptotic effect of PFK15 could be explained by cytotoxic calcium overload and subsequent cell death. Glycolysis inhibition reduces the amount of ATP required for plasma membrane calcium ATPases, responsible for the low intracellular calcium observed in pancreatic cells [
34], but this effect was not present in noncancerous human pancreatic stellate cells. Our previous study showed induction of apoptosis in cancer DLD1 cells due to an increased intracellular concentration of calcium released from the endoplasmic reticulum via enhanced expression of inositol 1,4,5-trisphosphate (IP3R1) receptors, but this effect was not observed in normal endothelial EA.hy 926 cells [
35].
We found reduced expression of effector caspase-3 after PFK15 treatment, which can be explained by cleavage of the total form of caspase to the active phosphorylated form. The Bcl-2 protein can stabilize the mitochondrial membrane and block the release of cytochrome C, while the proapoptotic protein Bax contributes to the release of cytochrome C from the mitochondria, thereby assisting in the activation of caspase-3 and -9 [
36]. Thus, our data are in line with published results, suggesting that PFKFB3 inhibits apoptosis and inhibition of PFKFB3 suppresses cell cycle progression and accumulation of cells in G0/G1phase [
3]. PFKFB3 is upregulated at the transition from the G1 to S phase of the cell cycle, promoting cell division in conditions of sufficient nutrient supply [
37]; therefore, suppressed PFKFB3 activity is related to decreased cell division. In contrast to abovementioned study [
3], we observed arrest in G2 phase after exposition to PFK15 for 24 or 48 h. We suppose that sensitivity of cells to PFK15 and concentration of the drug play a role in the impact on cell cycle. This phenomenon was also observed, for example, in berberine and its derivatives, when low concentrations induced G0/G1 arrest while higher concentrations induced accumulation of cells in G2/M phase [
38,
39,
40,
41].
Moreover, the proapoptotic effect of suppressed PFKFB3 activity can be explained by increased expression of cyclin-dependent kinase inhibitor 1B (p27) in cancer cells following PFKFB3 inhibition. Studies have supported the role of p27 in the regulation of cell proliferation and apoptosis, and increased levels of this protein were observed in cancer cells after PFKFB3 knock-out [
27].
Increased PFKFB3 is considered a prognostic marker of cancer [
4] because it is related to Ki-67 expression, which is a marker of cell proliferation. In our study, we did not find significant changes in PFKFB3 expression in either of the tested cell types treated with PFK15 for 24 h, indicating that the treatment predominantly affects the activity of this enzyme and not its expression. In lung adenocarcinoma cells, levels of PFKFB3 were downregulated after PFK15 treatment, and upregulated expression of PFKFB3 in breast cancer tissues assessed by immunohistochemistry was found to be associated with poor patient prognosis [
27]. The results obtained in the current study indicate that different cell types can differ in their sensitivity to PFK15.
In the next step, we focused on characterizing the expression of genes involved in cell cycle control in DLD1 cells treated with PFK15. We found a dose-dependent increase in p21 mRNA after PFK15 treatment. Because p21 negatively affects the activity of cyclin and cyclin-dependent kinases [
42], we next quantified cyclin D1 and B1 mRNA. We found a trend toward decreased expression of cyclin D1 mRNA after treatment with PFK15 at the highest concentrations. This cytostatic effect of p21 could be observed after p53 binding to its promotor, which contains several binding sites for p53, and for c-Jun, respectively. Tumor suppressor p53 induces cell cycle arrest after DNA damage, and conversely, activation of c-Jun induces apoptosis [
43]. Since p53 and c-Jun compete for binding site on p21 promotor, in HCT116 p53 -/- cells c-Jun may downregulate p21 expression. Induction of p21 by p53 may be also blocked by transcription factor myc, which promotes cell growth and proliferation [
44]. Overexpression of p21 in prostate cancer cells has been reported to reduce tumor volume in mice and induce apoptosis, with similar results found in cervical cancer cells [
45]. Moreover, blocking PFKFB3 activity with PFK15 resulted in a significant reduction in cyclin D1 expression in tumor cells isolated from lung adenocarcinoma after 24 h [
4].
As the in vitro data revealed antiproliferative and apoptotic effects of PFK15 on cancer and endothelial cells, we investigated the efficiency of PFK15 in reducing growth and progression of tumor xenografts in athymic mice implanted with DLD1 cells. The dose of the drug was chosen based on previously published studies [
3,
9]. PFK15 was administrated either in the morning, two hours after the onset of the light phase, or in the evening, two hours before the onset of the dark phase. We found a reduced tumor mass in the group that received the morning PFK15 treatment, while no significant effects were recorded after the evening treatment. The size of the tumor was reduced after 12 days of the morning treatment. It is possible that longer treatment with PFK15 can have a more profound effect on tumor progression, but it could also cause necrosis due to an insufficient nutrient supply. Administration of PFK15 for 15 days to nude mice with an induced gastric tumor resulted in a significant decrease in tumor size after 6 days [
3], suggesting specific efficacy of the treatment depending on the cell type. Although we found a reduction in tumor mass, we did not record any changes in the body weight of animals or their general appearance, suggesting that PFK15 is tolerated and can suppress tumor growth without serious side effects. Moreover, it was confirmed that the viability of normal epithelioid cells was suppressed less than 10% compared to cancer cells after PFK15 treatment [
4]. Obviously, more data are needed to prove the tolerability of this treatment, but it seems that specific inhibition of PFKFB3 does not substantially interfere with physiological processes at the system level; therefore, it represents a promising target for cancer treatment.
Marker Ki-67 is considered to be a prognostic marker for cancer due to its increased expression in tumor tissues, which is directly correlated with tumor differentiation, TNM classification, and five-year survival [
4]. Our results suggest that the reduction in tumor size after morning PFK15 treatment is related to decreased expression of Ki-67, which plays a role in cell proliferation. Increased Ki-67 expression has been shown to be a negative prognostic marker of patient survival with pulmonary adenocarcinoma [
4].
The different effects of morning and evening treatment with PFK15 on tumor growth and progression indicate that the chronopharmacological approach can be helpful in cancer treatment. Our data are in line with the results of recent study [
21], which reported circadian control of PFKFB3 mRNA levels in human tongue carcinoma cells, with increased levels in the early light (passive) phase of a 24 h day, with peak levels between ZT5 and ZT9 (ZT = Zeitgeber Time, ZT0 indicates the beginning of the light phase), and lowest levels between ZT17 and ZT21. PFKFB3 suppression by the glycolytic inhibitor 3PO at ZT7 led to enhanced glycolysis inhibition and reduced lactate production [
21]. Significant inhibition of tumor progression after morning glycolysis inhibition is in line with our results and data obtained in another animal model, in which nude mice were implanted with breast tumor cells into the femoral artery [
46]. These authors observed an increased uptake of arterial glucose by tumor cells (Warburg effect) and the release of lactate into the blood. Lactate production reflected an increase in glycolysis during the passive phase, reaching a peak two hours before lights off and a minimum in the middle of the active phase. Moreover, circadian oscillations in [
3H] thymidine incorporation and total DNA content suggest that the number of tumor cells increased during the light phase due to increased cell proliferative activity. In contrast, during the dark phase, the number of cells was lower, indicating cell apoptosis with a concomitant reduction in cell proliferation [
46]. These results are consistent with previous studies suggesting that tumor growth shows circadian variations [
47]. Because PFK15 predominantly affects proliferating cells, which are activated especially during the passive phase of the day [
46], we hypothesize that the more pronounced effect of PFK15 observed in the group that received morning administration of PFK15 is related to circadian processes peaking during the passive phase of the day.
4. Materials and Methods
4.1. Cell Culture and Cultivation
Human colorectal carcinoma cell line (DLD1; CCL-221, ATCC, USA) was cultured in RPMI GlutaMAX medium (Gibco, Gaithersburg, MD, USA) supplemented with 10% FBS (Biosera, Nuaille, France), 100 U/mL penicillin, and 100 μg/mL streptomycin (Biosera). Human umbilical vein endothelial cells (HUVEC) were isolated from fresh umbilical cords, as previously described [
24], and cultured in complete ECGM medium (PromoCell, Heidelberg, Germany) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Biosera). Cells were cultivated at 37 °C in a humidified incubator (Heal Force, Shanghai, China) containing 5% CO
2.
4.2. Drug Preparation
The PFK15 inhibitor was prepared in four synthetic steps by Biomagi Inc. (Bratislava, Slovak Republic), and the stock solution was prepared in 100% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Saint-Louis, MO, USA) at a concentration of 10 mM. The stock solution was subsequently diluted in RPMI or ECGM medium for in vitro experiments. Controls represent medium with DMSO at the highest concentration used for PFK15 dissolving. In addition, PFK15 was suspended in 5% DMSO, 45% PEG300, 1% Tween80, and 49% H2O for in vivo experiments.
4.3. IC50 of PFK15
Cells were seeded in a 96-well plate with serial dilutions of PFK15 (starting at 100 µM containing 1% DMSO) at a density of 3 × 103 cells/well. Cells were incubated for 3 days, and then 10 µM of MTS reagent was added, according to the manufacturer’s instructions (CellTiter 96® Aqueous MTS Reagent, Promega, Madison, WI, USA). The absorbance was measured at a wavelength of 490 nm (Apollo LB913, Berthold Technologies, Wien, Austria). The half maximal inhibitory concentration (IC50) was evaluated using Graph Pad Prism 6 software (Graph Pad, San Diego, CA, USA).
4.4. Colorimetric BrdU Assay
Cell proliferation was determined by BrdU assay based on bromodeoxyuridine incorporated during DNA synthesis. Briefly, cells were seeded in a 96-well plate at a density of 1 × 103 cells/well. After 24 h, cells were treated with 1, 2, 4, or 6 µM of PFK15 containing 1% DMSO. A colorimetric BrdU assay (Roche, Meylan, France) was performed after 24 h according to the manufacturer’s manual.
4.5. Detection of Apoptosis by Annexin V Assay
Cells were seeded on 24-well plates (4 × 104/well). The next day, PFK15 was added at required concentrations to the respective wells, and cells were treated for 48 h. Harvested cells (also the ones from supernatant) were washed in PBS. The pellet was subsequently resuspended in binding buffer containing PE-conjugated Annexin V (eBioscience, San Diego, CA, USA) and incubated for 15 min at room temperature, protected from light. 7-Amino-Actinomycin D (7AAD; 2 μg/mL) was added to detect dead cells. Analysis was performed on BD FACSCanto™ II flow cytometer (BD Bioscience, San Jose, CA, USA), data were analyzed with FCS Express program.
4.6. Analysis of Cell Cycle
Cell cycle was determined by flow cytometric measurement of DNA content. Cells were seeded on a 12-well plate (8 × 104 for DLD1; 12 × 104 for HUVEC) and cultured overnight. Cells were subsequently cultured in the presence of PFK15 at required concentration for 24 and 48 h. For analysis, cells were collected by trypsinization, washed with cold PBS, resuspended in 300 µL PBS with 0.05% Triton X-100 and RNA-se A (100 µg/mL), and incubated for 20 min at 37 °C. Afterwards, cells were cooled on ice for 1 min and subsequently, propidium iodide (PI, 40 µg/mL) was added. Cells were analyzed using a CytoFLEX S flow cytometer (Beckman Coulter, Brea, CA, USA). Forward/side light scatter characteristic was used to exclude the cell debris from the analysis. Doublets were excluded from analysis based on 690/50 peak area vs. peak height, lin 585/42 was used for DNA cell cycle histogram. Data were analyzed by Kaluza software (Beckman Coulter).
4.7. Western Blot
Cultured DLD1 and HUVEC cells were lysed in lysis buffer containing 0.02 M Tris, 0.15 M NaCl, 0.09 M KCl, 0.002 M EDTA, 5% Igepal, 0.5% Triton-X 100, 0.0002 M Na3VO4, 0.01 M NaF, and 0.1% protease inhibitor (Sigma-Aldrich, Saint-Louis, MO, USA). Total protein concentration was determined using the bicinchoninic acid assay (BCA assay kit; Sigma-Aldrich). Proteins were separated by SDS-PAGE (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to a 0.22 µm or 0.45 µm nitrocellulose membrane (Thermo Fisher Scientific, Waltham, MA, USA). The membrane was blocked with 5% bovine serum albumin (BSA; BioWest, Nuaillé, France) for 1 h at room temperature to prevent nonspecific binding of antibodies. The blots were incubated with anti-p 44/42 MAPK (ERK1/2; 1:1000), anti-phospho-p44/42 MAPK (pERK1/2; 1:1000), anti-Akt, anti-phospho-Akt (1:2000), anti-caspase-3 (1:1000), anti-PFKFB3 (1:1000), anti-Bcl-2 (1:1000), anti-Bax (1:1000), and anti-GAPDH (1:2000) antibodies, followed by incubation with goat anti-rabbit HRP (1:2000) or horse anti-mouse HRP (1:2000) secondary antibodies. All antibodies were purchased from Cell Signaling Technologies (Danvers, MA, USA) except anti-caspase-3 (Abcam, Cambridge, UK) and anti-GAPDH (Merck Millipore, Burlington, MA, USA) antibodies. Bands were detected using the Clarity Western ECL Substrate (Bio-Rad Laboratories) chemiluminescence system with a Vü-C (Pop-Bio IMAGING, Cambridge, UK) imaging system, and were quantified using Image Studio Lite Version 5.2 (LI-COR Biosciences, Lincoln, NE, USA).
4.8. Quantitative PCR
Total RNA isolation from DLD1 cells was performed using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA). The RNA concentration and purity were determined using NanoDrop One (Thermo Fisher Scientific). Synthesis of cDNA was performed using Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Fisher Scientific) according to the manufacturer’s protocol. Quantitative PCR (qPCR) was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) in the CFX Connect Real-Time Detection System (Bio-Rad Laboratories). Data were analyzed using CFX Manager Software (Bio-Rad Laboratories) and LinRegPCR software [
48]. All Ct values were normalized to geometrical means of rpl13a Ct values. Data are presented as relative changes, obtained using the ∆∆Ct method [
49]. The list of primers is presented in
Table 2.
4.9. In Vivo Experiment
The experiment was approved by the Institutional Ethics Committee of the Biomedical Research Center, Slovak Academy of Sciences, Bratislava and the State Veterinary and Food Administration of the Slovak Republic (Project Registration No. Ro 1289/18 - 221) in compliance with the Directive 2010/63/EU and the Regulation 377/2012 on the protection of animals used for scientific purposes in the approved animal facility (License No. SK UCH 02017). Male athymic nude mice (NU(NCr)-Foxn1nu) were purchased from Charles River (Velaz, Czech Republic). The mice were housed in sterile individually ventilated cages (room temperature 24 °C, 12:12 LD cycle, lights on at 7:00 h). Sterilized water and food were provided ad libitum. Tumor DLD1 cells were collected from the exponential growth phase and resuspended in PBS. Mice were injected subcutaneously with 0.1 mL of cell suspension (4 × 10
6 cells) to the left and right site of the body (5 animals/untreated group; 6 animals/treated group). Monitoring of tumor growth was performed twice per week using a Vernier caliper. Tumor mass was determined according to the following formula: mass (mg) = (width, mm)
2 × (length, mm)/2 [
6]. After 3 days, mice were intraperitoneally treated with PFK15 at 25 mg/kg or a vehicle for 14 days (
Figure 9). PFK15 administration was performed at two different times: morning group, 2 h after the onset of a light phase (ZT 02); evening group, injected 2 h before the onset of a dark phase (ZT 10). The mice were monitored daily, and their body weight was recorded before each PFK15 or vehicle injection. At the indicated time, mice were sacrificed by cervical dislocation. Tumors were harvested for histology and additional molecular analysis according to standard procedures.
4.10. Tissue Specimens
The tissue specimens were fixed in 10% neutral buffered formalin and paraffin embedded for histological examination with hematoxylin and eosin staining.
4.11. Immunohistochemistry
Paraffin blocks of formalin-fixed tissues were cut into 4 µm thick serial slices. The Ki-67 antigen and PECAM-1 (CD31) were detected immunohistochemically. Monoclonal mouse anti-human Ki-67 antigen (clone MIB-1, isotype IgG1, kappa; Agilent Technologies, Inc., Dako, Glostrup, Denmark) was used at a dilution of 1:100, and monoclonal rabbit anti-mouse PECAM-1 (CD31) antibody (EPR17259; ab182981, Abcam) at a dilution of 1:2000 (incubation time: 20 min at room temperature using an automated immunostainer; Dako Autostainer Plus S 3400; Dako). Antigen enhancement (epitope retrieval) was performed by immersing the slides in citrate buffer (Dako EnVisionTM FLEX Target Retrieval Solution, low pH, 50×) and heating (in a steamer as well as using the automatic Dako PT Link Pre-Treatment Module for Autostainer; Dako). A conjugated reporter enzyme (peroxidase) and polymer (EnVision; Dako) [
50] were used for detection with a chromogenic (diaminobenzidine) substrate (H
2O
2), then tissues were counterstained with hematoxylin. The prepared histological slides were semiquantitatively evaluated using an optical microscope (Leica DM2000; Leica Microsystems, Mannheim, Germany). The Ki-67 staining was given an immunoreactive score based on the intensity of nuclear staining and the number of cells stained according to a subjective grading system [
51]. The staining intensity was classified into four categories, with the most strongly staining case as the upper limit: 0, negative; 1, weakly positive; 2, moderately positive; 3, strongly positive. The quantity of stained cells was scored according to the percentage of stained tumor nuclei: 0, 0%; 1, 1–10%; 2, 11–50%; 3, 51–80%; 4, >80%. The total immunoreactive score was expressed as a product of the scores obtained for staining intensity and quantity. A total score of 0 was considered as negative, 1–3 as weak, 4–6 as moderate, and 8–12 as strong immunoreactivity.
4.12. Tumor Parenchyma Cell Regressive Changes Morphometry
The prepared histological slides were evaluated by optical microscope (Leica DM2000, Leica Microsystems, Mannheim, Germany) semiquantitatively. The quantity (proportion, area) of cell regressive changes was scored as follows: 0%, 1–10%, 11–25%, 26–49%, ≥50% of tumor parenchyma cell regressive changed proportion/area in the field of view at low magnification of the microscope.
4.13. Statistical Analysis
The results are presented as mean ± SEM, and each value represents the average of at least three wells from at least three independent experiments. Normality was tested by Shapiro–Wilk test. Statistical differences among groups were determined by one-way ANOVA followed by Tukey’s post hoc test or by Kruskal–Wallis test. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using STATISTICA 7.0 (StatSoft Inc., Tulsa, OK, USA) and GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA).