Decoy Technology as a Promising Therapeutic Tool for Atherosclerosis
Abstract
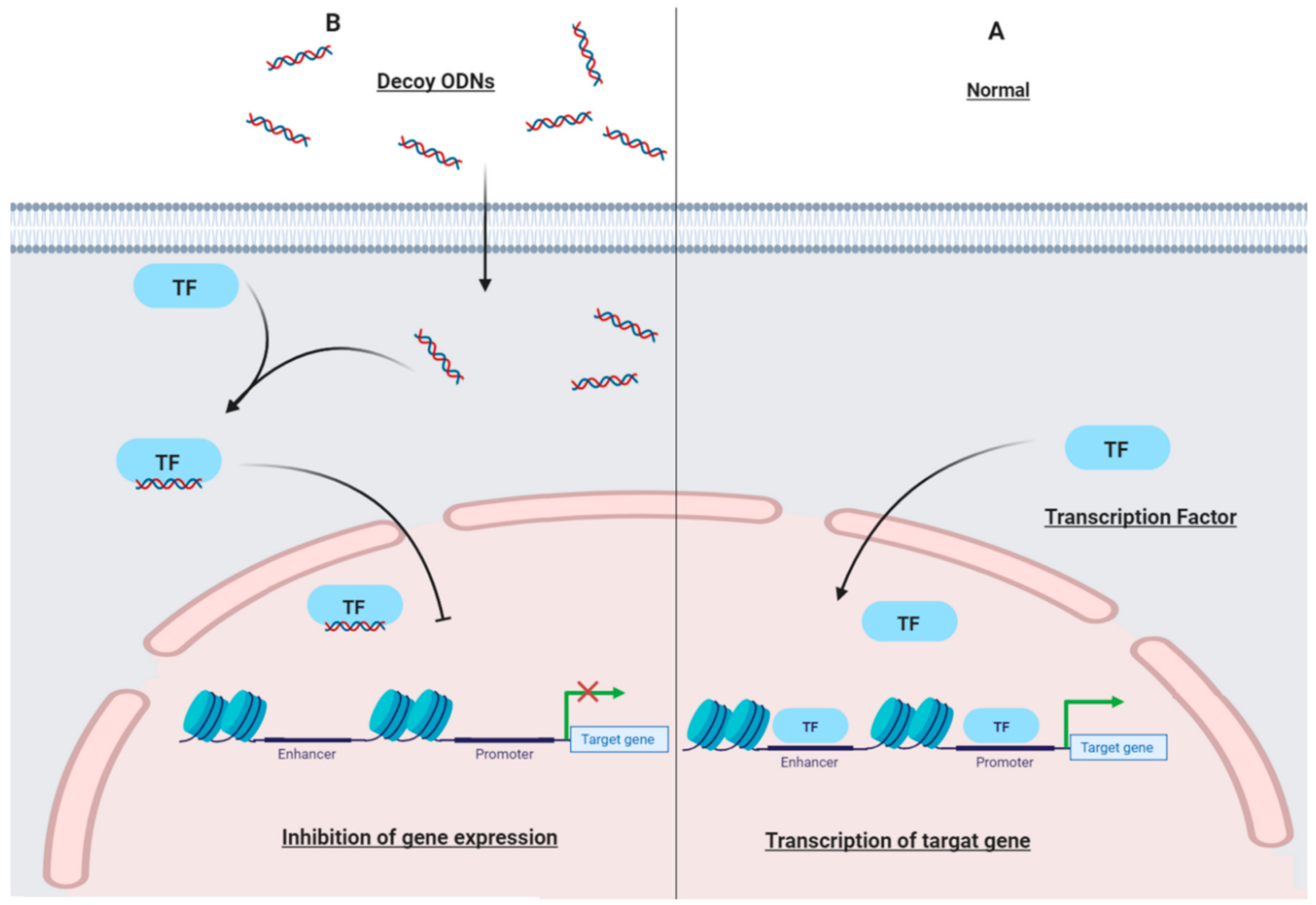
:1. Introduction
2. Therapeutic Approaches for Atherosclerosis
2.1. Lipid-Lowering Therapies
2.2. Antioxidant Interventions
2.3. Anti-Inflammatory Interventions
2.4. Potential Vaccinations
3. Decoy
3.1. Decoy Oligodeoxynucleotides (ODN)
3.2. Decoy Peptide
4. Targets
4.1. Protein Phosphatase 1
4.2. Macrophage Scavenger Receptors
4.3. Activator Protein-1
4.4. Cyclic Adenosine Monophosphate Response Element
4.5. Early Growth Response Factor-1
4.6. E2F
4.7. NF-κB
4.8. Smad
4.9. TNF-Like Cytokine 1A
4.10. Sterol Regulatory Element Binding Protein (SREBP)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertrand, M.J.; Tardif, J.C. Inflammation and beyond: New directions and emerging drugs for treating atherosclerosis. Expert Opin. Emerg. Drugs 2017, 22, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Flores-Gomez, D.; Bekkering, S.; Netea, M.G.; Riksen, N.P. Trained Immunity in Atherosclerotic Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kypreos, K.E.; Bitzur, R.; Karavia, E.A.; Xepapadaki, E.; Panayiotakopoulos, G.; Constantinou, C. Pharmacological Management of Dyslipidemia in Atherosclerosis: Limitations, Challenges, and New Therapeutic Opportunities. Angiology 2019, 70, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Ikitimur, B.; Karadag, B.; Barman, H.A.; Atici, A.; Koca, D.; Raimoglu, U.; Karaca, O.F.; Mutlu, D.; Ongen, Z. The impact of atherosclerotic risk factors on disease progression in patients with previously diagnosed nonobstructive coronary artery disease: Factors affecting coronary artery disease progression. Coron. Artery Dis. 2020, 31, 365–371. [Google Scholar] [CrossRef]
- Amarenco, P.; Hobeanu, C.; Labreuche, J.; Charles, H.; Giroud, M.; Meseguer, E.; Lavallée, P.C.; Gabriel Steg, P.; Vicaut, É.; Bruckert, E. Carotid atherosclerosis evolution when targeting a low-density lipoprotein cholesterol concentration <70 mg/dL after an ischemic stroke of atherosclerotic origin. Circulation 2020, 142, 748–757. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Lopes da Silva, A.; dos Santos Samary, C.; Leme Silva, P.; Dal Pizzol, F.; Pelosi, P.; Rocco, P.R.M. Brain–heart interaction after acute ischemic stroke. Crit. Care 2020, 24, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Whelton, S.P.; Deal, J.A.; Zikusoka, M.; Jacobson, L.P.; Sarkar, S.; Palella, F.J., Jr.; Kingsley, L.; Budoff, M.; Witt, M.D.; Brown, T.T. Associations between lipids and subclinical coronary atherosclerosis. Aids 2019, 33, 1053–1061. [Google Scholar] [CrossRef]
- Henning, R.J. Recognition and treatment of ischemic heart diseases in women. Future Cardiol. 2019, 15, 197–225. [Google Scholar] [CrossRef]
- Gupta, M.; Blumenthal, C.; Chatterjee, S.; Bandyopadhyay, D.; Jain, V.; Lavie, C.J.; Virani, S.S.; Ray, K.K.; Aronow, W.S.; Ghosh, R.K. Novel emerging therapies in atherosclerosis targeting lipid metabolism. Expert Opin. Investig. Drugs 2020, 29, 611–622. [Google Scholar] [CrossRef]
- Afshari, A.R.; Mollazadeh, H.; Henney, N.C.; Jamialahmad, T.; Sahebkar, A. Effects of statins on brain tumors: A review. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. The effects of statins on microglial cells to protect against neurodegenerative disorders: A mechanistic review. BioFactors 2020, 46, 309–325. [Google Scholar] [CrossRef]
- Reiner, Ž.; Hatamipour, M.; Banach, M.; Pirro, M.; Al-Rasadi, K.; Jamialahmadi, T.; Radenkovic, D.; Montecucco, F.; Sahebkar, A. Statins and the Covid-19 main protease: In silico evidence on direct interaction. Arch. Med. Sci. 2020, 16, 490–496. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Undas, A.; Lip, G.Y.H.; Bittner, V.; Ray, K.K.; Watts, G.F.; Kees Hovingh, G.; et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen: Systematic review and meta-analysis of Randomised placebo-controlled trials. Thromb. Haemost. 2016, 115, 520–532. [Google Scholar] [CrossRef]
- Banach, M.; Penson, P.E. Lipid-lowering therapies: Better together. Atherosclerosis 2021, 320, 86–88. [Google Scholar] [CrossRef]
- Libby, P.; Everett, B.M. Novel antiatherosclerotic therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Stojanović, S.D.; Fiedler, J.; Bauersachs, J.; Thum, T.; Sedding, D.G. Senescence-induced inflammation: An important player and key therapeutic target in atherosclerosis. Eur. Heart J. 2020, 41, 2983–2996. [Google Scholar] [CrossRef] [Green Version]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2020, 100694. [Google Scholar] [CrossRef] [PubMed]
- Nettersheim, F.S.; De Vore, L.; Winkels, H. Vaccination in Atherosclerosis. Cells 2020, 9, 2560. [Google Scholar] [CrossRef]
- Chyu, K.-Y.; Shah, P.K. In Pursuit of an Atherosclerosis Vaccine. Circ. Res. 2018, 123, 1121–1123. [Google Scholar] [CrossRef]
- Ito, M.K.; Watts, G.F. Challenges in the Diagnosis and Treatment of Homozygous Familial Hypercholesterolemia. Drugs 2015, 75, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Naci, H.; Brugts, J.; Ades, T. Comparative tolerability and harms of individual statins: A study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions: An update. Expert Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions. Expert Opin. Drug Saf. 2012, 11, 933–946. [Google Scholar] [CrossRef]
- Landmesser, U.; Poller, W.; Tsimikas, S.; Most, P.; Paneni, F.; Lüscher, T.F. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur. Heart J. 2020, 41, 3884–3899. [Google Scholar] [CrossRef]
- Mäkinen, P.; Ruotsalainen, A.-K.; Ylä-Herttuala, S. Nucleic acid–based therapies for atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Vahdat Lasemi, F.; Mahjoubin-Tehran, M.; Aghaee-Bakhtiari, S.H.; Jalili, A.; Jaafari, M.R.; Sahebkar, A. Harnessing nucleic acid-based therapeutics for atherosclerotic cardiovascular disease: State of the art. Drug Discov. Today 2019, 24, 1116–1131. [Google Scholar] [CrossRef]
- Hecker, M.; Wagner, A.H. Transcription factor decoy technology: A therapeutic update. Biochem. Pharmacol. 2017, 144, 29–34. [Google Scholar] [CrossRef]
- Gambari, R. New trends in the development of transcription factor decoy (TFD) pharmacotherapy. Curr. Drug Targets 2004, 5, 419–430. [Google Scholar] [CrossRef]
- Crinelli, R.; Bianchi, M.; Gentilini, L.; Palma, L.; Magnani, M. Locked nucleic acids (LNA): Versatile tools for designing oligonucleotide decoys with high stability and affinity. Curr. Drug Targets 2004, 5, 745–752. [Google Scholar] [CrossRef]
- Farahmand, L.; Darvishi, B.; Majidzadeh, A.K. Suppression of chronic inflammation with engineered nanomaterials delivering nuclear factor κB transcription factor decoy oligodeoxynucleotides. Drug Deliv. 2017, 24, 1249–1261. [Google Scholar] [CrossRef] [Green Version]
- Rad, S.M.A.H.; Langroudi, L.; Kouhkan, F.; Yazdani, L.; Koupaee, A.N.; Asgharpour, S.; Shojaei, Z.; Bamdad, T.; Arefian, E. Transcription factor decoy: A pre-transcriptional approach for gene downregulation purpose in cancer. Tumor Biol. 2015, 36, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubin-Tehran, M.; Rezaei, S.; Jalili, A.; Aghaee-Bakhtiari, S.H.; Sahebkar, A. Decoy oligodeoxynucleotide technology: An emerging paradigm for breast cancer treatment. Drug Discov. Today 2020, 25, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Suzuki, J.I.; Ogawa, M.; Taniyama, Y.; Morishita, R.; Isobe, M. Ultrasound-microbubble-mediated NF-κB decoy transfection attenuates neointimal formation after arterial injury in mice. J. Vasc. Res. 2005, 43, 12–18. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; Rezaei, S.; Jalili, A.; Aghaee-Bakhtiari, S.H.; Orafai, H.M.; Jamialahmadi, T.; Sahebkar, A. Peptide decoys: A new technology offering therapeutic opportunities for breast cancer. Drug Discov. Today 2020, 25, 593–598. [Google Scholar] [CrossRef]
- Mantovani, A.; Locati, M.; Vecchi, A.; Sozzani, S.; Allavena, P. Decoy receptors: A strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001, 22, 328–336. [Google Scholar] [CrossRef]
- Jimeno, A.; Gordon, M.; Chugh, R.; Messersmith, W.; Mendelson, D.; Dupont, J.; Stagg, R.; Kapoun, A.M.; Xu, L.; Uttamsingh, S. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin. Cancer Res. 2017, 23, 7490–7497. [Google Scholar] [CrossRef] [Green Version]
- Chandra, N.; Frängsmyr, L.; Arnberg, N. Decoy receptor interactions as novel drug targets against EKC-causing human adenovirus. Viruses 2019, 11, 242. [Google Scholar] [CrossRef] [Green Version]
- Lillehoj, E.P.; Guang, W.; Hyun, S.W.; Liu, A.; Hegerle, N.; Simon, R.; Cross, A.S.; Ishida, H.; Luzina, I.G.; Atamas, S.P. Neuraminidase 1–mediated desialylation of the mucin 1 ectodomain releases a decoy receptor that protects against Pseudomonas aeruginosa lung infection. J. Biol. Chem. 2019, 294, 662–678. [Google Scholar] [CrossRef] [Green Version]
- Albulescu, L.-O.; Kazandjian, T.; Slagboom, J.; Bruyneel, B.; Ainsworth, S.; Alsolaiss, J.; Wagstaff, S.C.; Whiteley, G.; Harrison, R.A.; Ulens, C. A decoy-receptor approach using nicotinic acetylcholine receptor mimics reveals their potential as novel therapeutics against neurotoxic snakebite. Front. Pharmacol. 2019, 10, 848. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, P.; Li, A.; Ladd, T.B.; Strickland, M.R.; Koller, E.J.; Burgess, J.D.; Funk, C.C.; Cruz, P.E.; Allen, M.; Yaroshenko, M. TLR5 decoy receptor as a novel anti-amyloid therapeutic for Alzheimer’s disease. J. Exp. Med. 2018, 215, 2247–2264. [Google Scholar] [CrossRef]
- Vallot, O.; Combettes, L.; Jourdon, P.; Inamo, J.; Marty, I.; Claret, M.; Lompré, A.M. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1225–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipskaia, L.; del Monte, F.; Capiod, T.; Yacoubi, S.; Hadri, L.; Hours, M.; Hajjar, R.J.; Lompré, A.M. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ. Res. 2005, 97, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenaart, N.A.; Ganim, J.R.; Di Salvo, J.; Kranias, E.G. The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch. Biochem. Biophys. 1992, 293, 17–24. [Google Scholar] [CrossRef]
- Jang, S.P.; Oh, J.G.; Kang, D.H.; Kang, J.Y.; Kang, S.W.; Hajjar, R.J.; Park, W.J. A decoy peptide targeted to protein phosphatase 1 attenuates degradation of serca2a in vascular smooth muscle cells. PLoS ONE 2016, 11, e0165569. [Google Scholar] [CrossRef]
- Laukkanen, J.; Lehtolainen, P.; Gough, P.J.; Greaves, D.R.; Gordon, S.; Ylä-Herttuala, S. Adenovirus-mediated gene transfer of a secreted form of human macrophage scavenger receptor inhibits modified low-density lipoprotein degradation and foam-cell formation in macrophages. Circulation 2000, 101, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Jalkanen, J.; Leppänen, P.; Pajusola, K.; Närvänen, O.; Mähönen, A.; Vähäkangas, E.; Greaves, D.R.; Büeler, H.; Ylä-Herttuala, S. Adeno-associated virus-mediated gene transfer of a secreted decoy human macrophage scavenger receptor reduces atherosclerotic lesion formation in LDL receptor knockout mice. Mol. Ther. 2003, 8, 903–910. [Google Scholar] [CrossRef]
- Jalkanen, J.; Leppänen, P.; Närvänen, O.; Greaves, D.R.; Ylä-Herttuala, S. Adenovirus-mediated gene transfer of a secreted decoy human macrophage scavenger receptor (SR-AI) in LDL receptor knock-out mice. Atherosclerosis 2003, 169, 95–103. [Google Scholar] [CrossRef]
- Kume, M.; Komori, K.; Matsumoto, T.; Onohara, T.; Takeuchi, K.; Yonemitsu, Y.; Sugimachi, K. Administration of a decoy against the activator protein-1 binding site suppresses neointimal thickening in rabbit balloon-injured arteries. Circulation 2002, 105, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Nikol, S.; Isner, J.M.; Pickering, J.G.; Kearney, M.; Leclerc, G.; Weir, L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J. Clin. Investig. 1992, 90, 1582–1592. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.D.; Morishita, R.; Kaneda, Y.; Lee, S.J.; Kwon, K.Y.; Choi, S.Y.; Lee, K.U.; Park, J.Y.; Moon, I.J.; Park, J.G.; et al. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ. Res. 2002, 90, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.H.; Kim, H.T.; Koo, J.H.; Lee, I.K. Effect of AP-1 Decoy Using Hemagglutinating Virus of Japan-Liposome on the Intimal Hyperplasia of the Autogenous Vein Graft in Mongrel Dogs. Transplant. Proc. 2006, 38, 2161–2163. [Google Scholar] [CrossRef]
- Xie, S.; Nie, R.; Wang, J.; Li, F.; Yuan, W. Transcription factor decoys for activator protein-1 (AP-1) inhibit oxidative stress-induced proliferation and matrix metalloproteinases in rat cardiac fibroblasts. Transl. Res. 2009, 153, 17–23. [Google Scholar] [CrossRef]
- Nakanishi, K.; Saito, Y.; Azuma, N.; Sasajima, T. Cyclic adenosine monophosphate response-element binding protein activation by mitogen-activated protein kinase-activated protein kinase 3 and four-and-a-half LIM domains 5 plays a key role for vein graft intimal hyperplasia. J. Vasc. Surg. 2013, 57, 182–193.e110. [Google Scholar] [CrossRef] [Green Version]
- Uchida, D.; Saito, Y.; Kikuchi, S.; Yoshida, Y.; Hirata, S.; Sasajima, T.; Azuma, N. Development of gene therapy with a cyclic adenosine monophosphate response element decoy oligodeoxynucleotide to prevent vascular intimal hyperplasia. J. Vasc. Surg. 2010, 71, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, K.; Egashira, K.; Usui, M.; Ishibashi, M.; Hiasa, K.I.; Zhao, Q.; Aoki, M.; Kaneda, Y.; Morishita, R.; Takeshita, A. Inhibition of neointimal hyperplasia after balloon injury by cis-element ‘decoy’ of early growth response gene-1 in hypercholesterolemic rabbits. Gene Ther. 2004, 11, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Mei, Y.; Ji, Q.; Feng, J.; Cai, J.; Xie, S. Early growth response gene-1 decoy oligonucleotides inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia of autogenous vein graft in rabbits. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Liu, G.N. EGR-1 decoy ODNs inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia of balloon-injured arteries in rat. Life Sci. 2010, 86, 234–243. [Google Scholar] [CrossRef]
- Peroulis, M.; Kakisis, J.; Kapelouzou, A.; Giagini, A.; Giaglis, S.; Mantziaras, G.; Kostomitsopoulos, N.; Karayannacos, P.; MacHeras, A. The Role of ex-vivo Gene Therapy of Vein Grafts with Egr-1 Decoy in the Suppression of Intimal Hyperplasia. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Yao, W.; Zhu, J.; Mu, K.; Zhang, J.; Zhang, J.-A. Resveratrol Attenuates High Glucose-Induced Vascular Endothelial Cell Injury by Activating the E2F3 Pathway. BioMed. Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Morishita, R.; Gibbons, G.H.; Horiuchi, M.; Ellison, K.E.; Nakajima, M.; Zhang, L.; Kaneda, Y.; Ogihara, T.; Dzau, V.J. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 5855–5859. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.H.; Hafley, G.; Harrington, R.A.; Peterson, E.D.; Ferguson, T.B., Jr.; Lorenz, T.J.; Goyal, A.; Gibson, M.; Mack, M.J.; Gennevois, D.; et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. J. Am. Med. Assoc. 2005, 294, 2446–2454. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.D.; Williams, J.B.; Mehta, R.H.; Reyes, E.M.; Hafley, G.E.; Allen, K.B.; MacK, M.J.; Peterson, E.D.; Harrington, R.A.; Gibson, C.M.; et al. Edifoligide and long-term outcomes after coronary artery bypass grafting: PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) 5-year results. Am. Heart J. 2012, 164, 379–386.e371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, M.J.; Whittemore, A.D.; Donaldson, M.C.; Belkin, M.; Conte, M.S.; Polak, J.F.; Orav, E.J.; Ehsan, A.; Dell’Acqua, G.; Dzau, V.J. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: The PREVENT single-centre, randomised, controlled trial. Lancet 1999, 354, 1493–1498. [Google Scholar] [CrossRef]
- Ehsan, A.; Mann, M.J.; Dell’Acqua, G.; Dzau, V.J. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J. Thorac. Cardiovasc. Surg. 2001, 121, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Grube, E.; Felderhoff, T.; Fitzgerald, P.; Terashima, M.; Gerckens, U.; Orav, E.; Lorenz, T.; Iversen, S. Phase II trial of the E2F decoy in coronary bypass grafting. In Proceedings of the American Heart Association Annual Meeting (Late Breaking Clinical Trials), Anaheim, CA, USA, 12 November 2001. [Google Scholar]
- Conte, M.S.; Bandyk, D.F.; Clowes, A.W.; Moneta, G.L.; Seely, L.; Lorenz, T.J.; Namini, H.; Hamdan, A.D.; Roddy, S.P.; Belkin, M.; et al. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 2006, 43, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Morishita, R.; Asai, T.; Tsuboniwa, N.; Aoki, M.; Sakonjo, H.; Yamasaki, K.; Hashiya, N.; Kaneda, Y.; Ogihara, T. Molecular strategy using cis-element ‘decoy’ of E2F binding site inhibits neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2002, 9, 488–494. [Google Scholar] [CrossRef]
- Kawauchi, M.; Suzuki, J.I.; Morishita, R.; Wada, Y.; Izawa, A.; Tomita, N.; Amano, J.; Kaneda, Y.; Ogihara, T.; Takamoto, S.; et al. Gene therapy for attenuating cardiac allograft arteriopathy using ex vivo E2F decoy transfection by HVJ-AVE-liposome method in mice and nonhuman primates. Circ. Res. 2000, 87, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-T.; Chen, L.-K.; Jian, D.-Y.; Hsu, T.-C.; Huang, W.-C.; Kuan, T.-T.; Wu, S.-Y.; Kwok, C.-F.; Ho, L.-T.; Juan, C.-C. Visfatin Promotes Monocyte Adhesion by Upregulating ICAM-1 and VCAM-1 Expression in Endothelial Cells via Activation of p38-PI3K-Akt Signaling and Subsequent ROS Production and IKK/NF-κB Activation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 52, 1398–1411. [Google Scholar]
- Suzuki, J.; Tezuka, D.; Morishita, R.; Isobe, M. Eight-year follow-up of an initial case with NF-kB decoy oligodeoxynucleotide transfection after coronary stent implantation. Immunol. Endocr. Metab. Agents Med. Chem. 2012, 12, 40–42. [Google Scholar] [CrossRef]
- Osako, M.K.; Tomita, N.; Nakagami, H.; Kunugiza, Y.; Yoshino, M.; Yuyama, K.; Tomita, T.; Yoshikawa, H.; Ogihara, T.; Morishita, R. Increase in nuclease resistance and incorporation of NF-κB decoy oligodeoxynucleotides by modification of the 3′-terminus. J. Gene Med. 2007, 9, 812–819. [Google Scholar] [CrossRef]
- Miyake, T.; Aoki, M.; Morishita, R. Inhibition of anastomotic intimal hyperplasia using a chimeric decoy strategy against NFκB and E2F in a rabbit model. Cardiovasc. Res. 2008, 79, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Park, J.H.; Kim, K.H.; Lee, W.R.; Lee, S.; Kwon, O.C.; Kim, K.S.; Park, K.K. Effect of NF-κB decoy oligodeoxynucleotide on LPS/high-fat diet-induced atherosclerosis in an animal model. Basic Clin. Pharmacol. Toxicol. 2010, 107, 925–930. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Park, Y.Y.; Kim, K.S.; Lee, C.K.; Min, B.K.; Park, K.K. Effects of Chimeric Decoy Oligodeoxynucleotide in the Regulation of Transcription Factors NF-κB and Sp1 in an Animal Model of Atherosclerosis. Basic Clin. Pharmacol. Toxicol. 2013, 112, 236–243. [Google Scholar] [CrossRef]
- Miyake, T.; Aoki, M.; Shiraya, S.; Tanemoto, K.; Ogihara, T.; Kaneda, Y.; Morishita, R. Inhibitory effects of NFκB decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. J. Mol. Cell. Cardiol. 2006, 41, 431–440. [Google Scholar] [CrossRef]
- Feeley, B.T.; Miniati, D.N.; Park, A.K.; Grant Hoyt, E.; Robbins, R.C. Nuclear factor-κB transcription factor decoy treatment inhibits graft coronary artery disease after cardiac transplantation in rodents. Transplantation 2000, 70, 1560–1568. [Google Scholar] [CrossRef] [Green Version]
- Miyake, T.; Ihara, S.; Miyake, T.; Tsukada, Y.; Watanabe, H.; Matsuda, H.; Kiguchi, H.; Tsujimoto, H.; Nakagami, H.; Morishita, R. Prevention of neointimal formation after angioplasty using nuclear factor-ΚB Decoy oligodeoxynucleotide-coated balloon catheter in rabbit model. Circ. Cardiovasc. Interv. 2014, 7, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Tomita, N.; Morishita, R.; Tomita, S.; Yamamoto, K.; Aoki, M.; Matsushita, H.; Hayashi, S.I.; Higaki, J.; Ogihara, T. Transcription factor decoy for nuclear factor-κB inhibits tumor necrosis factor-α-induced expression of interleukin-6 and intracellular adhesion molecule-1 in endothelial cells. J. Hypertens. 1998, 16, 993–1000. [Google Scholar] [CrossRef]
- Yoshimura, S.; Morishita, R.; Hayashi, K.; Yamamoto, K.; Nakagami, H.; Kaneda, Y.; Sakai, N.; Ogihara, T. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element ‘decoy’of nuclear factor-kB binding site as a novel molecular strategy. Gene Ther. 2001, 8, 1635. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Asai, T.; Shimizu, M.; Aoki, M.; Hashiya, N.; Sakonjo, H.; Makino, H.; Kaneda, Y.; Ogihara, T.; Morishita, R. Inhibition of NFκB activation using cis-element ‘decoy’of NFκB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2003, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; He, L.; Du, Y.; Zhu, P.; Huang, G.; Luo, J.; Yan, X.; Ye, B.; Li, C.; Xia, P. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell 2015, 16, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Al-Azab, M.; Qaed, E.; Ouyang, X.; Elkhider, A.; Walana, W.; Li, H.; Li, W.; Tang, Y.; Adlat, S.; Wei, J. TL1A/TNFR2-mediated mitochondrial dysfunction of fibroblast-like synoviocytes increases inflammatory response in patients with rheumatoid arthritis via reactive oxygen species generation. FEBS J. 2020, 287, 3088–3104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Hou, H.T.; Chen, H.X.; Wang, Z.Q.; He, G.W. Increased circulating levels of tumor necrosis factor-like cytokine 1A and decoy receptor 3 correlate with SYNTAX score in patients undergoing coronary surgery. J. Int. Med. Res. 2018, 46, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Hsu, C.Y.; Huang, P.H.; Chiang, C.H.; Leu, H.B.; Huang, C.C.; Chen, J.W.; Lin, S.J. Usefulness of Circulating Decoy Receptor 3 in Predicting Coronary Artery Disease Severity and Future Major Adverse Cardiovascular Events in Patients with Multivessel Coronary Artery Disease. Am. J. Cardiol. 2015, 116, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- An, H.-J.; Kim, J.-Y.; Gwon, M.-G.; Gu, H.; Kim, H.-J.; Leem, J.; Youn, S.W.; Park, K.-K. Beneficial Effects of SREBP Decoy Oligodeoxynucleotide in an Animal Model of Hyperlipidemia. Int. J. Mol. Sci. 2020, 21, 552. [Google Scholar] [CrossRef] [Green Version]
- Tomita, N.; Tomita, T.; Yuyama, K.; Tougan, T.; Tajima, T.; Ogihara, T.; Morishita, R. Development of novel decoy oligonucleotides: Advantages of circular dumb-bell decoy. Curr. Opin. Mol. Ther. 2003, 5, 107–112. [Google Scholar] [PubMed]
- Crinelli, R.; Bianchi, M.; Gentilini, L.; Palma, L.; Sørensen, M.D.; Bryld, T.; Babu, R.B.; Arar, K.; Wengel, J.; Magnani, M. Transcription factor decoy oligonucleotides modified with locked nucleic acids: An in vitro study to reconcile biostability with binding affinity. Nucleic Acids Res. 2004, 32, 1874–1885. [Google Scholar] [CrossRef] [Green Version]


| Decoy Name | Type of CVD | Target Up/Down ↑ ↓ | Concentration/Dose µM mg/kg/day | Decoy Type | Model/Cell Line | Delivery Method | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| ΨPLB-SE | Injury of the carotid artery (vascular proliferative disorders) | protein phosphatase 1 ↓ | 5 μg in 200 μl buffer injection for 15 min | Peptide | Balloon-injured rat carotid arteries | - | -Preventing the SERCA2a degradation in VSMC-reduced neointimal growth carotid artery | [44] |
| sMSR | Atherosclerosis | MSR ↓ | 2.5 mg/mL | Peptide | RAW 264 cells and peritoneal mouse macrophages | Adenovirus | -Decreased adhesion of monocyte/macrophage to the endothelial cells -Prevented the formation macrophage foam cell | [45] |
| sMSR | Atherosclerosis | MSR ↓ | Single injection of 7.5 × 109 AAVsMSR particles | Peptide | LDLR knockout mice | AAV | -Reduced atherosclerotic lesion in the aorta | [46] |
| sMSR | Atherosclerosis | MSR ↓ | 1 × 109 | Peptide | Hypercholesterolemic LDLR knockout mice | Recombinant adenovirus | -Reduces atherosclerotic lesion area | [47] |
| AP-1 decoy | - restenosis -injured carotid arteries -neointimal thickening | AP-1 ↓ | 15 nmol/L | ODN | -Balloon-injured rabbit carotid arteries -human aortic SMCs | HVJ liposomes | -Reduced the neointimal area. -Decreased SMCs cell number -Decreased TGF-β1 production of SMCs | [48] |
| AP-1 Decoy | Restenosisafter angioplasty Neointimal formation Intimal Hyperplasia | AP-1 ↓ | - | ODN | Balloon-injured Rats | HVJ-liposome | -Inhibited VSMC proliferation and migration. -Abolished neointimal formation after balloon injury | [50] |
| AP-1 Decoy | Intimal Hyperplasia | AP-1 ↓ | - | ODN | Mongrel dogs | HVJ-liposome | -Inhibited intimal hyperplasia | [51] |
| AP-1 decoy | Oxidative stress-induced proliferation and MMPs in rat cardiac fibroblasts | AP-1 ↓ | - | ODN | Rat cardiac fibroblasts | LipofectAMINE 2000 | -Inhibited XXO-induced CF proliferation and MMP gene expression | [52] |
| CRE decoy | Intimal hyperplasia | CRE ↓ | 7.814 pmol/mL | ODN | Mice | ultrasound-sonoporation | -Decreased VSMC proliferation and migration -Suppressed the intimal hyperplasia formation | [54] |
| EGR-1 decoy | Atherosclerosis and restenosis | EGR-1 ↓ | 80 µM | ODN | Hypercholesterolemic rabbits | - | -The Egr-1 decoy reduced inflammation, cell proliferation and later neointimal hyperplasia | [55] |
| EGR-1 decoy | Vein graft failure intimal hyperplasia | EGR-1 ↓ | 500 µg | ODN | Rabbits | Fugene6 transfection reagent | -Reduced VSMC proliferation and intimal hyperplasia | [56] |
| EGR-1 decoy | Neointimal hyperplasia | EGR-1↓ | 0.1 μM | ODN | Balloon-injured rat VSMCs | FuGene6 | -Inhibited VSMC proliferation and neointimal hyperplasia | [57] |
| EGR-1 decoy | Intimal Hyperplasia | EGR-1 ↓ | 40 µmol/l | ODN | Hypercholesterolaemic rabbits | - | -Suppressed intimal hyperplasia | [58] |
| E2F decoy | -Carotid injury -Abnormal growth of vascular cells | E2F ↓ | 3 µM | ODN | -Rat carotid injury -Rat aortic VSMCs | HVJ liposomes | -Inhibited proliferation of SMC -Inhibited formation of vascular lesion | [60] |
| E2F decoy | Neointimal hyperplasia and vein graft failure | E2F ↓ | 0.38 mg/mL (40 µmol/L) | ODN | Human | pressure-mediated delivery system | -Edifoligide is no more effective than placebo in preventing of vein graft Failure | [61] |
| E2F decoy | Atherosclerosis neointimal thickness | E2F ↓ PCNA ↓ | 40 µmol/L | ODN | Cholesterol-fed rabbits | nondistending pressure-mediated transfection | -Reduced neointimal thickness -Inhibited plaqe formation | [64] |
| E2F decoy | Atherosclerosis graft failure | E2F ↓ PCNA ↓ c-myc ↓ | 40 µmol/L | ODN | Human | pressure-mediated DNA transfection | -Decreased stenosis | [63] |
| E2F decoy | Atherosclerosis | E2F ↓ | 40 µmol/L | ODN | Human | - | -Reduced critical stenosis and neointimal volume | [65] |
| Edifoligide | Atherosclerosis | E2F ↓ | 40 µmol/L | ODN | Human | - | -Improvement in secondary graft patency -Did not showed any protection against vein graft failure | [66] |
| E2F decoy | Atherosclerosis intimal hyperplasia | E2F ↓ | 1 mg/pig | ODN | Balloon-injured pig | hydrogel catheter | -Reduced plaque area -Increased luminal and total vessel areas | [67] |
| E2F decoy | Neointimal formation Cardiac Allograft Arteriopathy | E2F ↓ | ODN | Mice and Japanese monkeys | HVJ | -Suppressed neointimal formation and prevented expression of cell-cycle regulatory genes -Reduced Cardiac allograft arteriopathy | [68] | |
| chimeric decoy | Neointimal formation | NF-κB ↓ E2F↓ | 200 nM | ODN | Cholesterol-fed rabbits | - | -Suppressed anastomotic intimal hyperplasia -accelerated re -endothelialization -Inhibited macrophage accumulation -Repressed the expression of VCAM-1 and MCP-1 gene -Inhibited VSMC proliferation Chimeric decoy was more than two others. | [72] |
| NF-κB decoy | NF-B↓ | 600 nM | ||||||
| E2F decoy | E2F↓ | 600 nM | ||||||
| NF-κB decoy | Remodeling of vascular neointimal formation restenosis | NF-κB ↓ | 1 mg | ODN | Human | remedy catheter | -Suppressed the development of neointimal formation -reduced lesion | [70] |
| NF-κB decoy | Atherosclerosis | NF-κB ↓ | 0.4 mg ⁄ kg | ODN | LPS/Fat-induced mice | - | -Decreased pro-inflammatory cytokines and inflammatory markers, VCAM-1 and ICAM-1 | [73] |
| Chimeric decoy | Atherosclerosis | NF-κB ↓ Sp1 ↓ | 10 µg per mouse | ODN | LPS/atherogenic diet-induced mice | - | -Decreased TG and TC -improved atherosclerotic changes | [74] |
| NF-κB decoy | Neointimal hyperplasia Atherosclerosis | NF-κB ↓ | 40 µmol/l | ODN | Hypercholesterolemic rabbits | pressure-mediated transfection | -Inhibited the development of neointimal hyperplasia -Suppressed inflammatory changes and accumulation of VSMC | [75] |
| NF-κB decoys (NF-ICAM, NF-VCAM, NF-ESEL) | Graft coronary artery disease (GCAD) | NF-κB ↓ ICAM ↓ VCAM ↓ ESEL ↓ | 160 µmol/L | ODN | Rat | pressure-mediated | -Blocked adhesion molcule expression and reperfusion injury -Prolongs allograft survival and decreases GCAD | [76] |
| NF-κB decoy | Restenosis Neointimal Formation neointimal hyperplasia | NF-κB ↓ | - | ODN | Rabbits | chitosan-modifed PLGA NS | -Inhibited neointimal formation -Restored ECMs -Inhibited macrophage recruitment -Inhibited VSMCs growth | [77] |
| NF-κB decoy | Inflammation in atherosclerotic | NF-κB ↓ | 2 µmol/l | ODN | Mouse brain microvascular endothelial cells | cationic liposome | -Inhibited TNF-induced expression of interleukin-6 and ICAM-1 in endothelial cells | [78] |
| NF-κB Decoy | Neointimal Formation | NF-κB ↓ | 20 µg | ODN | Arterial injured mice | ultrasound-microbubble-mediated | -Reduced the neointima/media areas. The expression of inflammatory factors | [33] |
| R-ODN | Cardiovascular diseases | NF-κB ↓ | 10 nM | ODN | VSMC | lipofectamine | -Expression of MMP-9 and the proliferation of VSMC were inhibited | [71] |
| NF-κB decoy | Atherosclerosis /lesion formation after vascular injury/ intimal hyperplasia | NF-κB ↓ | 15 µM | ODN | Balloon-injured rat | HVJ-liposome | -Apoptosis was upregulated -ICAM-1 and VCAM-1 expression was decreased -The migration of T-lymphocytes and macrophages into the media and neointima was inhibited | [79] |
| NF-κB decoy | Intimal hyperplasia neointimal formation | NF-κB ↓ | 1 mg/pig | ODN | Balloon-injured pigs | hydrogel balloon catheter | -Decoy inhibited the proliferation of VSMC- Reduced the neointimal area -Decrease the expression of ICAM | [80] |
| Smad decoy | Atherosclerosis | TGF-β1 ↓ PAI-1 ↓ α-SMA ↓ | - | ODN | Shear stress-induced ApoE-/-mice | Trans IT In vivo Gene Delivery System | -Suppressed the histological atherosclerotic changes -Prevented the extracellular matrix deposition | [81] |
| DcR3 (Biomarker) | Atherosclerosis | - | - | Peptide | Human | - | -Circulating levels of DcR3 in CAD patients require coronary artery bypass grafting are high | [83] |
| DcR3 (Biomarker) | Coronary Artery Disease Severity | Peptide | -Increased level of circulating DcR3 are associated with CAD severity and predict future MACE in patients with multivessel CAD | [84] | ||||
| SREBP decoy | Atherosclerosis | SREBP-1c ↓ FAS, SCD-1 ↓ ACC1 ↓ HMGCR ↓ | 10 μg every two weeks for 12 weeks | ODN | High-fat diet fed hyperlipidemic mice | - | -Regulated lipid metabolism and inhibited lipogenesis -Decreased pro-inflammatory cytokines | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahjoubin-Tehran, M.; Teng, Y.; Jalili, A.; Aghaee-Bakhtiari, S.H.; Markin, A.M.; Sahebkar, A. Decoy Technology as a Promising Therapeutic Tool for Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 4420. https://doi.org/10.3390/ijms22094420
Mahjoubin-Tehran M, Teng Y, Jalili A, Aghaee-Bakhtiari SH, Markin AM, Sahebkar A. Decoy Technology as a Promising Therapeutic Tool for Atherosclerosis. International Journal of Molecular Sciences. 2021; 22(9):4420. https://doi.org/10.3390/ijms22094420
Chicago/Turabian StyleMahjoubin-Tehran, Maryam, Yong Teng, Amin Jalili, Seyed Hamid Aghaee-Bakhtiari, Alexander M. Markin, and Amirhossein Sahebkar. 2021. "Decoy Technology as a Promising Therapeutic Tool for Atherosclerosis" International Journal of Molecular Sciences 22, no. 9: 4420. https://doi.org/10.3390/ijms22094420
APA StyleMahjoubin-Tehran, M., Teng, Y., Jalili, A., Aghaee-Bakhtiari, S. H., Markin, A. M., & Sahebkar, A. (2021). Decoy Technology as a Promising Therapeutic Tool for Atherosclerosis. International Journal of Molecular Sciences, 22(9), 4420. https://doi.org/10.3390/ijms22094420








