The Bone Marrow Niche in B-Cell Acute Lymphoblastic Leukemia: The Role of Microenvironment from Pre-Leukemia to Overt Leukemia
Abstract
1. B-ALL Is a Genetic Disease
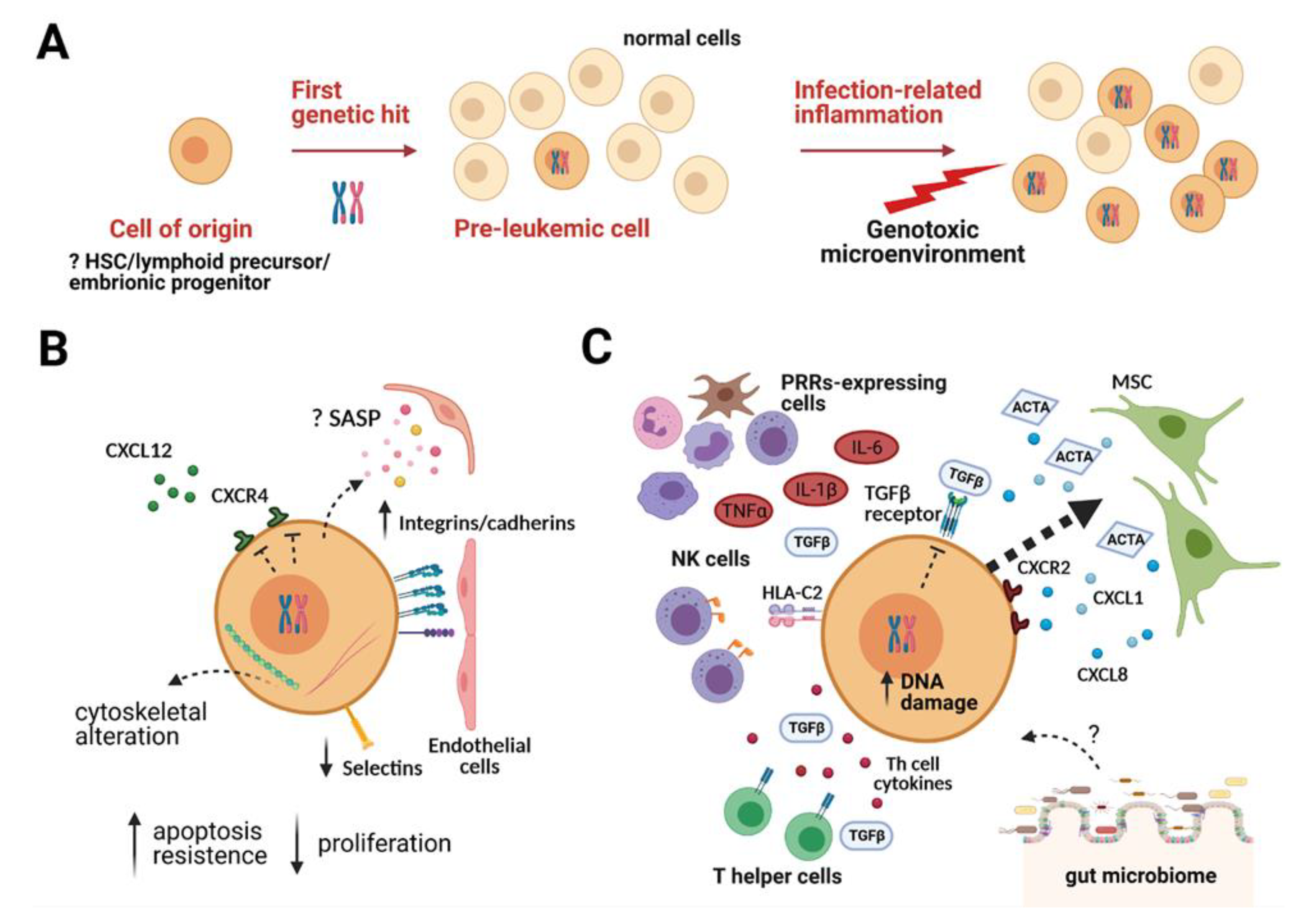
2. The Two-Step Model for Childhood B-ALL: The Pre-Leukemic Phase and the Overt Leukemia
3. The Pre-Leukemic Cell and Its Interaction with the Microenvironment
3.1. The Pre-Leukemic Cell
3.2. The Bone Marrow (BM) Microenvironment
3.3. The Pre-Leukemic Cell in the BM Niche
4. The Role of the Microenvironment in the Transition from Pre-Leukemia to Overt Leukemia
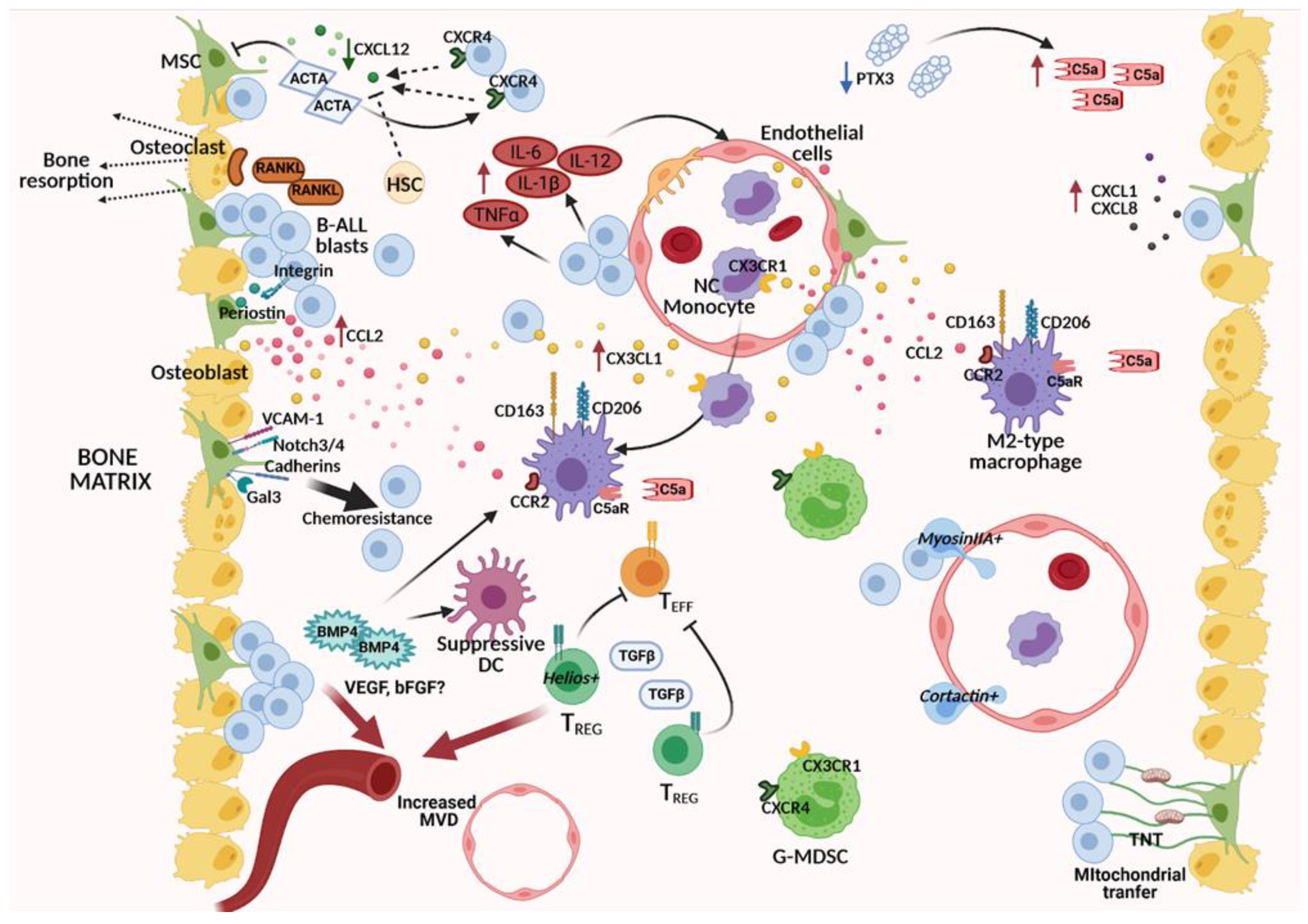
5. The BM Niche in Overt B-ALL: A Corrupted Microenvironment Reprogrammed to Sustain Leukemic Cells
5.1. Altered Molecular Pathways in the Cross-Talk between BM Stroma and B-ALL Cells
5.1.1. Deregulated Chemokine Axes
5.1.2. Altered Soluble Mediators of the TGFβ Family: Activin A, BMP4
5.1.3. MSC-Induced Chemoresistance and Metabolic Exchanges with B-ALL Cells
5.2. Interaction with Other Stromal Components: Bone Effects and Matrix Remodeling
5.3. Altered Molecular Pathways in the Cross-Talk between Vascular Cells and B-ALL Cells
5.4. Deregulation of Immunity-Related Components within the B-ALL Microenvironment
5.4.1. Monocytes and Macrophages
5.4.2. Myeloid-Derived Suppressor Cells (MDSCs), Regulatory T Cells (Tregs), and Dendritic Cells (DCs)
5.4.3. Inflammatory Mediators
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tran, T.H.; Hunger, S.P. The genomic landscape of pediatric acute lymphoblastic leukemia and precision medicine opportunities. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Stanulla, M.; Dagdan, E.; Zaliova, M.; Moricke, A.; Palmi, C.; Cazzaniga, G.; Eckert, C.; Te Kronnie, G.; Bourquin, J.P.; Bornhauser, B.; et al. IKZF1(plus) Defines a New Minimal Residual Disease-Dependent Very-Poor Prognostic Profile in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2018, 36, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Zelent, A.; Greaves, M.; Enver, T. Role of the TEL-AML1 fusion gene in the molecular pathogenesis of childhood acute lymphoblastic leukaemia. Oncogene 2004, 23, 4275–4283. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Hein, D.; Borkhardt, A.; Fischer, U. Insights into the prenatal origin of childhood acute lymphoblastic leukemia. Cancer Metastasis Rev. 2020, 39, 161–171. [Google Scholar] [CrossRef]
- Greaves, M.F.; Maia, A.T.; Wiemels, J.L.; Ford, A.M. Leukemia in twins: Lessons in natural history. Blood 2003, 102, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Fueller, E.; Schaefer, D.; Fischer, U.; Krell, P.F.; Stanulla, M.; Borkhardt, A.; Slany, R.K. Genomic inverse PCR for exploration of ligated breakpoints (GIPFEL), a new method to detect translocations in leukemia. PLoS ONE 2014, 9, e104419. [Google Scholar] [CrossRef]
- Hein, D.; Dreisig, K.; Metzler, M.; Izraeli, S.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. The preleukemic TCF3-PBX1 gene fusion can be generated in utero and is present in approximately 0.6% of healthy newborns. Blood 2019, 134, 1355–1358. [Google Scholar] [CrossRef]
- Hong, D.; Gupta, R.; Ancliff, P.; Atzberger, A.; Brown, J.; Soneji, S.; Green, J.; Colman, S.; Piacibello, W.; Buckle, V.; et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science 2008, 319, 336–339. [Google Scholar] [CrossRef]
- Hotfilder, M.; Rottgers, S.; Rosemann, A.; Schrauder, A.; Schrappe, M.; Pieters, R.; Jurgens, H.; Harbott, J.; Vormoor, J. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19- cells. Cancer Res. 2005, 65, 1442–1449. [Google Scholar] [CrossRef]
- Cobaleda, C.; Sanchez-Garcia, I. B-cell acute lymphoblastic leukaemia: Towards understanding its cellular origin. Bioessays 2009, 31, 600–609. [Google Scholar] [CrossRef]
- Vicente-Duenas, C.; Romero-Camarero, I.; Cobaleda, C.; Sanchez-Garcia, I. Function of oncogenes in cancer development: A changing paradigm. EMBO J. 2013, 32, 1502–1513. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, G.; Hauer, J.; Martin-Lorenzo, A.; Schafer, D.; Bartenhagen, C.; Garcia-Ramirez, I.; Auer, F.; Gonzalez-Herrero, I.; Ruiz-Roca, L.; Gombert, M.; et al. Infection Exposure Promotes ETV6-RUNX1 Precursor B-cell Leukemia via Impaired H3K4 Demethylases. Cancer Res. 2017, 77, 4365–4377. [Google Scholar] [CrossRef]
- Boiers, C.; Richardson, S.E.; Laycock, E.; Zriwil, A.; Turati, V.A.; Brown, J.; Wray, J.P.; Wang, D.; James, C.; Herrero, J.; et al. A Human IPS Model Implicates Embryonic B-Myeloid Fate Restriction as Developmental Susceptibility to B Acute Lymphoblastic Leukemia-Associated ETV6-RUNX1. Dev. Cell 2018, 44, 362–377.e367. [Google Scholar] [CrossRef]
- Zuna, J.; Ford, A.M.; Peham, M.; Patel, N.; Saha, V.; Eckert, C.; Kochling, J.; Panzer-Grumayer, R.; Trka, J.; Greaves, M. TEL deletion analysis supports a novel view of relapse in childhood acute lymphoblastic leukemia. Clin. Cancer Res. 2004, 10, 5355–5360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ford, A.M.; Palmi, C.; Bueno, C.; Hong, D.; Cardus, P.; Knight, D.; Cazzaniga, G.; Enver, T.; Greaves, M. The TEL-AML1 leukemia fusion gene dysregulates the TGF-beta pathway in early B lineage progenitor cells. J. Clin. Investig. 2009, 119, 826–836. [Google Scholar] [CrossRef]
- Beneforti, L.; Dander, E.; Bresolin, S.; Bueno, C.; Acunzo, D.; Bertagna, M.; Ford, A.; Gentner, B.; Kronnie, G.T.; Vergani, P.; et al. Pro-inflammatory cytokines favor the emergence of ETV6-RUNX1-positive pre-leukemic cells in a model of mesenchymal niche. Br. J. Haematol. 2020, 190, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Diakos, C.; Krapf, G.; Gerner, C.; Inthal, A.; Lemberger, C.; Ban, J.; Dohnal, A.M.; Panzer-Gruemayer, E.R. RNAi-mediated silencing of TEL/AML1 reveals a heat-shock protein- and survivin-dependent mechanism for survival. Blood 2007, 109, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Inthal, A.; Krapf, G.; Beck, D.; Joas, R.; Kauer, M.O.; Orel, L.; Fuka, G.; Mann, G.; Panzer-Grumayer, E.R. Role of the erythropoietin receptor in ETV6/RUNX1-positive acute lymphoblastic leukemia. Clin. Cancer Res. 2008, 14, 7196–7204. [Google Scholar] [CrossRef] [PubMed]
- Gefen, N.; Binder, V.; Zaliova, M.; Linka, Y.; Morrow, M.; Novosel, A.; Edry, L.; Hertzberg, L.; Shomron, N.; Williams, O.; et al. Hsa-mir-125b-2 is highly expressed in childhood ETV6/RUNX1 (TEL/AML1) leukemias and confers survival advantage to growth inhibitory signals independent of p53. Leukemia 2010, 24, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Colman, S.M.; Xiao, Z.; Ford, A.M.; Healy, L.E.; Donaldson, C.; Hows, J.M.; Navarrete, C.; Greaves, M. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc. Natl. Acad. Sci. USA 2002, 99, 8242–8247. [Google Scholar] [CrossRef] [PubMed]
- Congrains, A.; Bianco, J.; Rosa, R.G.; Mancuso, R.I.; Saad, S.T.O. 3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives. Materials 2021, 14, 569. [Google Scholar] [CrossRef]
- Man, Y.; Yao, X.; Yang, T.; Wang, Y. Hematopoietic Stem Cell Niche During Homeostasis, Malignancy, and Bone Marrow Transplantation. Front. Cell Dev. Biol. 2021, 9, 621214. [Google Scholar] [CrossRef]
- Crippa, S.; Bernardo, M.E. Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoietic Stem Cell Transplantation. Hemasphere 2018, 2, e151. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar] [PubMed]
- Kiel, M.J.; Radice, G.L.; Morrison, S.J. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 2007, 1, 204–217. [Google Scholar] [CrossRef]
- Ding, L.; Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013, 495, 231–235. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef]
- Itkin, T.; Gur-Cohen, S.; Spencer, J.A.; Schajnovitz, A.; Ramasamy, S.K.; Kusumbe, A.P.; Ledergor, G.; Jung, Y.; Milo, I.; Poulos, M.G.; et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 2016, 532, 323–328. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Zhao, M.; Perry, J.M.; Marshall, H.; Venkatraman, A.; Qian, P.; He, X.C.; Ahamed, J.; Li, L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 2014, 20, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Zhang, J.; Lucas, D. The Role of the Bone Marrow Microenvironment in the Response to Infection. Front. Immunol. 2020, 11, 585402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Supakorndej, T.; Krambs, J.R.; Rao, M.; Abou-Ezzi, G.; Ye, R.Y.; Li, S.; Trinkaus, K.; Link, D.C. Bone marrow dendritic cells regulate hematopoietic stem/progenitor cell trafficking. J. Clin. Investig. 2019, 129, 2920–2931. [Google Scholar] [CrossRef]
- Palmi, C.; Fazio, G.; Savino, A.M.; Procter, J.; Howell, L.; Cazzaniga, V.; Vieri, M.; Longinotti, G.; Brunati, I.; Andre, V.; et al. Cytoskeletal regulatory gene expression and migratory properties of B-cell progenitors are affected by the ETV6-RUNX1 rearrangement. Mol. Cancer Res. 2014, 12, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Malik, M.; Tomkowicz, B.E.; Collman, R.G.; Ptasznik, A. BCR-ABL1 alters SDF-1alpha-mediated adhesive responses through the beta2 integrin LFA-1 in leukemia cells. Blood 2008, 111, 5182–5186. [Google Scholar] [CrossRef]
- Salgia, R.; Quackenbush, E.; Lin, J.; Souchkova, N.; Sattler, M.; Ewaniuk, D.S.; Klucher, K.M.; Daley, G.Q.; Kraeft, S.K.; Sackstein, R.; et al. The BCR/ABL oncogene alters the chemotactic response to stromal-derived factor-1alpha. Blood 1999, 94, 4233–4246. [Google Scholar]
- Chang, Y.C.; Tien, S.C.; Tien, H.F.; Zhang, H.; Bokoch, G.M.; Chang, Z.F. p210(Bcr-Abl) desensitizes Cdc42 GTPase signaling for SDF-1alpha-directed migration in chronic myeloid leukemia cells. Oncogene 2009, 28, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Lazarides, K.; Lewis, J.B.; von Andrian, U.H.; Van Etten, R.A. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood 2014, 123, 1361–1371. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Li, Z.; Xi, Y. Identification of hub genes and molecular mechanisms in infant acute lymphoblastic leukemia with MLL gene rearrangement. PeerJ 2019, 7, e7628. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Jung, S.K.; Kurre, P. Hematopoietic stem and progenitor cell signaling in the niche. Leukemia 2020, 34, 3136–3148. [Google Scholar] [CrossRef] [PubMed]
- Chandeck, C.; Mooi, W.J. Oncogene-induced cellular senescence. Adv. Anat. Pathol. 2010, 17, 42–48. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Liu, X.L.; Ding, J.; Meng, L.H. Oncogene-induced senescence: A double edged sword in cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Hernandez, M.; Mayado, A.; Casado-Garcia, A.; Martinez-Cano, J.; Palmi, C.; Fazio, G.; Orfao, A.; Ribera, J.; Ribera, J.M.; Zamora, L.; et al. Inhibition of inflammatory signaling in Pax5 mutant cells mitigates B-cell leukemogenesis. Sci. Rep. 2020, 10, 19189. [Google Scholar] [CrossRef]
- Soegaard, S.H.; Rostgaard, K.; Skogstrand, K.; Wiemels, J.L.; Schmiegelow, K.; Hjalgrim, H. Neonatal Inflammatory Markers Are Associated with Childhood B-cell Precursor Acute Lymphoblastic Leukemia. Cancer Res. 2018, 78, 5458–5463. [Google Scholar] [CrossRef]
- Chang, J.S.; Zhou, M.; Buffler, P.A.; Chokkalingam, A.P.; Metayer, C.; Wiemels, J.L. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, A.; Hauer, J.; Vicente-Duenas, C.; Auer, F.; Gonzalez-Herrero, I.; Garcia-Ramirez, I.; Ginzel, S.; Thiele, R.; Constantinescu, S.N.; Bartenhagen, C.; et al. Infection Exposure is a Causal Factor in B-cell Precursor Acute Lymphoblastic Leukemia as a Result of Pax5-Inherited Susceptibility. Cancer Discov. 2015, 5, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H.; Manz, M.G. Impact of inflammation on early hematopoiesis and the microenvironment. Int. J. Hematol. 2017, 106, 27–33. [Google Scholar] [CrossRef]
- Pietras, E.M. Inflammation: A key regulator of hematopoietic stem cell fate in health and disease. Blood 2017, 130, 1693–1698. [Google Scholar] [CrossRef]
- Portale, F.; Beneforti, L.; Fallati, A.; Biondi, A.; Palmi, C.; Cazzaniga, G.; Dander, E.; D’Amico, G. Activin A contributes to the definition of a pro-oncogenic bone marrow microenvironment in t(12;21) preleukemia. Exp. Hematol. 2019, 73, 7–12.e4. [Google Scholar] [CrossRef]
- Raaijmakers, M.H.; Mukherjee, S.; Guo, S.; Zhang, S.; Kobayashi, T.; Schoonmaker, J.A.; Ebert, B.L.; Al-Shahrour, F.; Hasserjian, R.P.; Scadden, E.O.; et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010, 464, 852–857. [Google Scholar] [CrossRef]
- Zambetti, N.A.; Ping, Z.; Chen, S.; Kenswil, K.J.G.; Mylona, M.A.; Sanders, M.A.; Hoogenboezem, R.M.; Bindels, E.M.J.; Adisty, M.N.; Van Strien, P.M.H.; et al. Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell 2016, 19, 613–627. [Google Scholar] [CrossRef]
- Swiergiel, A.H.; Dunn, A.J. The roles of IL-1, IL-6, and TNFalpha in the feeding responses to endotoxin and influenza virus infection in mice. Brain Behav. Immun. 1999, 13, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Burgler, S.; Nadal, D. Pediatric precursor B acute lymphoblastic leukemia: Are T helper cells the missing link in the infectious etiology theory? Mol. Cell Pediatr. 2017, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.K.; Prakash, S.; Moulik, N.R.; Kumar, A.; Agrawal, S. Genetic associations of killer immunoglobulin like receptors and class I human leukocyte antigens on childhood acute lymphoblastic leukemia among north Indians. Hum. Immunol. 2016, 77, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Miko, E.; Ujlaki, G.; Sari, Z.; Bai, P. The Microbiome as a Component of the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 137–153. [Google Scholar] [CrossRef]
- Vicente-Duenas, C.; Janssen, S.; Oldenburg, M.; Auer, F.; Gonzalez-Herrero, I.; Casado-Garcia, A.; Isidro-Hernandez, M.; Raboso-Gallego, J.; Westhoff, P.; Pandyra, A.A.; et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 2020, 136, 2003–2017. [Google Scholar] [CrossRef]
- Conforti, A.; Biagini, S.; Del Bufalo, F.; Sirleto, P.; Angioni, A.; Starc, N.; Li Pira, G.; Moretta, F.; Proia, A.; Contoli, B.; et al. Biological, functional and genetic characterization of bone marrow-derived mesenchymal stromal cells from pediatric patients affected by acute lymphoblastic leukemia. PLoS ONE 2013, 8, e76989. [Google Scholar] [CrossRef]
- Menendez, P.; Catalina, P.; Rodriguez, R.; Melen, G.J.; Bueno, C.; Arriero, M.; Garcia-Sanchez, F.; Lassaletta, A.; Garcia-Sanz, R.; Garcia-Castro, J. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J. Exp. Med. 2009, 206, 3131–3141. [Google Scholar] [CrossRef]
- Vicente Lopez, A.; Vazquez Garcia, M.N.; Melen, G.J.; Entrena Martinez, A.; Cubillo Moreno, I.; Garcia-Castro, J.; Orellana, M.R.; Gonzalez, A.G. Mesenchymal stromal cells derived from the bone marrow of acute lymphoblastic leukemia patients show altered BMP4 production: Correlations with the course of disease. PLoS ONE 2014, 9, e84496. [Google Scholar] [CrossRef]
- van den Berk, L.C.; van der Veer, A.; Willemse, M.E.; Theeuwes, M.J.; Luijendijk, M.W.; Tong, W.H.; van der Sluis, I.M.; Pieters, R.; den Boer, M.L. Disturbed CXCR4/CXCL12 axis in paediatric precursor B-cell acute lymphoblastic leukaemia. Br. J. Haematol. 2014, 166, 240–249. [Google Scholar] [CrossRef]
- de Rooij, B.; Polak, R.; van den Berk, L.C.J.; Stalpers, F.; Pieters, R.; den Boer, M.L. Acute lymphoblastic leukemia cells create a leukemic niche without affecting the CXCR4/CXCL12 axis. Haematologica 2017, 102, e389–e393. [Google Scholar] [CrossRef]
- Portale, F.; Cricri, G.; Bresolin, S.; Lupi, M.; Gaspari, S.; Silvestri, D.; Russo, B.; Marino, N.; Ubezio, P.; Pagni, F.; et al. ActivinA: A new leukemia-promoting factor conferring migratory advantage to B-cell precursor-acute lymphoblastic leukemic cells. Haematologica 2019, 104, 533–545. [Google Scholar] [CrossRef]
- Balandran, J.C.; Purizaca, J.; Enciso, J.; Dozal, D.; Sandoval, A.; Jimenez-Hernandez, E.; Aleman-Lazarini, L.; Perez-Koldenkova, V.; Quintela-Nunez Del Prado, H.; Rios de Los Rios, J.; et al. Pro-inflammatory-Related Loss of CXCL12 Niche Promotes Acute Lymphoblastic Leukemic Progression at the Expense of Normal Lymphopoiesis. Front. Immunol. 2016, 7, 666. [Google Scholar] [CrossRef]
- Colmone, A.; Amorim, M.; Pontier, A.L.; Wang, S.; Jablonski, E.; Sipkins, D.A. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 2008, 322, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Juarez, J.; Dela Pena, A.; Baraz, R.; Hewson, J.; Khoo, M.; Cisterne, A.; Fricker, S.; Fujii, N.; Bradstock, K.F.; Bendall, L.J. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia 2007, 21, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos, J.F.; Laranjeira, A.B.; Zanchin, N.I.; Otubo, R.; Vaz, T.H.; Cardoso, A.A.; Brandalise, S.R.; Yunes, J.A. Increased CCL2 and IL-8 in the bone marrow microenvironment in acute lymphoblastic leukemia. Pediatr. Blood Cancer 2011, 56, 568–577. [Google Scholar] [CrossRef]
- Polak, R.; de Rooij, B.; Pieters, R.; den Boer, M.L. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 2015, 126, 2404–2414. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, X.; Deng, M.; Huang, Z.; Wang, J.; Wu, Y.; Cui, D.; Liu, Y.; Liu, R.; Ouyang, G. Bone Marrow Mesenchymal Stromal Cell-Derived Periostin Promotes B-ALL Progression by Modulating CCL2 in Leukemia Cells. Cell Rep. 2019, 26, 1533–1543.e1534. [Google Scholar] [CrossRef] [PubMed]
- Dander, E.; Fallati, A.; Gulic, T.; Pagni, F.; Gaspari, S.; Silvestri, D.; Cricri, G.; Bedini, G.; Portale, F.; Buracchi, C.; et al. Monocyte-macrophage polarization and recruitment pathways in the tumour microenvironment of B-cell acute lymphoblastic leukaemia. Br. J. Haematol. 2021. [Google Scholar] [CrossRef]
- Crazzolara, R.; Kreczy, A.; Mann, G.; Heitger, A.; Eibl, G.; Fink, F.M.; Mohle, R.; Meister, B. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2001, 115, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Arduino, N.; Ferretti, E.; Pistorio, A.; Spinelli, M.; Ottonello, L.; Dallegri, F.; Basso, G.; Pistoia, V. Chemokine receptor expression and function in childhood acute lymphoblastic leukemia of B-lineage. Leuk. Res. 2006, 30, 365–372. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, X.; Huang, J.; Jia, X.; Deng, M.; Cui, D.; Du, Z.; Fu, G.; Ouyang, G.; Xiao, C. A critical role of periostin in B-cell acute lymphoblastic leukemia. Leukemia 2017, 31, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Bataller, A.; Montalban-Bravo, G.; Soltysiak, K.A.; Garcia-Manero, G. The role of TGFbeta in hematopoiesis and myeloid disorders. Leukemia 2019, 33, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Hikita, A.; Inoue, Y. The roles of TGF-beta signaling in carcinogenesis and breast cancer metastasis. Breast Cancer 2012, 19, 118–124. [Google Scholar] [CrossRef]
- Bellam, N.; Pasche, B. Tgf-beta signaling alterations and colon cancer. Cancer Treat. Res. 2010, 155, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Naka, K.; Hoshii, T.; Muraguchi, T.; Tadokoro, Y.; Ooshio, T.; Kondo, Y.; Nakao, S.; Motoyama, N.; Hirao, A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 2010, 463, 676–680. [Google Scholar] [CrossRef]
- Geyh, S.; Rodriguez-Paredes, M.; Jager, P.; Koch, A.; Bormann, F.; Gutekunst, J.; Zilkens, C.; Germing, U.; Kobbe, G.; Lyko, F.; et al. Transforming growth factor beta1-mediated functional inhibition of mesenchymal stromal cells in myelodysplastic syndromes and acute myeloid leukemia. Haematologica 2018, 103, 1462–1471. [Google Scholar] [CrossRef]
- Valencia, J.; Fernández-Sevilla, L.M.; Fraile-Ramos, A.; Sacedon, R.; Jimenez, E.; Vicente, A.; Varas, A. Acute Lymphoblastic Leukaemia Cells Impair Dendritic Cell and Macrophage Differentiation: Role of BMP4. Cells 2019, 8, 722. [Google Scholar] [CrossRef]
- Manabe, A.; Murti, K.G.; Coustan-Smith, E.; Kumagai, M.; Behm, F.G.; Raimondi, S.C.; Campana, D. Adhesion-dependent survival of normal and leukemic human B lymphoblasts on bone marrow stromal cells. Blood 1994, 83, 758–766. [Google Scholar] [CrossRef]
- Shalapour, S.; Hof, J.; Kirschner-Schwabe, R.; Bastian, L.; Eckert, C.; Prada, J.; Henze, G.; von Stackelberg, A.; Seeger, K. High VLA-4 expression is associated with adverse outcome and distinct gene expression changes in childhood B-cell precursor acute lymphoblastic leukemia at first relapse. Haematologica 2011, 96, 1627–1635. [Google Scholar] [CrossRef]
- Jacamo, R.; Chen, Y.; Wang, Z.; Ma, W.; Zhang, M.; Spaeth, E.L.; Wang, Y.; Battula, V.L.; Mak, P.Y.; Schallmoser, K.; et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-kappaB mediates chemoresistance. Blood 2014, 123, 2691–2702. [Google Scholar] [CrossRef]
- Mudry, R.E.; Fortney, J.E.; York, T.; Hall, B.M.; Gibson, L.F. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood 2000, 96, 1926–1932. [Google Scholar] [CrossRef]
- Yu, W.; Yang, L.; Li, T.; Zhang, Y. Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target. Front. Oncol. 2019, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mallampati, S.; Sun, B.; Zhang, J.; Kim, S.B.; Lee, J.S.; Gong, Y.; Cai, Z.; Sun, X. Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett. 2013, 333, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, S.; Romanos-Sirakis, E.; Pais, F.; Bhatla, T.; Jones, C.; Bourgeois, W.; Hunger, S.P.; Raetz, E.A.; Hermiston, M.L.; Dasgupta, R.; et al. Wnt inhibition leads to improved chemosensitivity in paediatric acute lymphoblastic leukaemia. Br. J. Haematol. 2014, 167, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Nygren, M.K.; Dosen-Dahl, G.; Stubberud, H.; Walchli, S.; Munthe, E.; Rian, E. beta-catenin is involved in N-cadherin-dependent adhesion, but not in canonical Wnt signaling in E2A-PBX1-positive B acute lymphoblastic leukemia cells. Exp. Hematol. 2009, 37, 225–233. [Google Scholar] [CrossRef]
- Nwabo Kamdje, A.H.; Mosna, F.; Bifari, F.; Lisi, V.; Bassi, G.; Malpeli, G.; Ricciardi, M.; Perbellini, O.; Scupoli, M.T.; Pizzolo, G.; et al. Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood 2011, 118, 380–389. [Google Scholar] [CrossRef]
- Fei, F.; Joo, E.J.; Tarighat, S.S.; Schiffer, I.; Paz, H.; Fabbri, M.; Abdel-Azim, H.; Groffen, J.; Heisterkamp, N. B-cell precursor acute lymphoblastic leukemia and stromal cells communicate through Galectin-3. Oncotarget 2015, 6, 11378–11394. [Google Scholar] [CrossRef]
- Fei, F.; Abdel-Azim, H.; Lim, M.; Arutyunyan, A.; von Itzstein, M.; Groffen, J.; Heisterkamp, N. Galectin-3 in pre-B acute lymphoblastic leukemia. Leukemia 2013, 27, 2385–2388. [Google Scholar] [CrossRef]
- Hu, K.; Gu, Y.; Lou, L.; Liu, L.; Hu, Y.; Wang, B.; Luo, Y.; Shi, J.; Yu, X.; Huang, H. Galectin-3 mediates bone marrow microenvironment-induced drug resistance in acute leukemia cells via Wnt/beta-catenin signaling pathway. J. Hematol. Oncol. 2015, 8, 1. [Google Scholar] [CrossRef]
- Usmani, S.; Sivagnanalingam, U.; Tkachenko, O.; Nunez, L.; Shand, J.C.; Mullen, C.A. Support of acute lymphoblastic leukemia cells by nonmalignant bone marrow stromal cells. Oncol. Lett. 2019, 17, 5039–5049. [Google Scholar] [CrossRef] [PubMed]
- Burt, R.; Dey, A.; Aref, S.; Aguiar, M.; Akarca, A.; Bailey, K.; Day, W.; Hooper, S.; Kirkwood, A.; Kirschner, K.; et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019, 134, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Le, P.M.; Andreeff, M.; Battula, V.L. Osteogenic niche in the regulation of normal hematopoiesis and leukemogenesis. Haematologica 2018, 103, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, S.A.; Papp, E.; Lee, K.K.; Grandal, I.; Merico, D.; Liu, C.C.; Allo, B.; Zhang, L.; Grynpas, M.D.; Minden, M.D.; et al. B cell acute lymphoblastic leukemia cells mediate RANK-RANKL-dependent bone destruction. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Cheung, L.C.; Tickner, J.; Hughes, A.M.; Skut, P.; Howlett, M.; Foley, B.; Oommen, J.; Wells, J.E.; He, B.; Singh, S.; et al. New therapeutic opportunities from dissecting the pre-B leukemia bone marrow microenvironment. Leukemia 2018, 32, 2326–2338. [Google Scholar] [CrossRef]
- Schmidt, T.; Carmeliet, P. Angiogenesis: A target in solid tumors, also in leukemia? Hematol. Am. Soc. Hematol. Educ. Program. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Butler, J.M.; O’Donnell, R.; Kobayashi, M.; Ding, B.S.; Bonner, B.; Chiu, V.K.; Nolan, D.J.; Shido, K.; Benjamin, L.; et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 2010, 12, 1046–1056. [Google Scholar] [CrossRef]
- Ahsberg, J.; Xiao, P.; Okuyama, K.; Somasundaram, R.; Strid, T.; Qian, H.; Sigvardsson, M. Progression of progenitor B-cell leukemia is associated with alterations of the bone marrow micro-environment. Haematologica 2020, 105, e102–e106. [Google Scholar] [CrossRef] [PubMed]
- Pule, M.A.; Gullmann, C.; Dennis, D.; McMahon, C.; Jeffers, M.; Smith, O.P. Increased angiogenesis in bone marrow of children with acute lymphoblastic leukaemia has no prognostic significance. Br. J. Haematol. 2002, 118, 991–998. [Google Scholar] [CrossRef]
- Aguayo, A.; Kantarjian, H.; Manshouri, T.; Gidel, C.; Estey, E.; Thomas, D.; Koller, C.; Estrov, Z.; O’Brien, S.; Keating, M.; et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 2000, 96, 2240–2245. [Google Scholar] [CrossRef]
- Schneider, P.; Dubus, I.; Gouel, F.; Legrand, E.; Vannier, J.P.; Vasse, M. What role for angiogenesis in childhood acute lymphoblastic leukaemia? Adv. Hematol. 2011, 2011, 274628. [Google Scholar] [CrossRef]
- Wigton, E.J.; Thompson, S.B.; Long, R.A.; Jacobelli, J. Myosin-IIA regulates leukemia engraftment and brain infiltration in a mouse model of acute lymphoblastic leukemia. J. Leukoc. Biol. 2016, 100, 143–153. [Google Scholar] [CrossRef]
- Velazquez-Avila, M.; Balandran, J.C.; Ramirez-Ramirez, D.; Velazquez-Avila, M.; Sandoval, A.; Felipe-Lopez, A.; Nava, P.; Alvarado-Moreno, J.A.; Dozal, D.; Prieto-Chavez, J.L.; et al. High cortactin expression in B-cell acute lymphoblastic leukemia is associated with increased transendothelial migration and bone marrow relapse. Leukemia 2019, 33, 1337–1348. [Google Scholar] [CrossRef]
- Mantovani, A.; Ponzetta, A.; Inforzato, A.; Jaillon, S. Innate immunity, inflammation and tumour progression: Double-edged swords. J. Intern. Med. 2019, 285, 524–532. [Google Scholar] [CrossRef]
- Boissard, F.; Fournie, J.J.; Laurent, C.; Poupot, M.; Ysebaert, L. Nurse like cells: Chronic lymphocytic leukemia associated macrophages. Leuk. Lymphoma 2015, 56, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Boissard, F.; Laurent, C.; Ramsay, A.G.; Quillet-Mary, A.; Fournie, J.J.; Poupot, M.; Ysebaert, L. Nurse-like cells impact on disease progression in chronic lymphocytic leukemia. Blood Cancer J. 2016, 6, e381. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Morande, P.E.; Dergan-Dylon, S.; Borge, M.; Toscano, M.A.; Stupirski, J.C.; Bezares, R.F.; Avalos, J.S.; Narbaitz, M.; Gamberale, R.; et al. Nurse-like cells control the activity of chronic lymphocytic leukemia B cells via galectin-1. Leukemia 2013, 27, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Fiorcari, S.; Martinelli, S.; Bulgarelli, J.; Audrito, V.; Zucchini, P.; Colaci, E.; Potenza, L.; Narni, F.; Luppi, M.; Deaglio, S.; et al. Lenalidomide interferes with tumor-promoting properties of nurse-like cells in chronic lymphocytic leukemia. Haematologica 2015, 100, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chittezhath, M.; Andre, V.; Zhao, H.; Poidinger, M.; Biondi, A.; D’Amico, G.; Biswas, S.K. Protumoral role of monocytes in human B-cell precursor acute lymphoblastic leukemia: Involvement of the chemokine CXCL10. Blood 2012, 119, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.T.; Dolgalev, I.; Evensen, N.A.; Ma, C.; Chambers, T.; Roberts, K.G.; Sreeram, S.; Dai, Y.; Tikhonova, A.N.; Lasry, A.; et al. Extensive Remodeling of the Immune Microenvironment in B Cell Acute Lymphoblastic Leukemia. Cancer Cell 2020. [Google Scholar] [CrossRef]
- Ancuta, P.; Rao, R.; Moses, A.; Mehle, A.; Shaw, S.K.; Luscinskas, F.W.; Gabuzda, D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 2003, 197, 1701–1707. [Google Scholar] [CrossRef]
- Ferretti, E.; Bertolotto, M.; Deaglio, S.; Tripodo, C.; Ribatti, D.; Audrito, V.; Blengio, F.; Matis, S.; Zupo, S.; Rossi, D.; et al. A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment. Leukemia 2011, 25, 1268–1277. [Google Scholar] [CrossRef]
- Marchica, V.; Toscani, D.; Corcione, A.; Bolzoni, M.; Storti, P.; Vescovini, R.; Ferretti, E.; Dalla Palma, B.; Vicario, E.; Accardi, F.; et al. Bone Marrow CX3CL1/Fractalkine is a New Player of the Pro-Angiogenic Microenvironment in Multiple Myeloma Patients. Cancers 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Scielzo, C.; Barbaglio, F.; Rodriguez, T.V.; Riba, M.; Lazarevic, D.; Cittaro, D.; Simonetti, G.; Ranghetti, P.; Scarfo, L.; et al. Targeting Macrophages Sensitizes Chronic Lymphocytic Leukemia to Apoptosis and Inhibits Disease Progression. Cell Rep. 2016, 14, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Filardi, E.; Nieto, C.; Dominguez-Soto, A.; Barroso, R.; Sanchez-Mateos, P.; Puig-Kroger, A.; Lopez-Bravo, M.; Joven, J.; Ardavin, C.; Rodriguez-Fernandez, J.L.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Li, T.; Wang, Q.; Qian, J.; Lu, Y.; Zhang, M.; Bi, E.; Yang, M.; Reu, F.; et al. Chemokines CCL2, 3, 14 stimulate macrophage bone marrow homing, proliferation, and polarization in multiple myeloma. Oncotarget 2015, 6, 24218–24229. [Google Scholar] [CrossRef]
- Hohtari, H.; Bruck, O.; Blom, S.; Turkki, R.; Sinisalo, M.; Kovanen, P.E.; Kallioniemi, O.; Pellinen, T.; Porkka, K.; Mustjoki, S. Immune cell constitution in bone marrow microenvironment predicts outcome in adult ALL. Leukemia 2019, 33, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Chen, Y.Y.; He, Y.Y.; Wang, J.Y.; Yang, J.P.; Zhong, S.L.; Jiang, N.; Zhou, P.; Jiang, H.; Zhou, J. Expansion and activation of granulocytic, myeloid-derived suppressor cells in childhood precursor B cell acute lymphoblastic leukemia. J. Leukoc Biol. 2017, 102, 449–458. [Google Scholar] [CrossRef]
- Salem, M.L.; El-Shanshory, M.R.; Abdou, S.H.; Attia, M.S.; Sobhy, S.M.; Zidan, M.F.; Zidan, A.A. Chemotherapy alters the increased numbers of myeloid-derived suppressor and regulatory T cells in children with acute lymphoblastic leukemia. Immunopharmacol. Immunotoxicol. 2018, 40, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kittang, A.O.; Kordasti, S.; Sand, K.E.; Costantini, B.; Kramer, A.M.; Perezabellan, P.; Seidl, T.; Rye, K.P.; Hagen, K.M.; Kulasekararaj, A.; et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology 2016, 5, e1062208. [Google Scholar] [CrossRef] [PubMed]
- Lustfeld, I.; Altvater, B.; Ahlmann, M.; Ligges, S.; Brinkrolf, P.; Rosemann, A.; Moericke, A.; Rossig, C. High proportions of CD4(+) T cells among residual bone marrow T cells in childhood acute lymphoblastic leukemia are associated with favorable early responses. Acta Haematol. 2014, 131, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.Z.; Hassan, N.; Lee, L.J.; Md Noor, S.; Osman, R.; Abdul-Jalil, M.; Nordin, A.J.; Abdullah, M. Increased regulatory T cells in acute lymphoblastic leukemia patients. Hematology 2015, 20, 523–529. [Google Scholar] [CrossRef]
- Wu, C.P.; Qing, X.; Wu, C.Y.; Zhu, H.; Zhou, H.Y. Immunophenotype and increased presence of CD4(+)CD25(+) regulatory T cells in patients with acute lymphoblastic leukemia. Oncol. Lett. 2012, 3, 421–424. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Huang, X.; Zhou, P.; Shi, Q.; Zhang, B.; Ju, X. Helios expression in regulatory T cells promotes immunosuppression, angiogenesis and the growth of leukemia cells in pediatric acute lymphoblastic leukemia. Leuk Res. 2018, 67, 60–66. [Google Scholar] [CrossRef]
- Duell, J.; Dittrich, M.; Bedke, T.; Mueller, T.; Eisele, F.; Rosenwald, A.; Rasche, L.; Hartmann, E.; Dandekar, T.; Einsele, H.; et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 2017, 31, 2181–2190. [Google Scholar] [CrossRef]
- Maecker, B.; Mougiakakos, D.; Zimmermann, M.; Behrens, M.; Hollander, S.; Schrauder, A.; Schrappe, M.; Welte, K.; Klein, C. Dendritic cell deficiencies in pediatric acute lymphoblastic leukemia patients. Leukemia 2006, 20, 645–649. [Google Scholar] [CrossRef]
- Mami, N.B.; Mohty, M.; Chambost, H.; Gaugler, B.; Olive, D. Blood dendritic cells in patients with acute lymphoblastic leukaemia. Br. J. Haematol. 2004, 126, 77–80. [Google Scholar] [CrossRef]
- Vilchis-Ordonez, A.; Contreras-Quiroz, A.; Vadillo, E.; Dorantes-Acosta, E.; Reyes-Lopez, A.; Quintela-Nunez del Prado, H.M.; Venegas-Vazquez, J.; Mayani, H.; Ortiz-Navarrete, V.; Lopez-Martinez, B.; et al. Bone Marrow Cells in Acute Lymphoblastic Leukemia Create a Proinflammatory Microenvironment Influencing Normal Hematopoietic Differentiation Fates. Biomed. Res. Int. 2015, 2015, 386165. [Google Scholar] [CrossRef]
- Verma, D.; Zanetti, C.; Godavarthy, P.S.; Kumar, R.; Minciacchi, V.R.; Pfeiffer, J.; Metzler, M.; Lefort, S.; Maguer-Satta, V.; Nicolini, F.E.; et al. Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia 2020, 34, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Witkowski, M.T.; Harris, J.; Dolgalev, I.; Sreeram, S.; Qian, W.; Tong, J.; Chen, X.; Aifantis, I.; Chen, W. Leukemia-on-a-chip: Dissecting the chemoresistance mechanisms in B cell acute lymphoblastic leukemia bone marrow niche. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Enciso, J.; Mayani, H.; Mendoza, L.; Pelayo, R. Modeling the Pro-inflammatory Tumor Microenvironment in Acute Lymphoblastic Leukemia Predicts a Breakdown of Hematopoietic-Mesenchymal Communication Networks. Front. Physiol. 2016, 7, 349. [Google Scholar] [CrossRef]
- Bonavita, E.; Gentile, S.; Rubino, M.; Maina, V.; Papait, R.; Kunderfranco, P.; Greco, C.; Feruglio, F.; Molgora, M.; Laface, I.; et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell 2015, 160, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset-Ismail, A.; Borkowska-Rzeszotek, S.; Kubis, E.; Bujko, K.; Brzezniakiewicz-Janus, K.; Bolkun, L.; Kloczko, J.; Moniuszko, M.; Basak, G.W.; Wiktor-Jedrzejczak, W.; et al. Activation of the complement cascade enhances motility of leukemic cells by downregulating expression of HO-1. Leukemia 2017, 31, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.; Zhang, W.M.; Li, T.T.; Zhang, C.C.; Qiu, S.; Liu, Y.; Liu, S.; Jin, M.; Jia, L.X.; Song, W.C.; et al. Complement 5a stimulates macrophage polarization and contributes to tumor metastases of colon cancer. Exp. Cell Res. 2018, 366, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Lynn, R.C.; Poussin, M.; Eiva, M.A.; Shaw, L.C.; O’Connor, R.S.; Minutolo, N.G.; Casado-Medrano, V.; Lopez, G.; Matsuyama, T.; et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 2021, 12, 877. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Palazon, A.; Noguera-Ortega, E.; Powell, D.J., Jr.; Guedan, S. CAR-T Cells Hit the Tumor Microenvironment: Strategies to Overcome Tumor Escape. Front. Immunol. 2020, 11, 1109. [Google Scholar] [CrossRef]
- Greaves, M.; Cazzaniga, V.; Ford, A. Can we prevent childhood Leukaemia? Leukemia 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dander, E.; Palmi, C.; D’Amico, G.; Cazzaniga, G. The Bone Marrow Niche in B-Cell Acute Lymphoblastic Leukemia: The Role of Microenvironment from Pre-Leukemia to Overt Leukemia. Int. J. Mol. Sci. 2021, 22, 4426. https://doi.org/10.3390/ijms22094426
Dander E, Palmi C, D’Amico G, Cazzaniga G. The Bone Marrow Niche in B-Cell Acute Lymphoblastic Leukemia: The Role of Microenvironment from Pre-Leukemia to Overt Leukemia. International Journal of Molecular Sciences. 2021; 22(9):4426. https://doi.org/10.3390/ijms22094426
Chicago/Turabian StyleDander, Erica, Chiara Palmi, Giovanna D’Amico, and Giovanni Cazzaniga. 2021. "The Bone Marrow Niche in B-Cell Acute Lymphoblastic Leukemia: The Role of Microenvironment from Pre-Leukemia to Overt Leukemia" International Journal of Molecular Sciences 22, no. 9: 4426. https://doi.org/10.3390/ijms22094426
APA StyleDander, E., Palmi, C., D’Amico, G., & Cazzaniga, G. (2021). The Bone Marrow Niche in B-Cell Acute Lymphoblastic Leukemia: The Role of Microenvironment from Pre-Leukemia to Overt Leukemia. International Journal of Molecular Sciences, 22(9), 4426. https://doi.org/10.3390/ijms22094426





