SGLT2is and Renal Protection: From Biological Mechanisms to Real-World Clinical Benefits
Abstract
1. Introduction
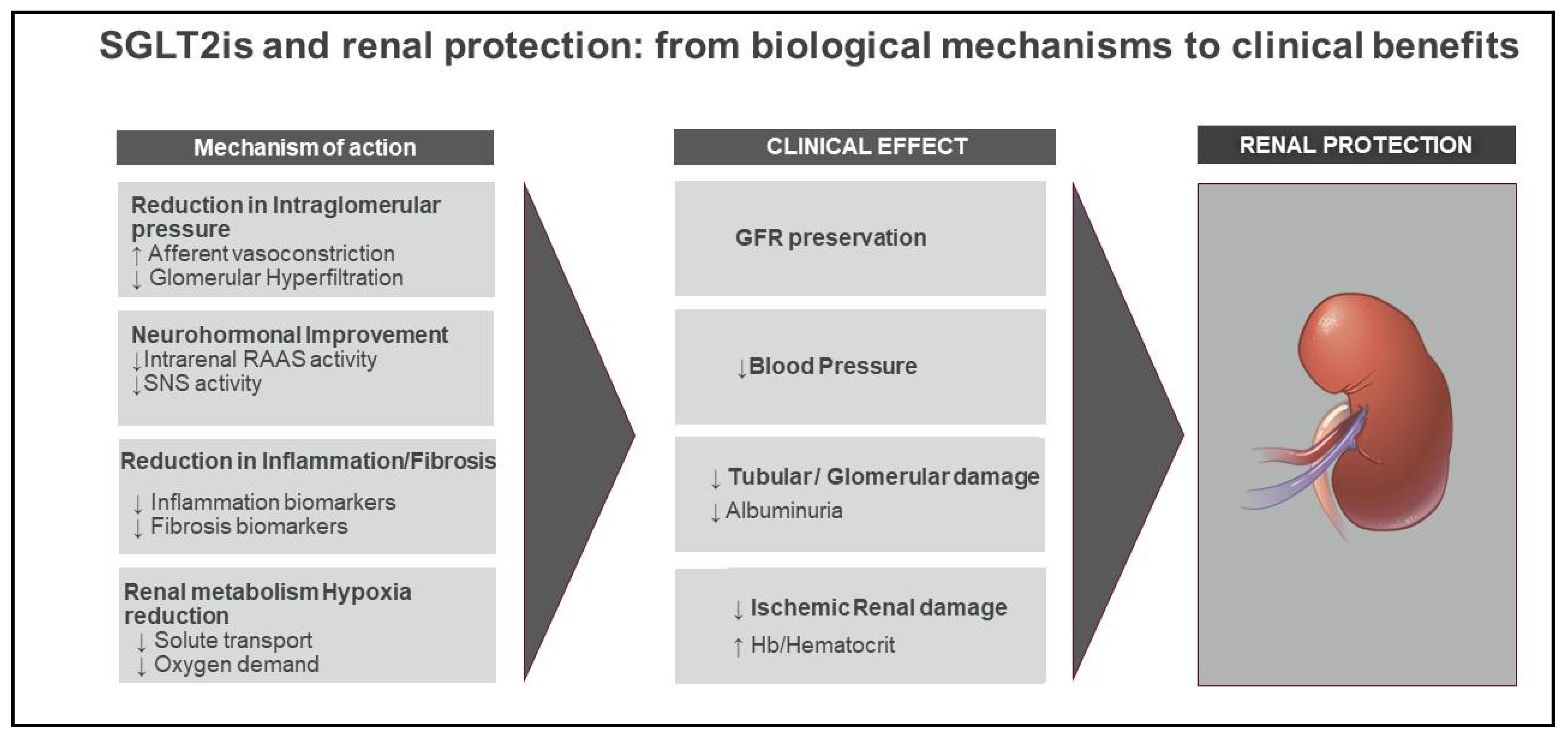
2. Mechanisms of Renal Protection with SGLT2
2.1. Renal Mechanisms
2.1.1. Glomerular Hemodynamics
2.1.2. Tubular Protection
2.2. Extrarenal Mechanisms
2.2.1. Blood Pressure Reduction
2.2.2. Natriuresis and Fluid Volume Reduction
2.2.3. Reduced Glucose and Lipo-Toxicity and Negative Caloric Balance
2.2.4. Uric Acid
2.2.5. Modulation of the Sympathetic Nervous and Renin Angiotensin Aldosterone Systems
3. Renal Protection by SGLT2is: Data from CVOTs
4. Renal Protection by SGLT2is: Data from Renal Trials
Ongoing Studies
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregg, E.W.; Li, Y.; Wang, J.; Burrows, N.R.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US renal data system 2019 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2020, 75, S1–S64. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry. Diabetes Mellitus-Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes; December 2008. Available online: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf (accessed on 26 March 2021).
- De Cosmo, S.; Viazzi, F.; Pacilli, A.; Giorda, C.; Ceriello, A.; Gentile, S.; Russo, G.; Rossi, M.C.; Nicolucci, A.; Guida, P.; et al. Achievement of therapeutic targets in patients with diabetes and chronic kidney disease: Insights from the Associazione Medici Diabetologi Annals initiative. Nephrol. Dial. Transplant. 2015, 30, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Van Bommel, E.J.M.; Lytvyn, Y.; Perkins, B.A.; Soleymanlou, N.; Fagan, N.M.; Koitka-Weber, A.; Joles, J.A.; Cherney, D.Z.I.; van Raalte, D.H. Renal hemodynamic effects of sodium-glucose cotransporter 2 inhibitors in hyperfiltering people with type 1 diabetes and people with type 2 diabetes and normal kidney function. Kidney Int. 2020, 97, 631–635. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021. [Google Scholar] [CrossRef]
- Onishi, A.; Fu, Y.; Patel, R.; Darshi, M.; Crespo-Masip, M.; Huang, W.; Song, P.; Freeman, B.; Kim, Y.C.; Soleimani, M.; et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol. Renal. Physiol. 2020, 319, F712–F728. [Google Scholar] [CrossRef]
- Foresto-Neto, O.; Albino, A.H.; Arias, S.C.A.; Faustino, V.D.; Zambom, F.F.F.; Cenedeze, M.A.; Elias, R.M.; Malheiros, D.M.A.C.; Camara, N.O.S.; Fujihara, C.K.; et al. NF-κB system is chronically activated and promotes glomerular injury in experimental type 1 diabetic kidney disease. Front Physiol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Brosius, F.C.; Tuttle, K.R.; Kretzler, M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 2016, 59, 1624–1647. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef]
- Jaikumkao, K.; Pongchaidecha, A.; Chueakula, N.; Thongnak, L.O.; Wanchai, K.; Chatsudthipong, V.; Chattipakorn, N.; Lungkaphin, A. Dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, slows the progression of renal complications through the suppression of renal inflammation, endoplasmic reticulum stress and apoptosis in prediabetic rats. Diabetes Obes. Metab. 2018, 20, 2617–2626. [Google Scholar] [CrossRef]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Renal. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Bajaj, M.; Yang, H.C.; Ye, Y. Combined SGLT2 and DPP4 inhibition reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic nephropathy in mice with type 2 diabetes. Cardiovasc. Drugs Ther. 2018, 32, 135–145. [Google Scholar] [CrossRef]
- Kogot-Levin, A.; Hinden, L.; Riahi, Y.; Israeli, T.; Tirosh, B.; Cerasi, E.; Mizrachi, E.B.; Tam, J.; Mosenzon, O.; Leibowitz, G. Proximal tubule mTORC1 is a central player in the pathophysiology of diabetic nephropathy and its correction by SGLT2 inhibitors. Cell. Rep. 2020, 32, 107954. [Google Scholar] [CrossRef]
- Bessho, R.; Takiyama, Y.; Takiyama, T.; Kitsunai, H.; Takeda, Y.; Sakagami, H.; Ota, T. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci. Rep. 2019, 9, 14754. [Google Scholar] [CrossRef]
- Packer, M. Role of ketogenic starvation sensors in mediating the renal protective effects of SGLT2 inhibitors in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107647. [Google Scholar] [CrossRef]
- Viollet, B. The energy sensor AMPK: Adaptations to exercise, nutritional and hormonal signals. In Hormones, Metabolism and the Benefits of Exercise; Spiegelman, B., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Hawley, S.A.; Ford, R.J.; Smith, B.K.; Gowans, G.J.; Mancini, S.J.; Pitt, R.D.; Day, E.A.; Salt, I.P.; Steinberg, G.R.; Hardie, D.G. The Na+/Glucose Cotransporter inhibitor canagliflozin activates ampk by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 2016, 65, 2784–2794. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Gao, H.K.; Kengne, A.P. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: A systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J. Am. Heart Assoc. 2017, 6, e004007. [Google Scholar] [CrossRef] [PubMed]
- Chilton, R.; Tikkanen, I.; Hehnke, U.; Woerle, H.J.; Johansen, O.E. Impact of empagliflozin on blood pressure in dipper and non-dipper patients with type 2 diabetes mellitus and hypertension. Diabetes Obes. Metab. 2017, 19, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E.; Fioretto, P.; Tang, W.; List, J.F. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014, 85, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Q.; Zhang, J.; Liu, L. Na+/H+ exchanger is required for hyperglycaemia-induced endothelial dysfunction via calcium-dependent calpain. Cardiovasc. Res. 2008, 80, 255–262. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Kario, K.; Ferdinand, K.C.; O’Keefe, J.H. Control of 24-h blood pressure with SGLT2 inhibitors to prevent cardiovascular disease. Prog. Cardiovasc. Dis. 2020, 63, 249–262. [Google Scholar] [CrossRef]
- Cooper, S.; Teoh, H.; Campeau, M.A.; Verma, S.; Leask, R.L. Empagliflozin restores the integrity of the endothelial glycocalyx in vitro. Mol. Cell Biochem. 2019, 459, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.I.; Drake-Holland, A.J.; Vink, H. Hypothesis: Arterial glycocalyx dysfunction is the first step in the atherothrombotic process. QJM Int. J. Med. 2008, 101, 513–518. [Google Scholar] [CrossRef]
- Ko, J.; Kang, H.J.; Kim, D.A.; Kim, M.J.; Ryu, E.S.; Lee, S.; Ryu, J.H.; Roncal, C.; Johnson, R.J.; Kang, D.H. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019, 33, 13334–13345. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Park, H.C.; Ha, S.K. Salt sensitivity and hypertension: A paradigm shift from kidney malfunction to vascular endothelial dysfunction. Electrolyte Blood Press. 2015, 13, 7–16. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Zinman, B.; Wanner, C.; Ferrari, R.; Fitchett, D.; Hantel, S.; Espadero, R.M.; Woerle, H.J.; Broedl, U.C.; Johansen, O.E. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab. Vasc. Dis. Res. 2015, 12, 90–100. [Google Scholar] [CrossRef]
- Machin, D.R.; Phuong, T.T.; Donato, A.J. The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr. Opin. Pharmacol. 2019, 45, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Maliha, G.; Townsend, R.R. SGLT2 inhibitors: Their potential reduction in blood pressure. J. Am. Soc. Hypertens. 2015, 9, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Titze, J.; Lang, R.; Ilies, C.; Schwind, K.H.; Kirsch, K.A.; Dietsch, P.; Luft, F.C.; Hilgers, K.F. Osmotically inactive skin Na+ storage in rats. Am. J. Physiol. Renal. Physiol. 2003, 285, F1108–F1117. [Google Scholar] [CrossRef] [PubMed]
- Petrykiv, S.; Sjöström, C.D.; Greasley, P.J.; Xu, J.; Persson, F.; Heerspink, H.J.L. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin. J. Am. Soc. Nephrol. 2017, 12, 751–759. [Google Scholar] [CrossRef]
- Wang, D.; Luo, Y.; Wang, X.; Orlicky, D.J.; Myakala, K.; Yang, P.; Levi, M. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents renal and liver disease in western diet induced obesity mice. Int. J. Mol. Sci. 2018, 19, 137. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Simental-Mendía, M.; Millán-Alanís, J.M.; Simental-Mendía, L.E. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: A systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol. Res. 2020, 160, 105068. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- Yuan, T.; Liu, S.; Dong, Y.; Fu, Y.; Tang, Y.; Zhao, W. Effects of dapagliflozin on serum and urinary uric acid levels in patients with type 2 diabetes: A prospective pilot trial. Diabetol. Metab. Syndr. 2020, 12, 92. [Google Scholar] [CrossRef]
- Ohashi, N.; Aoki, T.; Matsuyama, T.; Ishigaki, S.; Isobe, S.; Fujikura, T.; Hashimoto, T.; Tsuriya, D.; Morita, H.; Kato, A.; et al. Sodium-glucose cotransporter-2 inhibitor immediately decreases serum uric acid levels in type 2 diabetic patients. Med. Sci. Monit. 2020, 26, e926086. [Google Scholar] [CrossRef]
- Viazzi, F.; Garneri, D.; Leoncini, G.; Gonnella, A.; Muiesan, M.L.; Ambrosioni, E.; Costa, F.V.; Leonetti, G.; Pessina, A.C.; Trimarco, B.; et al. Serum uric acid and its relationship with metabolic syndrome and cardiovascular risk profile in patients with hypertension: Insights from the I-DEMAND study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 921–927. [Google Scholar] [CrossRef]
- Viazzi, F.; Rebora, P.; Giussani, M.; Orlando, A.; Stella, A.; Antolini, L.; Valsecchi, M.G.; Pontremoli, R.; Genovesi, S. Increased Serum Uric Acid Levels Blunt the Antihypertensive Efficacy of Lifestyle Modifications in Children at Cardiovascular Risk. Hypertension 2016, 67, 934–940. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the Uric Acid Thresholds Predicting an Increased Total and Cardiovascular Mortality Over 20 Years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Zheng, Y.; Yamada, H.; Sakamoto, K.; Horita, S.; Kunimi, M.; Endo, Y.; Li, Y.; Tobe, K.; Terauchi, Y.; Kadowaki, T.; et al. Roles of insulin receptor substrates in insulin-induced stimulation of renal proximal bicarbonate absorption. J. Am. Soc. Nephrol. 2005, 16, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Rong, S.; Wang, Q.; Sun, T.; Bao, W.; Chen, L.; Liu, L. Association between plasma uric acid and insulin resistance in type 2 diabetes: A Mendelian randomization analysis. Diabetes Res. Clin. Pract. 2021, 171, 108542. [Google Scholar] [CrossRef]
- Jordan, J.; Tank, J.; Heusser, K.; Heise, T.; Wanner, C.; Heer, M.; Macha, S.; Mattheus, M.; Lund, S.S.; Woerle, H.J.; et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J. Am. Soc. Hypertens. 2017, 11, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Wen, S.; Gong, M.; Yuan, X.; Xu, D.; Wang, C.; Jin, J.; Zhou, L. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting SGLT-2 in mice. Diabetes Metab. Syndr. Obes. 2020, 13, 2781–2799. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Zheng, C.M.; Yen, T.H.; Lu, K.C. Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Int. J. Mol. Sci. 2020, 21, 7833. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Cosentino, F.; Cannon, C.P.; Cherney, D.Z.I.; Masiukiewicz, U.; Pratley, R.; Dagogo-Jack, S.; Frederich, R.; Charbonnel, B.; Mancuso, J.; Shih, W.J.; et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: Results of the VERTIS CV Trial. Circulation 2020, 142, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.G.; Preiss, D.; Haynes, R.; von Eynatten, M.; Staplin, N.; Hauske, S.J.; George, J.T.; Green, J.B.; Landray, M.J.; Baigent, C.; et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: A rationale for the EMPA-KIDNEY study. Clin. Kidney J. 2018, 11, 749–761. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leoncini, G.; Russo, E.; Bussalino, E.; Barnini, C.; Viazzi, F.; Pontremoli, R. SGLT2is and Renal Protection: From Biological Mechanisms to Real-World Clinical Benefits. Int. J. Mol. Sci. 2021, 22, 4441. https://doi.org/10.3390/ijms22094441
Leoncini G, Russo E, Bussalino E, Barnini C, Viazzi F, Pontremoli R. SGLT2is and Renal Protection: From Biological Mechanisms to Real-World Clinical Benefits. International Journal of Molecular Sciences. 2021; 22(9):4441. https://doi.org/10.3390/ijms22094441
Chicago/Turabian StyleLeoncini, Giovanna, Elisa Russo, Elisabetta Bussalino, Cecilia Barnini, Francesca Viazzi, and Roberto Pontremoli. 2021. "SGLT2is and Renal Protection: From Biological Mechanisms to Real-World Clinical Benefits" International Journal of Molecular Sciences 22, no. 9: 4441. https://doi.org/10.3390/ijms22094441
APA StyleLeoncini, G., Russo, E., Bussalino, E., Barnini, C., Viazzi, F., & Pontremoli, R. (2021). SGLT2is and Renal Protection: From Biological Mechanisms to Real-World Clinical Benefits. International Journal of Molecular Sciences, 22(9), 4441. https://doi.org/10.3390/ijms22094441





