Indolyl Septanoside Synthesis for In Vivo Screening of Bacterial Septanoside Hydrolases
Abstract
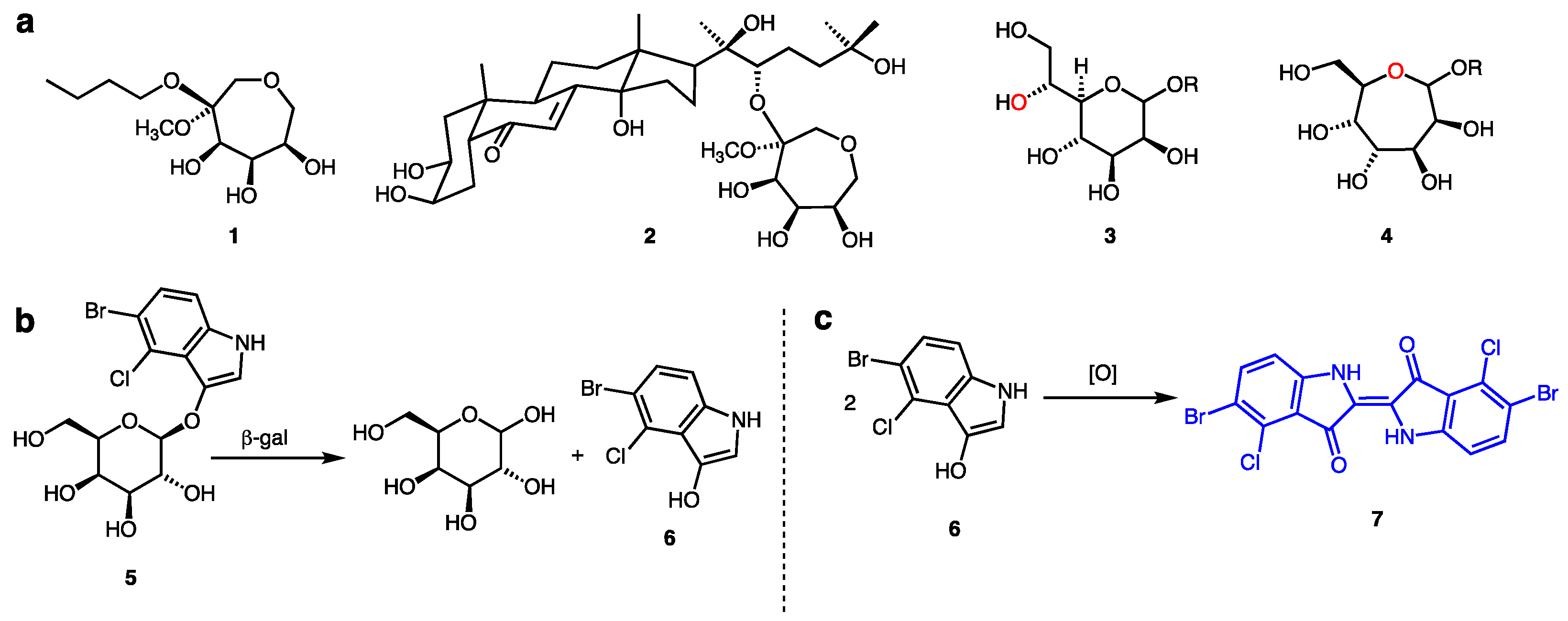
:1. Introduction
2. Results
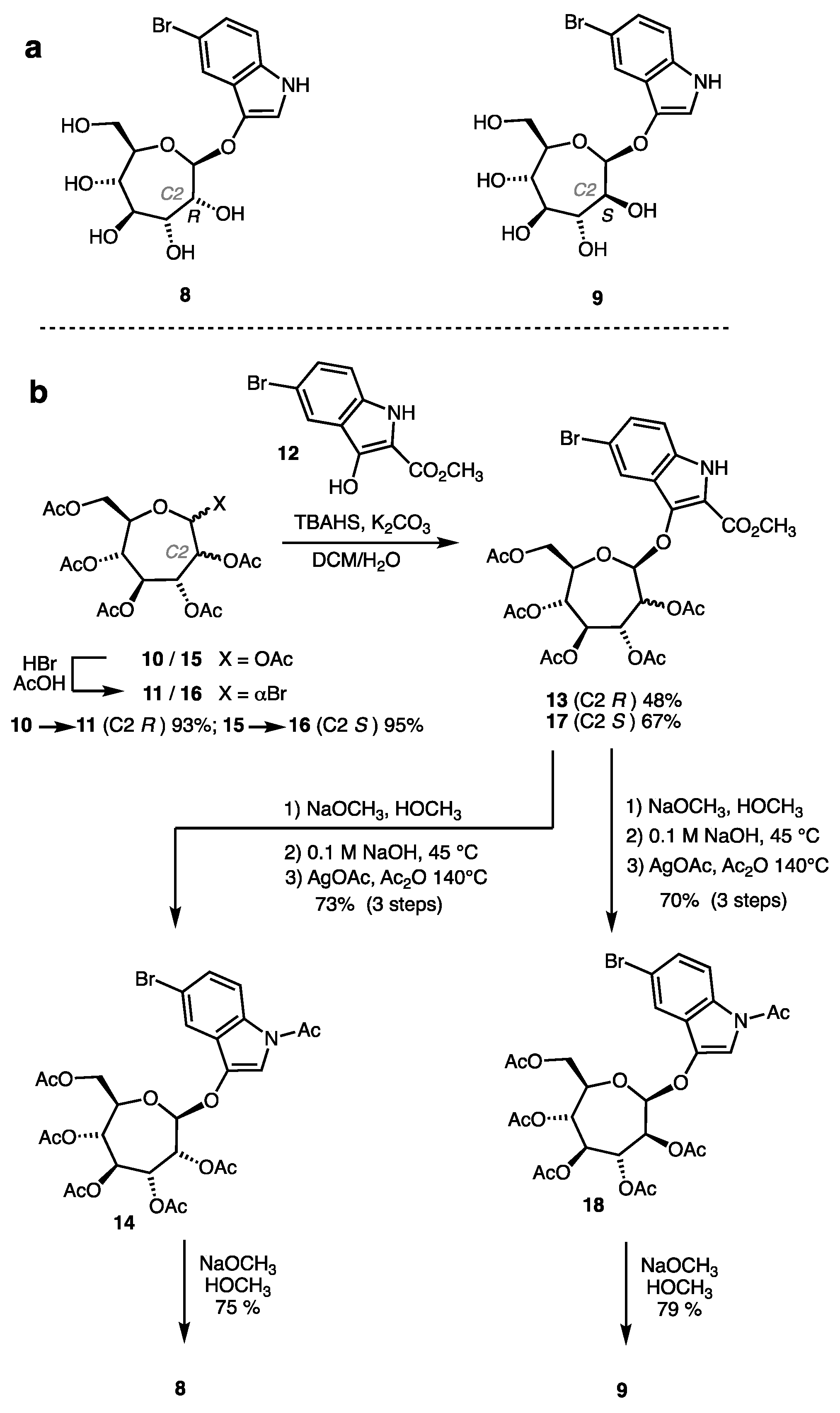
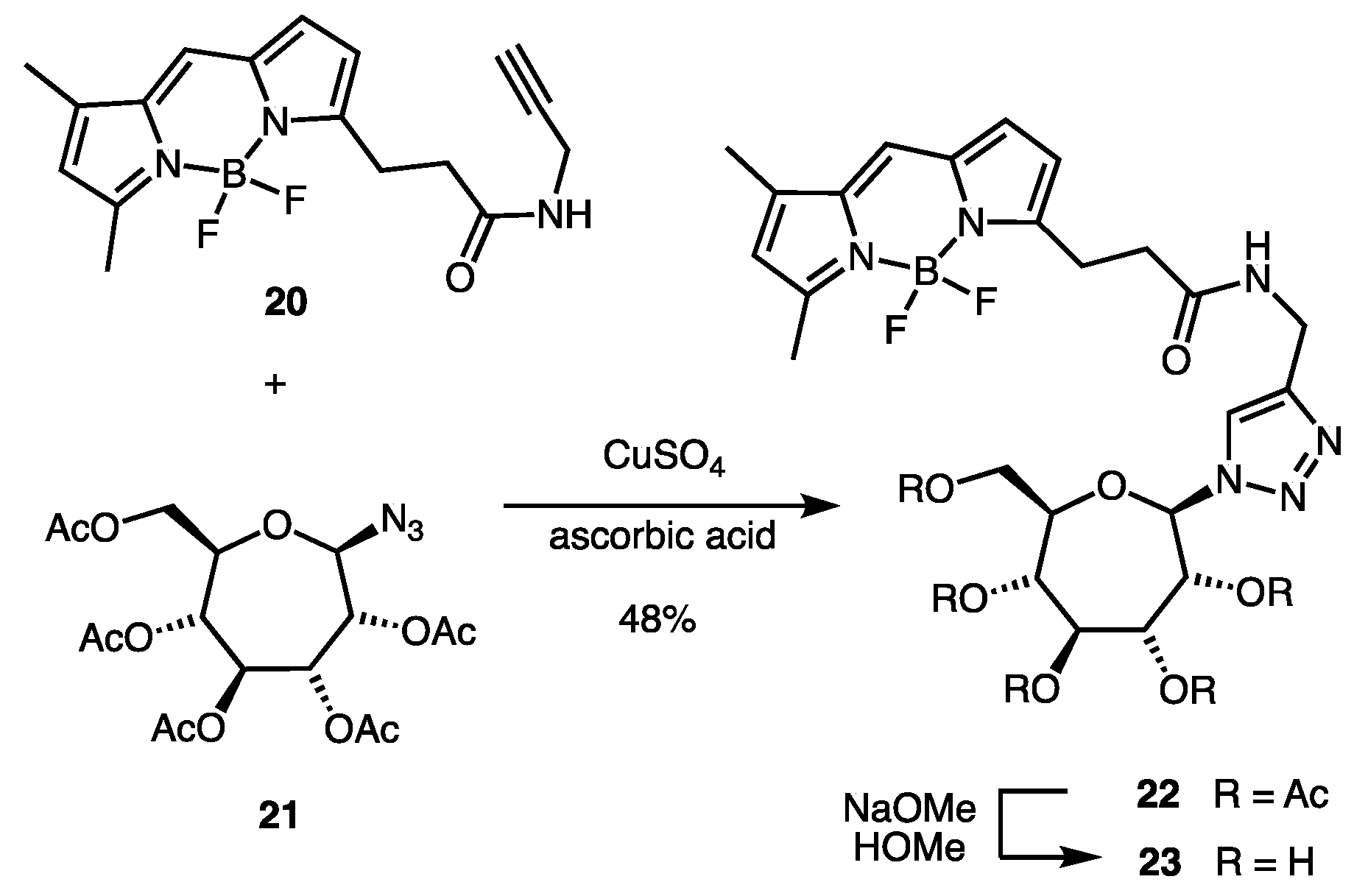
2.1. Synthesis of Indolyl Septanosides
2.2. Evaluation of Indolyl Septanoside 8 as a Substrate of Common Exo-Glycoside Hydrolases
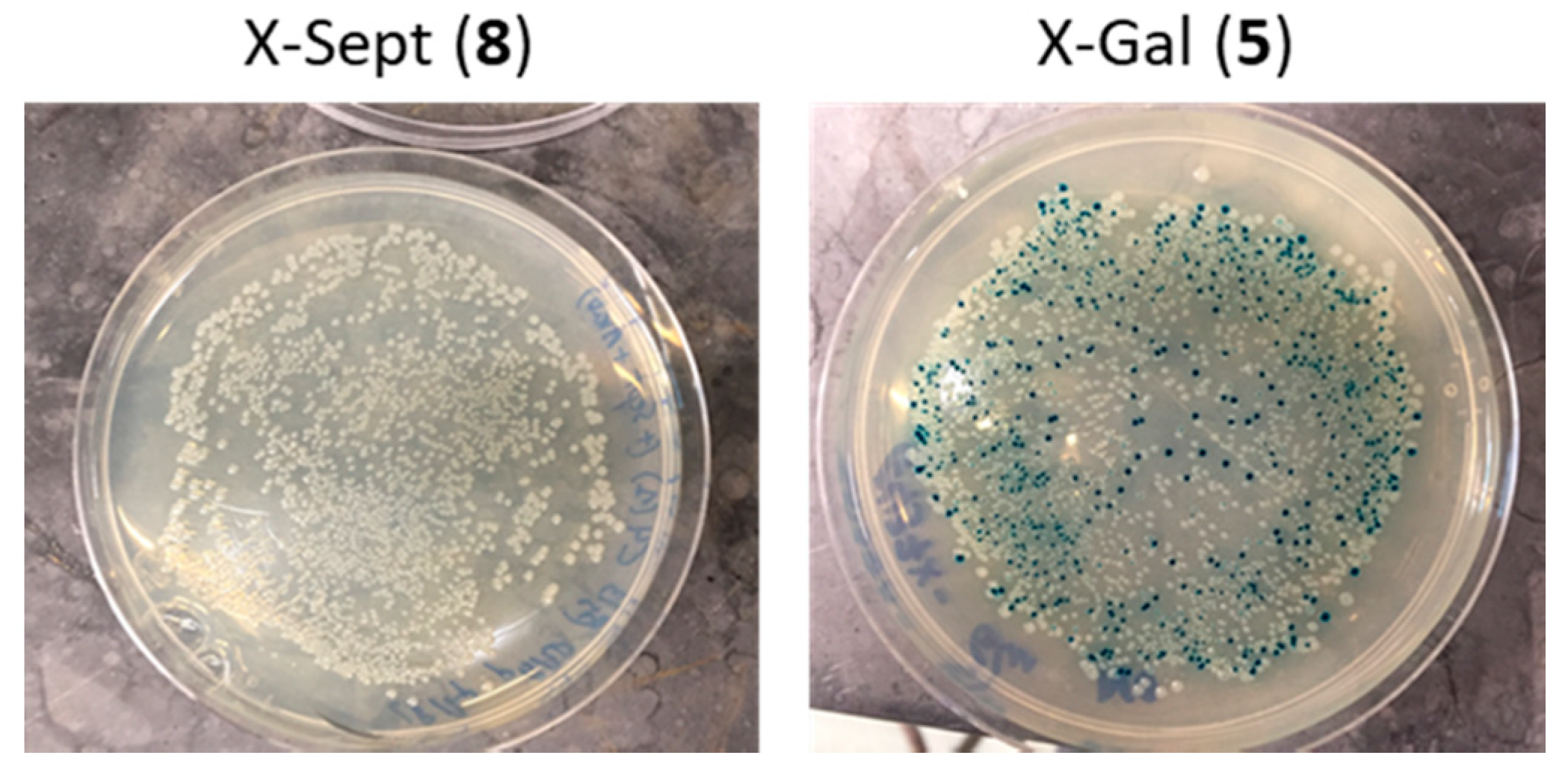
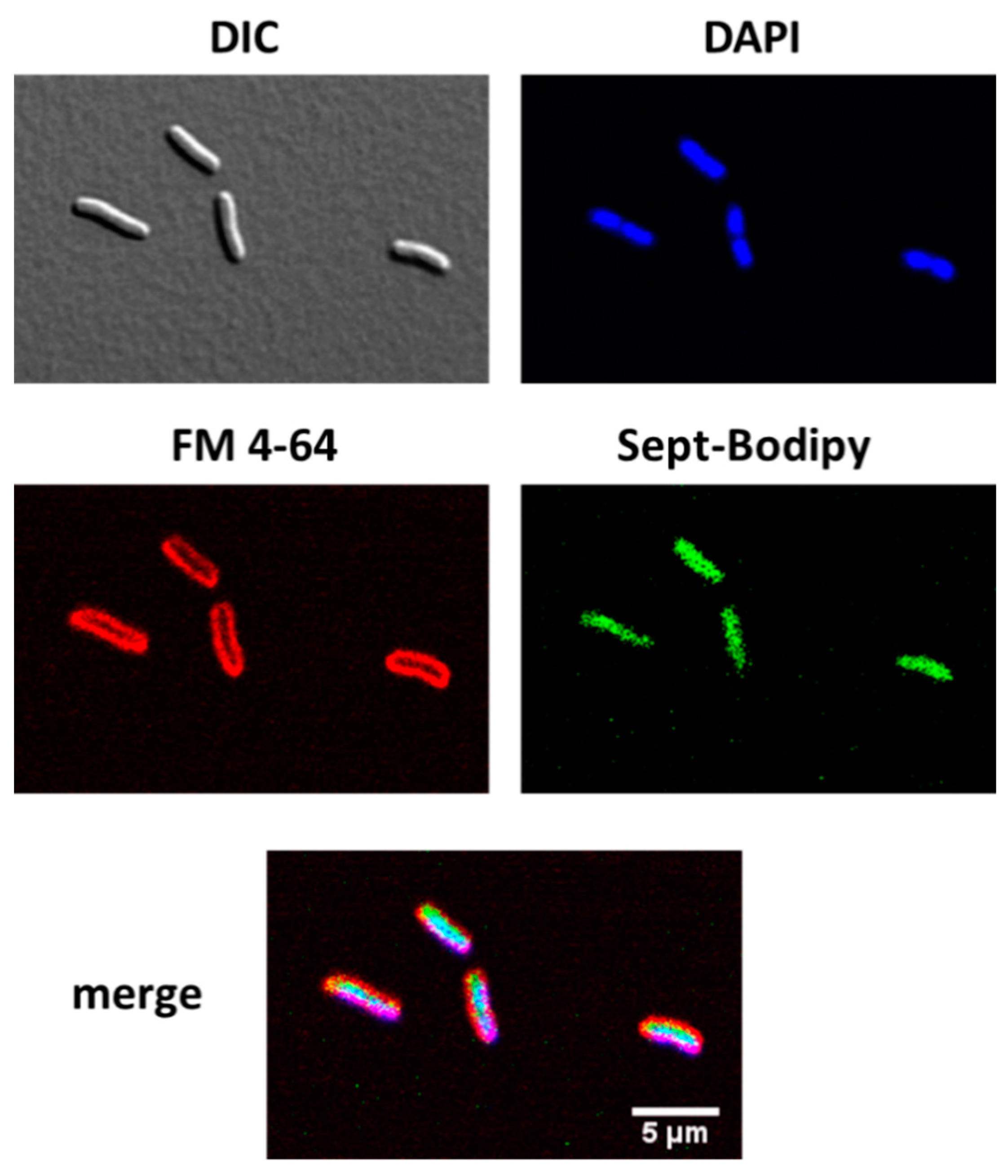
2.3. Transport of a BODIPY-Labeled Septanoside into E. coli Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental
4.2. Synthesis of Indolyl Septanosides 8 and 9
4.3. Synthesis of BODIPY-Labeled Septanoside 23
4.4. Glycosidase Activity Assay on E. coli Cells
4.5. Probing Glycoside Hydrolases for Septanoside Hydrolysis
4.6. Kinetics of Streptomyces β-Glucosidase with p-Nitrophenyl Septanoside 19
4.7. Cell Internalization of Compound 23 by Confocal Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pallister, E.; Gray, C.J.; Flitsch, S.L. Enzyme promiscuity of carbohydrate active enzymes and their applications in biocatalysis. Curr. Opin. Struct. Biol. 2020, 65, 184–192. [Google Scholar] [CrossRef]
- Kytidou, K.; Artola, M.; Overkleeft, H.S.; Aerts, J.M.F.G. Plant Glycosides and Glycosidases: A Treasure-Trove for Therapeutics. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Gloster, T.M. Exploitation of carbohydrate processing enzymes in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 180–188. [Google Scholar] [CrossRef]
- Davies, G.J.; Planas, A.; Rovira, C. Conformational Analyses of the Reaction Coordinate of Glycosidases. Acc. Chem. Res. 2012, 45, 308–316. [Google Scholar] [CrossRef]
- Sinnott, M.L. Catalytic Mechanisms of Enzymic Glycosyl Transfer. Chem. Rev. 1990, 90, 1171–1202. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.; Samanta, G.C.; Jayaraman, N. Advancements in synthetic and structural studies of septanoside sugars. In Recent Trends in Carbohydrate Chemistry; Rauter, A.P., Christensen, B., Somsak, L., Kosma, P., Adamo, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 217–251. [Google Scholar]
- Saha, J.; Peczuh, M.W. Synthesis and properties of septanose carbohydrates. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 2011; Volume 66, pp. 121–186. [Google Scholar]
- Ben Nejma, A.; Nguir, A.; Ben Jannet, H.; Hamza, M.A.; Daïch, A.; Othman, M.; Lawson, A.M. New septanoside and 20-hydroxyecdysone septanoside derivative from Atriplex portulacoides roots with preliminary biological activities. Bioorg. Med. Chem. Lett. 2015, 25, 1665–1670. [Google Scholar] [CrossRef]
- Le Merrer, Y.; Poitout, L.; Depezay, J.C.; Dosbaa, I.; Geoffroy, S.; Foglietti, M.J. Synthesis of azasugars as potent inhibitors of glycosidases. Bioorg. Med. Chem. 1997, 5, 519–533. [Google Scholar] [CrossRef]
- Tauss, A.; Steiner, A.J.; Stütz, A.E.; Tarling, C.A.; Withers, S.G.; Wrodnigg, T.M. l-Idoseptanosides: Substrates of d-glucosidases? Tetrahedron Asymmetry 2006, 17, 234–239. [Google Scholar] [CrossRef]
- Elshahawi, S.I.; Shaaban, K.A.; Kharel, M.K.; Thorson, J.S. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 2015, 44, 7591–7697. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Tang, Y.; Tang, W.; Chen, Y. Heptose-containing bacterial natural products: Structures, bioactivities, and biosyntheses. Nat. Prod. Rep. 2021. [Google Scholar] [CrossRef]
- Tang, W.; Guo, Z.; Cao, Z.; Wang, M.; Li, P.; Meng, X.; Zhao, X.; Xie, Z.; Wang, W.; Zhou, A.; et al. D-Sedoheptulose-7-phosphate is a common precursor for the heptoses of septacidin and hygromycin B. Proc. Natl. Acad. Sci. USA 2018, 115, 2818–2823. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, J.P.; Chua, J.; Cubby, R.J.; Tomson, A.J.; Da Rooge, M.A.; Fisher, B.E.; Mauricio, J.; Klundt, I. Substrates for Cytochemical Demonstration of Enzyme Activity. I. Some Substituted 3-Indolyl-β-D-glycopyranosides. J. Med. Chem. 1964, 7, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. Experiments in Molecular Genetics, 1st ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Kiernan, J. Indigogenic substrates for detection and localization of enzymes. Biotech. Histochem. 2007, 82, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Thiem, J. Indoxylic Acid Esters as Convenient Intermediates Towards Indoxyl Glycosides. Eur. J. Org. Chem. 2014, 2014, 564–574. [Google Scholar] [CrossRef]
- Wolk, J.L.; Frimer, A.A. A simple, safe and efficient synthesis of Tyrian purple (6,6’-Dibromoindigo). Molecules 2010, 15, 5561–5580. [Google Scholar] [CrossRef]
- Castro, S.; Duff, M.; Snyder, N.L.; Morton, M.; Kumar, C.V.; Peczuh, M.W. Recognition of septanose carbohydrates by concanavalin A. Org. Biomol. Chem. 2005, 3, 3869–3872. [Google Scholar] [CrossRef]
- Duff, M.R.; Fyvie, W.S.; Markad, S.D.; Frankel, A.E.; Kumar, C.V.; Gascón, J.A.; Peczuh, M.W. Computational and experimental investigations of mono-septanoside binding by Concanavalin A: Correlation of ligand stereochemistry to enthalpies of binding. Org. Biomol. Chem. 2011, 9, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Sager, C.P.; Fiege, B.; Zihlmann, P.; Vannam, R.; Rabbani, S.; Jakob, R.P.; Preston, R.C.; Zalewski, A.; Maier, T.; Peczuh, M.W.; et al. The price of flexibility—a case study on septanoses as pyranose mimetics. Chem. Sci. 2018, 9, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Nagata, S.; Tomida, H.; Iwai-Hirose, H.; Tanaka, H.N.; Ando, H.; Imamura, A.; Ishida, H. Synthesis of a 1,2-: Cis -indoxyl galactoside as a chromogenic glycosidase substrate. RSC Adv. 2019, 9, 28241–28247. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, S.; Thiem, J. Synthesis of indoxyl-glycosides for detection of glycosidase activities. J. Vis. Exp. 2015, 2015, e52442. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, S.; Hederos, M.; Champion, E.; Dékány, G.; Thiem, J. Novel efficient routes to indoxyl glycosides for monitoring glycosidase activities. Org. Lett. 2013, 15, 3766–3769. [Google Scholar] [CrossRef]
- Pote, A.R.; Vannam, R.; Richard, A.; Gascón, J.; Peczuh, M.W. Formation of and Glycosylation with Per-O-Acetyl Septanosyl Halides: Rationalizing Complex Reactivity En Route to p-Nitrophenyl Septanosides. Eur. J. Org. Chem. 2018, 2018, 1709–1719. [Google Scholar] [CrossRef]
- de Melo, J.S.S.; Rondão, R.; Burrows, H.D.; Melo, M.J.; Navaratnam, S.; Edge, R.; Voss, G. Spectral and Photophysical Studies of Substituted Indigo Derivatives in Their Keto Forms. ChemPhysChem 2006, 7, 2303–2311. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, K.; Somasundaram, M.; Saiganesh, R.; Balasubramanian, K.K. Bromination of deactivated aromatics: A simple and efficient method. J. Org. Chem. 2007, 72, 5867–5869. [Google Scholar] [CrossRef] [PubMed]
- Imming, P.; Imhof, I.; Zentgraf, M. An improved synthetic procedure for 6,6′-dibromoindigo (Tyrian Purple). Synth. Commun. 2001, 31, 3721–3727. [Google Scholar] [CrossRef]
- Vallmitjana, M.; Ferrer-Navarro, M.; Planell, R.; Abel, M.; Ausín, C.; Querol, E.; Planas, A.; Pérez-Pons, J.A. Mechanism of the family 1 β-glucosidase from Streptomyces sp: Catalytic residues and kinetic studies. Biochemistry 2001, 40, 5975–5982. [Google Scholar] [CrossRef] [PubMed]
- Stroobants, A.; Portetelle, D.; Vandenbol, M. New carbohydrate-active enzymes identified by screening two metagenomic libraries derived from the soil of a winter wheat field. J. Appl. Microbiol. 2014, 117, 1045–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Uppal, T.; Bhupathiraju, N.V.S.D.K.; Vicente, M.G.H. Synthesis and cellular properties of Near-IR BODIPY-PEG and carbohydrate conjugates. Tetrahedron 2013, 69, 4687–4693. [Google Scholar] [CrossRef]
- Aharoni, A.; Thieme, K.; Chiu, C.P.C.; Buchini, S.; Lairson, L.L.; Chen, H.; Strynadka, N.C.J.; Wakarchuk, W.W.; Withers, S.G. High-throughput screening methodology for the directed evolution of glycosyltransferases. Nat. Methods 2006, 3, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Novikova, N.; Simpson, M.C.; Timmer, M.S.M.; Stocker, B.L.; Söhnel, T.; Ware, D.C.; Brothers, P.J. Lighting up sugars: Fluorescent BODIPY-: Gluco -furanose and -septanose conjugates linked by direct B-O-C bonds. Org. Biomol. Chem. 2016, 14, 5205–5209. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Green, M.R.; Hughes, H.; Sambrook, J.; MacCallum, P. Molecular Cloning; A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Segel, I.H. Enzyme Kinetics: Behaviour and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems; John Wiley & Sons Ltd.: New York, NY, USA, 1975. [Google Scholar]
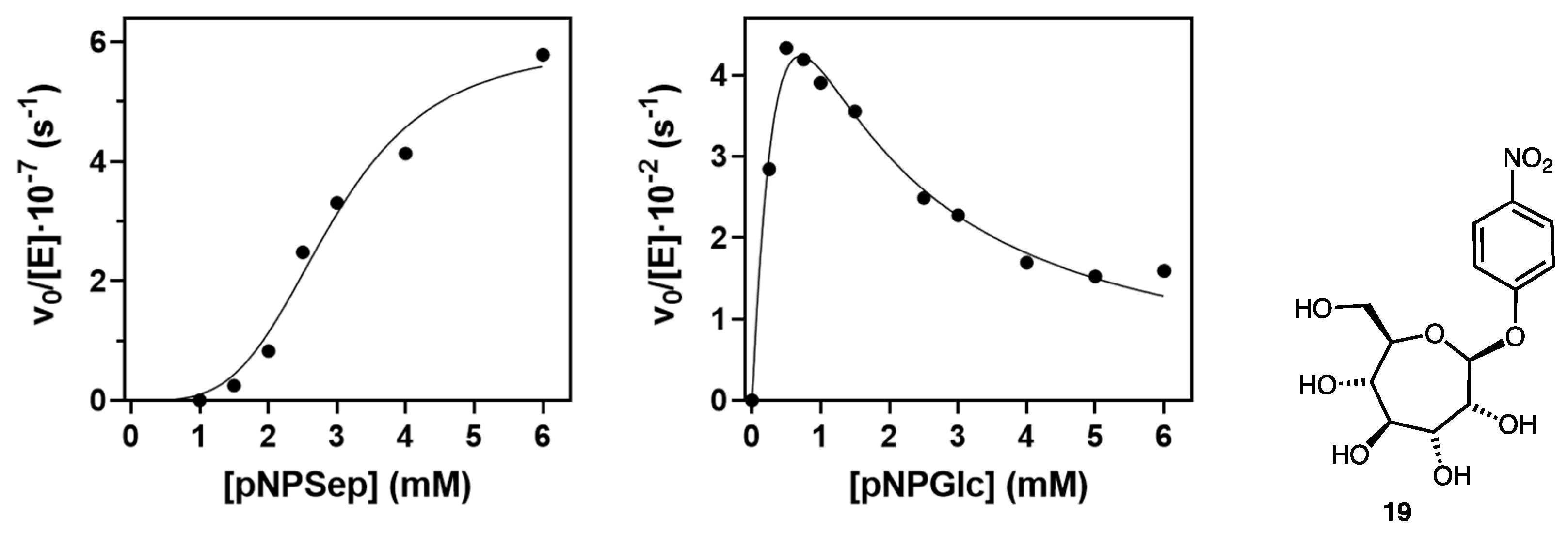
| Enzyme | Substrate | S.A. (U/mg) a | Conditions |
|---|---|---|---|
| β-Galactosidase (Escherichia coli) | pNP-Gal | 35 | Phosphate buffer (100 mM) pH 6.5 40 °C |
| 8 | n.d. b | ||
| 9 | n.d. | ||
| β-Galactosidase (Aspergillus niger) | pNP-Gal | 170 | Acetate buffer (100 mM) pH 4.5 40 °C |
| 8 | <0.001 | ||
| 9 | n.d. | ||
| β-Glucosidase (Streptomyces sp.) | pNP-Glc | 3.3 | Phosphate buffer (50 mM) pH 6.5 50 °C |
| 8 | <0.005 | ||
| 9 | n.d. | ||
| β-Glucosidase (Almonds) | pNP-Glc | 2 | Phosphate buffer (100 mM) pH 5.0 37 °C |
| 8 | n.d. | ||
| 9 | n.d. | ||
| β-Glucosidase (Thermatoga maritima) | pNP-Glc | 70 | Maleate buffer (50 mM) pH 6.5 40 °C |
| 8 | n.d. | ||
| 9 | n.d. | ||
| β-Glucosidase (Phanerochaete chyrosporium) | pNP-Glc | 100 | Acetate buffer (100 mM) pH 5.0 40 °C |
| 8 | n.d. | ||
| 9 | n.d. | ||
| β-Mannosidase (Cellulomonas fimi) | pNP-Man | 10 | Maleate buffer (100 mM) pH 6.5 35 °C |
| 8 | <0.001 | ||
| 9 | <0.001 | ||
| β-Glucuronidase (Escherichia coli) | pNP-GlcA | 110 | Tris·HCl buffer (100 mM) pH 7.5 37 °C |
| 8 | n.d. | ||
| 9 | n.d. | ||
| β-Xylosidase (Bacillus pumilus) | pNP-Xyl | 18 | Phosphate buffer (50 mM) pH 7.5 35 °C |
| 8 | n.d. | ||
| 9 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pote, A.R.; Pascual, S.; Planas, A.; Peczuh, M.W. Indolyl Septanoside Synthesis for In Vivo Screening of Bacterial Septanoside Hydrolases. Int. J. Mol. Sci. 2021, 22, 4497. https://doi.org/10.3390/ijms22094497
Pote AR, Pascual S, Planas A, Peczuh MW. Indolyl Septanoside Synthesis for In Vivo Screening of Bacterial Septanoside Hydrolases. International Journal of Molecular Sciences. 2021; 22(9):4497. https://doi.org/10.3390/ijms22094497
Chicago/Turabian StylePote, Aditya R., Sergi Pascual, Antoni Planas, and Mark W. Peczuh. 2021. "Indolyl Septanoside Synthesis for In Vivo Screening of Bacterial Septanoside Hydrolases" International Journal of Molecular Sciences 22, no. 9: 4497. https://doi.org/10.3390/ijms22094497
APA StylePote, A. R., Pascual, S., Planas, A., & Peczuh, M. W. (2021). Indolyl Septanoside Synthesis for In Vivo Screening of Bacterial Septanoside Hydrolases. International Journal of Molecular Sciences, 22(9), 4497. https://doi.org/10.3390/ijms22094497






