In Silico Analysis of the Molecular-Level Impact of SMPD1 Variants on Niemann-Pick Disease Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
- If a variant is annotated as U in one database and as D, A, B or N in another database, we used the latter (most informative) annotation.
- If a variant is annotated as D in one database and as A or B in another one, we used the latter (again most informative) annotation.
- In the case of strong conflicting annotations, i.e., when a variant is annotated as N in a database and as D, A or B in another database, we considered the variant as U.
- Variants annotated as NPDA-causing in one database and as NPDB-causing in another database were considered as D.
2.2. Residue Interactions
2.3. Features and 2-Class Generic Predictors
2.4. Prediction Method
2.5. Enzymatic Activity
3. Results
3.1. Molecular Effect of SMPD1 Variants
3.2. Three Class NPD Variant Classifier SMPD1-ZooM
3.3. Large-Scale Variant Analysis of SMPD1-ZooM
3.4. Heterozygous Variant Classification
3.5. Focus on SMPD1 Variants in Ashkenazi Jewish Individuals
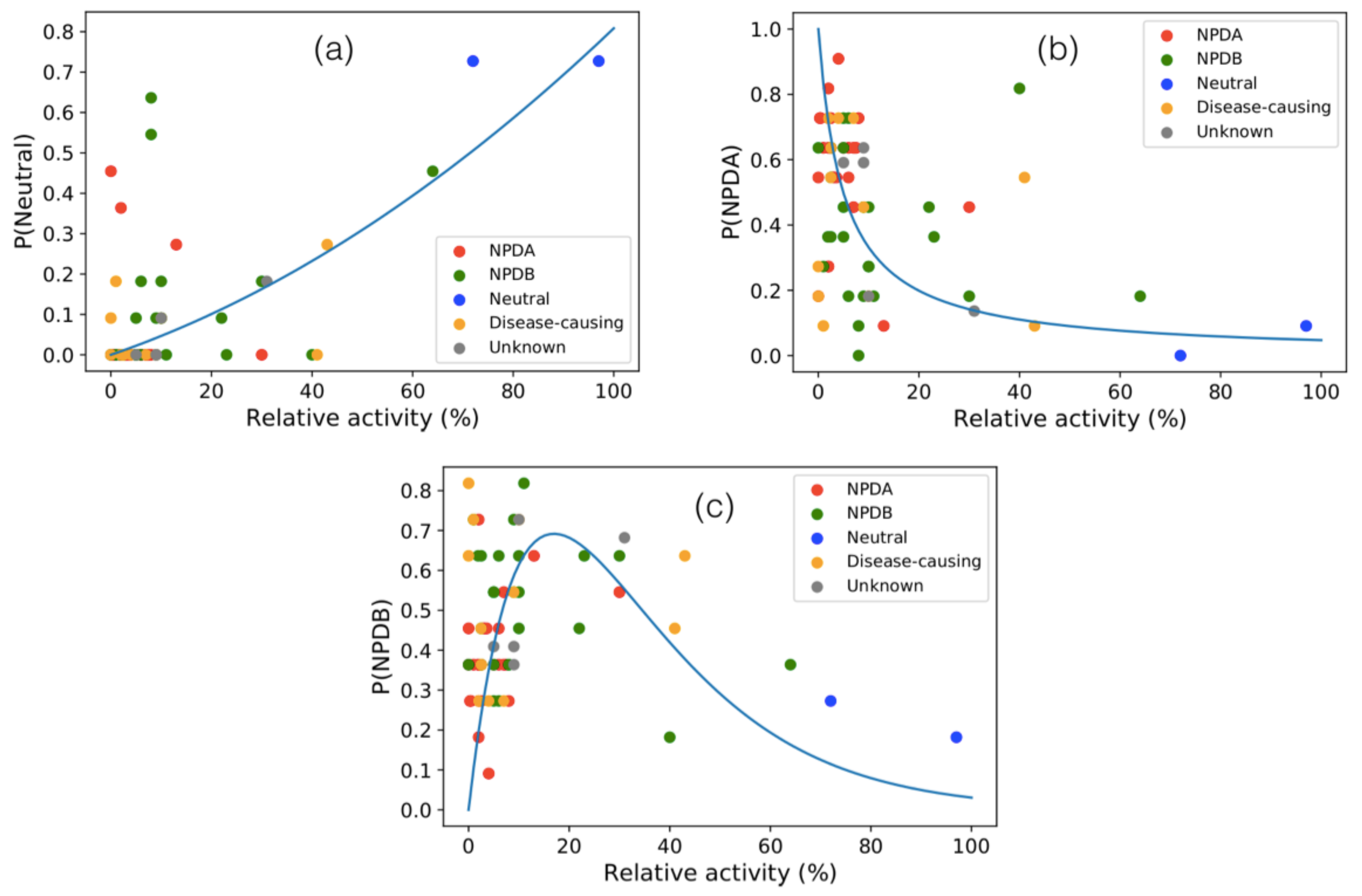
3.6. Activity of SMPD1 Variants
3.7. SMPD1 Activity and Parkinson Disease
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jana, A.; Pahan, K. Sphingolipids in multiple sclerosis. Neuromol. Med. 2010, 12, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Schissel, S.L.; Schuchman, E.H.; Williams, K.J.; Tabas, I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J. Biol. Chem. 1996, 271, 18431–18436. [Google Scholar] [CrossRef]
- Falcone, S.; Perrotta, C.; Palma, C.D.; Pisconti, A.; Sciorati, C.; Capobianco, A.; Rovere-Querini, P.; Manfredi, A.A.; Clementi, E. Activation of Acid Sphingomyelinase and Its Inhibition by the Nitric Oxide/Cyclic Guanosine 3,5-Monophosphate Pathway: Key Events in Escherichia coli- Elicited Apoptosis of Dendritic Cells. J. Immunol. 2004, 173, 4452–4463. [Google Scholar] [CrossRef]
- Park, M.H.; Jin, H.K.; Bae, J.S. Potential therapeutic target for aging and age-related neurodegenerative diseases: The role of acid sphingomyelinase. Exp. Mol. Med. 2020, 52, 380–389. [Google Scholar] [CrossRef]
- Levran, O.; Desnick, R.J.; Schuchman, E.H. Niemann-Pick disease: A frequent missense mutation in the acid sphingomyelinase gene of Ashkenazi Jewish type A and B patients. Proc. Natl. Acad. Sci. USA 1991, 88, 3748–3752. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.J.; Huang, J.; Poda, G.; Pomès, R.; Privé, G.G. Structure of Human Acid Sphingomyelinase Reveals the Role of the Saposin Domain in Activating Substrate Hydrolysis. J. Mol. Biol. 2016, 428, 3026–3042. [Google Scholar] [CrossRef]
- Schuchman, E.H.; Desnick, R.J. Types A and B Niemann-Pick Disease HHS Public Access. Mol. Genet. Metab. 2017, 120, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, M.P.; Diaz, G.A.; Lachmann, R.H.; Jouvin, M.H.; Nandy, I.; Ji, A.J.; Puga, A.C. Olipudase alfa for treatment of acid sphingomyelinase deficiency (ASMD): Safety and efficacy in adults treated for 30 months. J. Inherit. Metab. Dis. 2018, 41, 829–838. [Google Scholar] [CrossRef]
- Thurberg, B.L.; Diaz, G.A.; Lachmann, R.H.; Schiano, T.; Wasserstein, M.P.; Ji, A.J.; Zaher, A.; Peterschmitt, M.J. Long-term efficacy of olipudase alfa in adults with acid sphingomyelinase deficiency (ASMD): Further clearance of hepatic sphingomyelin is associated with additional improvements in pro- and anti-atherogenic lipid profiles after 42 months of treatment. Mol. Genet. Metab. 2020. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Mallett, V.; Vanderperre, B.; Tavassoly, O.; Dauvilliers, Y.; Wu, R.Y.; Ruskey, J.A.; Leblond, C.S.; Ambalavanan, A.; Laurent, S.B.; et al. SMPD1 mutations, activity, and α-synuclein accumulation in Parkinson’s disease. Mov. Disord. 2019, 34, 526–535. [Google Scholar] [CrossRef]
- Smolders, S.; Van Broeckhoven, C. Genetic perspective on the synergistic connection between vesicular transport, lysosomal and mitochondrial pathways associated with Parkinson’s disease pathogenesis. Acta Neuropathol. Commun. 2020, 8, 1–28. [Google Scholar] [CrossRef]
- Lee, J.K.; Jin, H.K.; Park, M.H.; Kim, B.R.; Lee, P.H.; Nakauchi, H.; Carter, J.E.; He, X.; Schuchman, E.H.; Bae, J.S. Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer’s disease. J. Exp. Med. 2014, 211, 1551–1570. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Medlin, A.; Bleich, S.; Jendrossek, V.; Henkel, A.; Wiltfang, J.; Gulbins, E. High activity of acid sphingomyelinase in major depression. J. Neural Transm. 2005, 112, 1583–1590. [Google Scholar] [CrossRef]
- Desnick, J.P.; Kim, J.; He, X.; Wasserstein, M.P.; Simonaro, C.M.; Schuchman, E.H. Identification and Characterization of Eight Novel SMPD1 Mutations Causing Types A and B Niemann-Pick Disease. Mol. Med. 2010, 16, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Vadlamudi, Y.; Muthu, K.; Suresh Kumar, M. Structural exploration of acid sphingomyelinase at different physiological pH through molecular dynamics and docking studies. RSC Adv. 2016, 6, 74859–74873. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Metcalf, M.C.; Garman, S.C.; Edmunds, T.; Qiu, H.; Wei, R.R. Human acid sphingomyelinase structures provide insight to molecular basis of Niemann–Pick disease. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Acu, M.; Castro-Fernandez, V.; Latorre, M.; Castro, J.; Schuchman, E.H.; Guix, V.; Gonzalez, M.; Zanlungo, S. Structural and functional analysis of the ASM p.Ala359Asp mutant that causes acid sphingomyelinase deficiency. Biochem. Biophys. Res. Commun. 2016, 479, 496–501. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, S.; Filocamo, M.; Pianta, A.; Lualdi, S.; Gort, L.; Coll, M.J.; Sinnott, R.; Geberhiwot, T.; Bembi, B.; Dardis, A. SMPD1 Mutation Update: Database and Comprehensive Analysis of Published and Novel Variants. Hum. Mutat. 2016, 37, 139–147. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef]
- Phan, L.; Jin, Y.; Zhang, H.; Qiang, W.; Shekhtman, E.; Shao, D.; Revoe, D.; Villamarin, R.; Ivanchenko, E.; Kimura, M.; et al. ALFA: Allele Frequency Aggregator. 2020. Available online: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (accessed on 2 April 2021).
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Weisburd, B.; Thomas, B.; Solomonson, M.; Ruderfer, D.M.; Kavanagh, D.; Hamamsy, T.; Lek, M.; Samocha, K.E.; Cummings, B.B.; et al. The ExAC browser: Displaying reference data information from over 60 000 exomes. Nucleic Acids Res. 2017, 45, D840–D845. [Google Scholar] [CrossRef] [PubMed]
- Belmont, J.W.; Hardenbol, P.; Willis, T.D.; Yu, F.; Yang, H.; Ch’Ang, L.Y.; Huang, W.; Liu, B.; Shen, Y.; Tam, P.K.H.; et al. The international HapMap project. Nature 2003, 426, 789–796. [Google Scholar] [CrossRef]
- Tina, K.; Bhadra, R.; Srinivasan, N. PIC: Protein interactions calculator. Nucleic Acids Res. 2007, 35, W473–W476. [Google Scholar] [CrossRef] [PubMed]
- Wintjens, R.; Liévin, J.; Rooman, M.; Buisine, E. Contribution of cation-π interactions to the stability of protein-DNA complexes. J. Mol. Biol. 2000, 302, 393–408. [Google Scholar] [CrossRef]
- Cauët, E.; Rooman, M.; Wintjens, R.; Liévin, J.; Biot, C. Histidine- aromatic interactions in proteins and protein- ligand complexes: Quantum chemical study of X-ray and model structures. J. Chem. Theory Comput. 2005, 1, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Sims, G.E.; Murphy, S.; Miller, J.R.; Chan, A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE 2012, 7, e46688. [Google Scholar] [CrossRef]
- Raimondi, D.; Tanyalcin, I.; Ferté, J.; Gazzo, A.; Orlando, G.; Lenaerts, T.; Rooman, M.; Vranken, W. DEOGEN2: Prediction and interactive visualization of single amino acid variant deleteriousness in human proteins. Nucleic Acids Res. 2017, 45, W201–W206. [Google Scholar] [CrossRef] [PubMed]
- Ancien, F.; Pucci, F.; Godfroid, M.; Rooman, M. Prediction and interpretation of deleterious coding variants in terms of protein structural stability. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, Y.; Kwasigroch, J.M.; Gilis, D.; Rooman, M. PoPMuSiC 2.1: A web server for the estimation of protein stability changes upon mutation and sequence optimality. BMC Bioinform. 2011, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, D.; Orlando, G.; Pancsa, R.; Khan, T.; Vranken, W.F. Exploring the Sequence-based Prediction of Folding Initiation Sites in Proteins. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Dasarathy, B.V. Nearest Neighbor (NN) Norms: NN Pattern Classification Techniques; IEEE Computer Society Press: Los Alamitos, CA, USA, 1991. [Google Scholar]
- Nadkarni, P. Core Technologies: Data Mining and “Big Data”; Clinical Research Computing: A Practitioner’s Handbook; Elsevier: Amsterdam, The Netherlands, 2016; pp. 187–204. [Google Scholar] [CrossRef]
- Urbanowicz, R.J.; Moore, J.H. ExSTraCS 2.0: Description and evaluation of a scalable learning classifier system. Evol. Intell. 2015, 8, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, Y.; Gilis, D.; Rooman, M. A new generation of statistical potentials for proteins. Biophys. J. 2006, 90, 4010–4017. [Google Scholar] [CrossRef]
- Makwana, K.M.; Mahalakshmi, R. Implications of aromatic–aromatic interactions: From protein structures to peptide models. Protein Sci. 2015, 24, 1920–1933. [Google Scholar] [CrossRef]
- Ninković, D.; Blagojević Filipović, J.; Hall, M.; Brothers, E.; Zarić, S.D. What Is Special about Aromatic-Aromatic Interactions? Significant Attraction at Large Horizontal Displacement. ACS Cent. Sci. 2020, 25, 420–425. [Google Scholar] [CrossRef]
- Vernon, R.M.; Chong, P.A.; Tsang, B.; Kim, T.H.; Bah, A.; Farber, P.; Lin, H.; Forman-Kay, J.D. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. eLife 2018, 7, e31486. [Google Scholar] [CrossRef]
- Saunders, C.T.; Baker, D. Evaluation of structural and evolutionary contributions to deleterious mutation prediction. J. Mol. Biol. 2002, 322, 891–901. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Canals, D.; Hannun, Y.A. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell. Signal. 2009, 21, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Pavlů-Pereira, H.; Asfaw, B.; Poupětová, H.; Ledvinová, J.; Sikora, J.; Vanier, M.T.; Sandhoff, K.; Zeman, J.; Novotná, Z.; Chudoba, D.; et al. Acid sphingomyelinase deficiency. Phenotype variability with prevalence of intermediate phenotype in a series of twenty-five Czech and Slovak patients. A multi-approach study. J. Inherit. Metab. Dis. 2005, 28, 203–227. [Google Scholar] [CrossRef]
- Wasserstein, M.P.; Aron, A.; Brodie, S.E.; Simonaro, C.; Desnick, R.J.; McGovern, M.M. Acid sphingomyelinase deficiency: Prevalence and characterization of an intermediate phenotype of Niemann-Pick disease. J. Pediatr. 2006, 149, 554–559. [Google Scholar] [CrossRef]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Reva, B.; Antipin, Y.; Sander, C. Predicting the functional impact of protein mutations: Application to cancer genomics. Nucleic Acids Res. 2011, 39, 1–14. [Google Scholar] [CrossRef]
- Di Resta, C.; Manzoni, M.; Berisso, M.; Siciliano, G.; Benedetti, S.; Ferrari, M. Evaluation of damaging effects of splicing mutations: Validation of an in vitro method for diagnostic laboratories. Clin. Chim. Acta 2014, 436, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, A.; Illes, K.; Heinz, L.X.; Superti-Furga, G.; Nagar, B. Crystal structure of mammalian acid sphingomyelinase. Nat. Commun. 2016, 7, 12196. [Google Scholar] [CrossRef]
- Ranganath, P.; Matta, D.; Bhavani, G.S.; Wangnekar, S.; Jain, J.M.N.; Verma, I.C.; Kabra, M.; Puri, R.D.; Danda, S.; Gupta, N.; et al. Spectrum of SMPD1 mutations in Asian-Indian patients with acid sphingomyelinase (ASM)-deficient Niemann–Pick disease. Am. J. Med. Genet. Part A 2016, 170, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Pittis, M.; Ricci, V.; Guerci, V.I.; Marçais, C.; Ciana, G.; Dardis, A.; Gerin, F.; Stroppiano, M.; Vanier, M.; Filocamo, M.; et al. Acid sphingomyelinase: Identification of nine novel mutations among Italian Niemann Pick type B patients and characterization of in vivo functional in-frame start codon. Hum. Mutat. 2004, 24, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Hollak, C.E.; de Sonnaville, E.S.; Cassiman, D.; Linthorst, G.E.; Groener, J.E.; Morava, E.; Wevers, R.A.; Mannens, M.; Aerts, J.M.; Meersseman, W.; et al. Acid sphingomyelinase (Asm) deficiency patients in The Netherlands and Belgium: Disease spectrum and natural course in attenuated patients. Mol. Genet. Metab. 2012, 107, 526–533. [Google Scholar] [CrossRef]
- Levran, O.; Desnick, R.J.; Schuchman, E.H. Identification and expression of a common missense mutation (L302P) in the acid sphingomyelinase gene of Ashkenazi Jewish type A Niemann-Pick disease patients. Blood 1992, 80, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Edelmann, L.; Liu, L.; Luo, M.; Desnick, R.J.; Kornreich, R. Experience with carrier screening and prenatal diagnosis for 16 Ashkenazi Jewish genetic diseases. Hum. Mutat. 2010, 31, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, S.; Albi, E.; Parnetti, L.; Beccari, T. Lysosomal ceramide metabolism disorders: Implications in Parkinson’s disease. J. Clin. Med. 2020, 9, 594. [Google Scholar] [CrossRef]
- Mihaylova, V.; Hantke, J.; Sinigerska, I.; Cherninkova, S.; Raicheva, M.; Bouwer, S.; Tincheva, R.; Khuyomdziev, D.; Bertranpetit, J.; Chandler, D.; et al. Highly variable neural involvement in sphingomyelinase-deficient Niemann–Pick disease caused by an ancestral Gypsy mutation. Brain 2007, 130, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Malentacchi, F.; Mancini, I.; Brandslund, I.; Vermeersch, P.; Schwab, M.; Marc, J.; van Schaik, R.; Siest, G.; Theodorsson, E.; Pazzagli, M.; et al. Is laboratory medicine ready for the era of personalized medicine? A survey addressed to laboratory directors of hospitals/academic schools of medicine in Europes. Clin. Chem. Lab. Med. 2015, 7, 981–988. [Google Scholar]
- Karas Kuželički, N.; Prodan Žitnik, I.; Gurwitz, D.; Llerena, A.; Cascorbi, I.; Siest, S.; Simmaco, M.; Ansari, M.; Pazzagli, M.; Di Resta, C.; et al. Pharmacogenomics education in medical and pharmacy schools: Conclusions of a global survey. Pharmacogenomics 2019, 9, 643–657. [Google Scholar] [CrossRef]

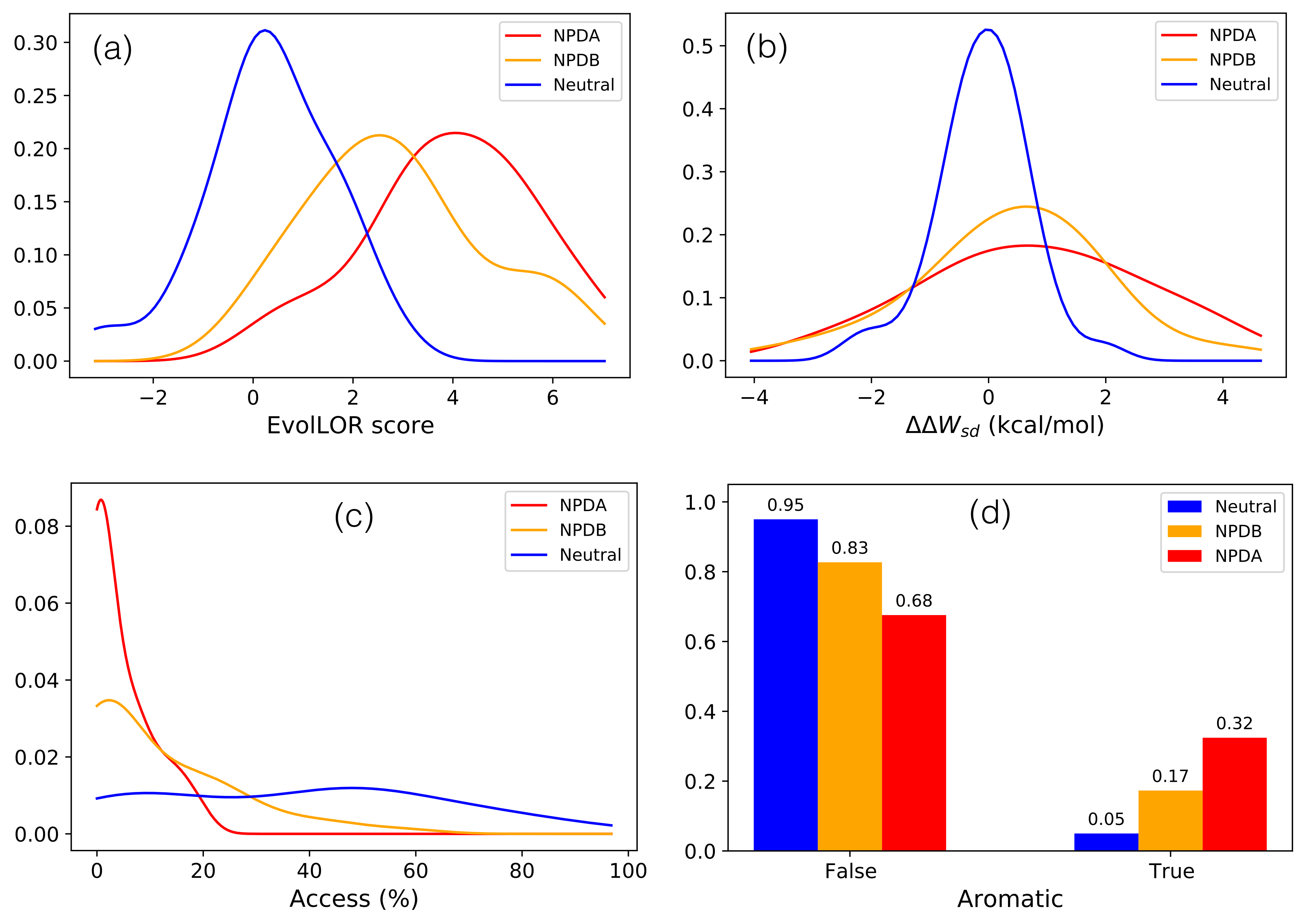
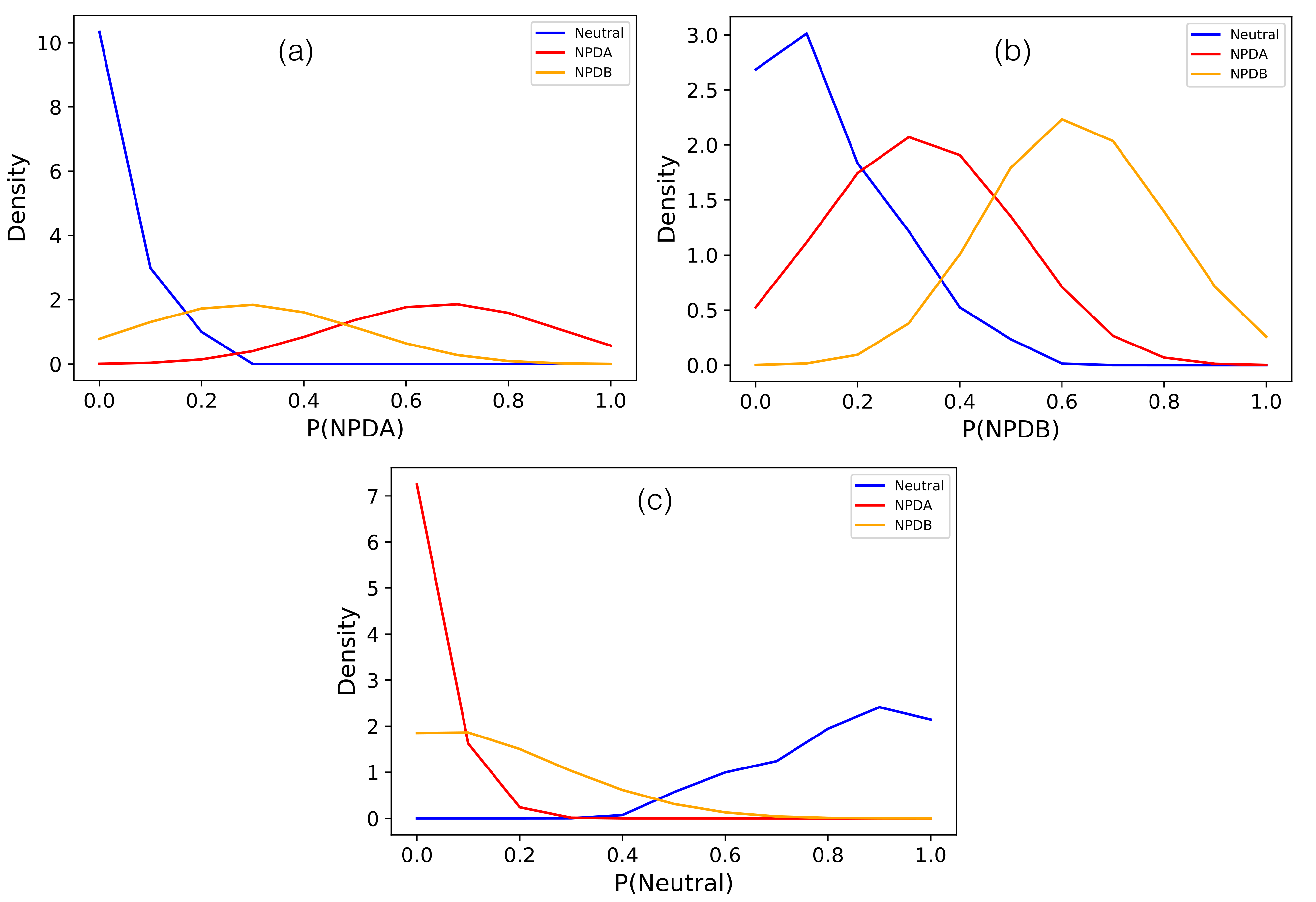
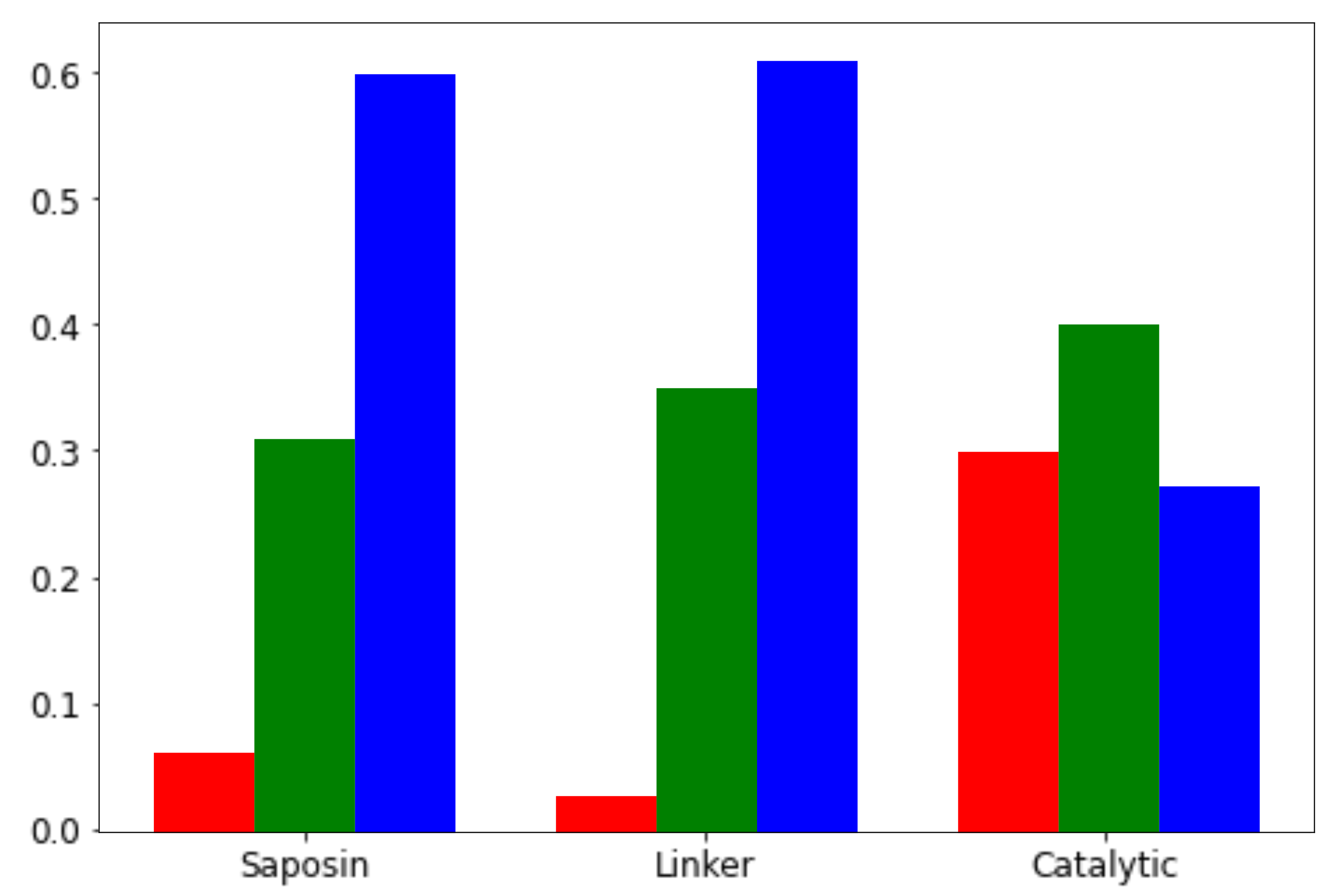

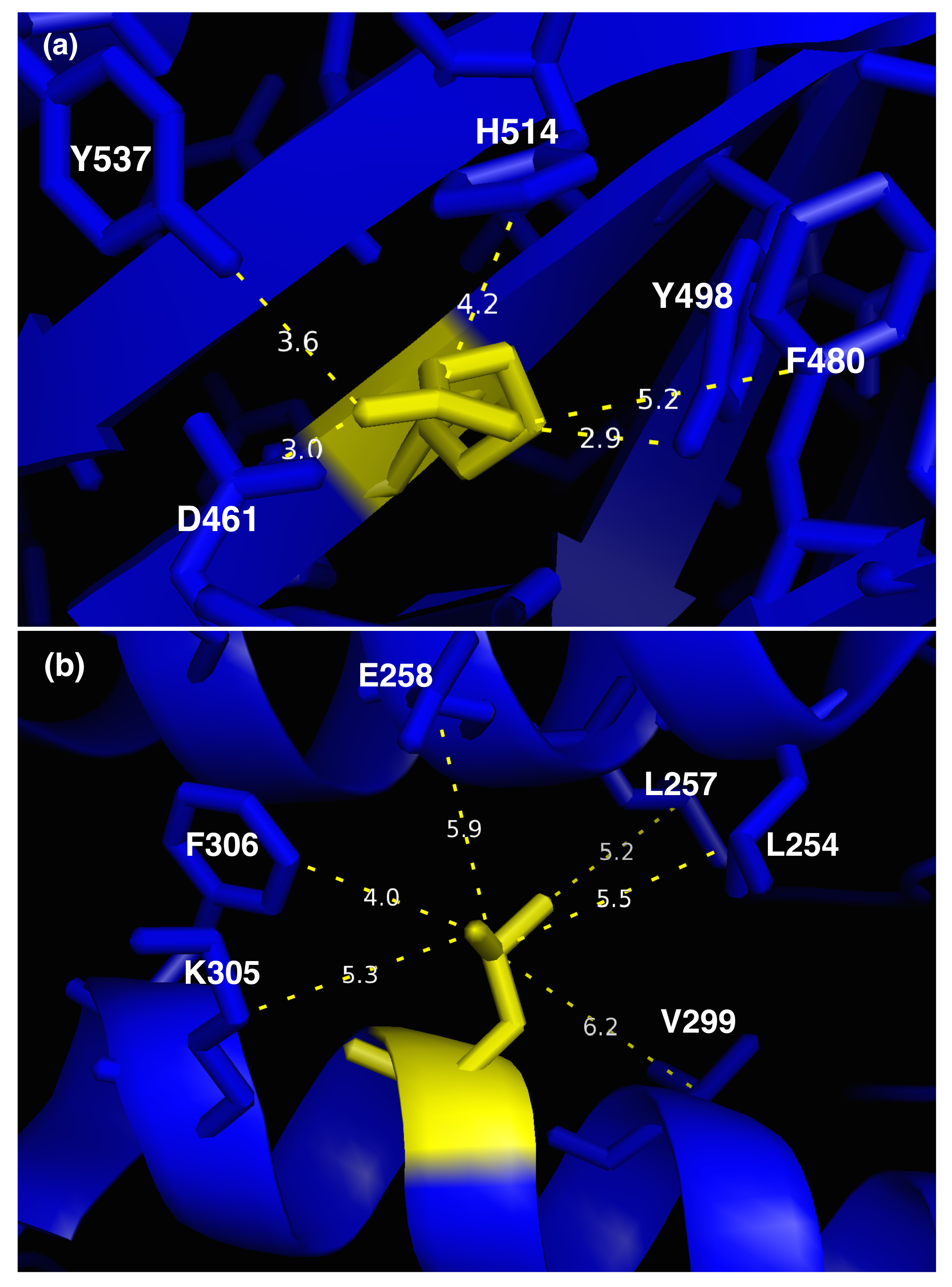
| Feature/Predictor | Description | Data Type | p-Value |
|---|---|---|---|
| PROVEAN | Deleterious variant predictor | Sequence/Evolution | <0.001 |
| DEOGEN2 | Deleterious variant predictor | Sequence/Evolution/Context | <0.001 |
| SNPMuSiC | Deleterious variant predictor | Structure/Stability | <0.001 |
| PoPMuSiC | Variant stability change predictor | Structure/Stability | <0.001 |
| Change in sd folding free energy | Structure/Stability | 0.046 | |
| Change in sds folding free energy | Structure/Stability | <0.001 | |
| Change in sad folding free energy | Structure/Stability | 0.018 | |
| Access | Variant solvent accessibility | Structure | <0.001 |
| Metal | Variant’s distance from Zn ions | Structure | <0.001 |
| Carbohyd | Variant’s distance from glycosylation site | Structure | 0.002 |
| Disulfide | Variant’s distance from disulfide bridge | Structure | 0.009 |
| EvolCI | Evolutionary conservation index | Sequence/Evolution | <0.001 |
| EvolLOR | Evolutionary Log-odd ratio | Sequence/Evolution | <0.001 |
| Aromatic | Aromaticity variation | Sequence | 0.017 |
| SMPD1-ZooM (3-Class) | |||
|---|---|---|---|
| Sensitivity | Specificity | BACC | AUROC |
| 73.3% | 86.0% | 79.7% | 86.9% |
| Method | Sensitivity | Specificity | BACC | AUROC |
|---|---|---|---|---|
| SMPD1-ZooM (2-class) | 89.4% | 97.5% | 93.5% | 97.8% |
| SNPMuSiC [31] | 85.4% | 87.5% | 86.5% | 94.0% |
| SNPMuSiC [31] | 82.8% | 67.5% | 75.1% | 86.2% |
| DEOGEN2 [30] | 96.0% | 80.0% | 88.0% | 96.9% |
| SIFT [46] | 60.9% | 95.0% | 78.0% | 88.8% |
| PolyPhen2 [47] | 96.7% | 85.0% | 90.8% | 96.6% |
| PROVEAN [29] | 87.4% | 97.5% | 92.5% | 96.1% |
| MutationAssessor [48] | 86.8% | 82.5% | 84.6% | 93.8% |
| Predictor | L302P | R496L |
|---|---|---|
| SMPD1-ZooM (3-class) | NPDA | NPDA |
| DEOGEN2 | 0.88 | 0.83 |
| PROVEAN | −4.39 | −6.65 |
| SNPMuSiC | 0.25 | 0.24 |
| PoPMuSiC | 3.93 kcal/mol | 0.24 kcal/mol |
| P(NDPA) | −0.38 (p ) | 0.30 |
| P(NPDB) | −0.11 (p ) | 0.26 |
| P(Neutral) | 0.61 (p ) | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ancien, F.; Pucci, F.; Rooman, M. In Silico Analysis of the Molecular-Level Impact of SMPD1 Variants on Niemann-Pick Disease Severity. Int. J. Mol. Sci. 2021, 22, 4516. https://doi.org/10.3390/ijms22094516
Ancien F, Pucci F, Rooman M. In Silico Analysis of the Molecular-Level Impact of SMPD1 Variants on Niemann-Pick Disease Severity. International Journal of Molecular Sciences. 2021; 22(9):4516. https://doi.org/10.3390/ijms22094516
Chicago/Turabian StyleAncien, François, Fabrizio Pucci, and Marianne Rooman. 2021. "In Silico Analysis of the Molecular-Level Impact of SMPD1 Variants on Niemann-Pick Disease Severity" International Journal of Molecular Sciences 22, no. 9: 4516. https://doi.org/10.3390/ijms22094516
APA StyleAncien, F., Pucci, F., & Rooman, M. (2021). In Silico Analysis of the Molecular-Level Impact of SMPD1 Variants on Niemann-Pick Disease Severity. International Journal of Molecular Sciences, 22(9), 4516. https://doi.org/10.3390/ijms22094516






