Molecular Characterization of Xyloglucanase cel74a from Trichoderma reesei
Abstract
1. Introduction
2. Results
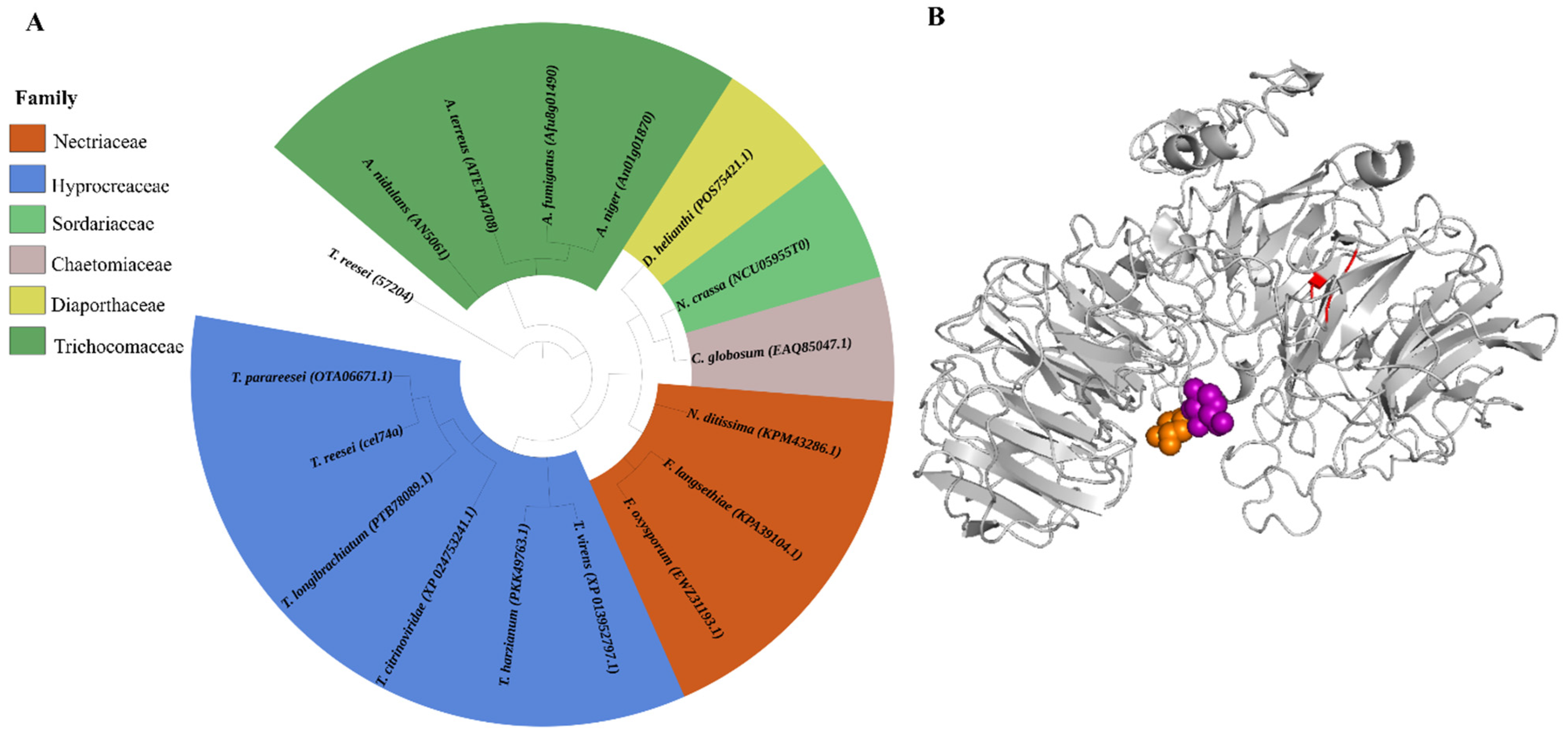
2.1. Phylogenetic Analysis and 3D Structure Analysis
2.2. Xyloglucanase CEL74A Activity is Regulated by Calcium
2.3. cel74a Gene Is Upregulated in T. reesei in the Presence of SCB
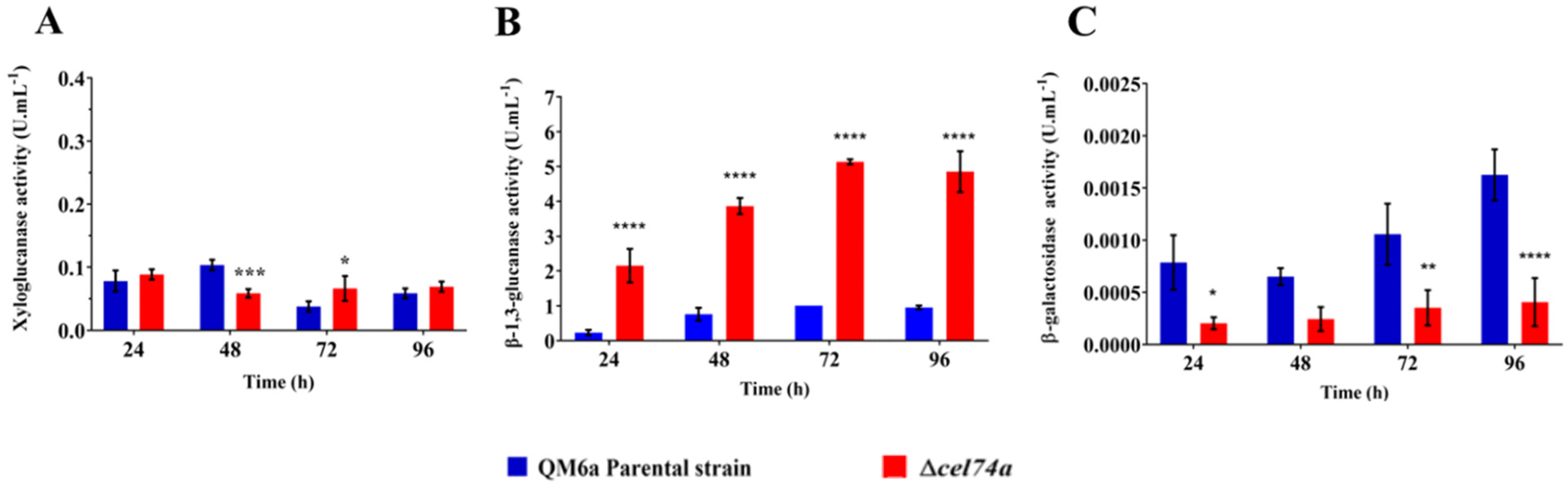
2.4. Xylose, Glucose, and Galactose Release Is Altered by cel74a Deletion
2.5. Absence of Xyloglucanase cel74a Affects Holocellulolytic Gene Expression in T. reesei
2.6. Absence of Xyloglucanase cel74a Affects Holocellulase Activities in T. reesei
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Phenotypic Characterization
4.3. Vector Construction for Gene Deletion
4.4. Transformation of T. reesei
4.5. Gene Expression Analysis
4.6. RNA Extraction and Transcript Analyis Using Quantitative PCR (RT-qPCR)
4.7. Enzyme Activity Assays
4.8. Total Protein Quantification
4.9. Xyloglucan Sugar Release Profile of T. reesei Δcel74a
4.10. D Structure Prediction and Phylogenetic Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.K.; Kim, S.H.; Yoon, J.J.; Yang, Y.H. Current status and strategies for second generation biofuel production using microbial systems. Energy Convers. Manag. 2017, 148, 1142–1156. [Google Scholar] [CrossRef]
- Martins-Santana, L.; Nora, L.C.; Sanches-Medeiros, A.; Lovate, G.L.; Cassiano, M.H.A.; Silva-Rocha, R. Systems and synthetic biology approaches to engineer fungi for fine chemical production. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kubicek, C.; Berrin, J.; Wilson, D.; Couturier, M.; Berlin, A.; Filho, E.; Ezeji, T. Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem. Sci. 2016, 41, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, M.S.; De Souza, A.P. Advances of basic science for second generation bioethanol from sugarcane. Adv. Basic Sci. Second Gener. Bioethanol Sugarcane 2017, 1–219. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kubicek, C.P. Genetic engineering of Trichoderma reesei cellulases and their production. Microb. Biotechnol. 2017, 10, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, E.; Aubry, N.; Ben Chaabane, F.; Cohen, C.; Tayeb, J.; Rémond, C. Contrasted enzymatic cocktails reveal the importance of cellulases and hemicellulases activity ratios for the hydrolysis of cellulose in presence of xylans. AMB Express 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Rashmi, R.; Siddalingamurthy, K.R. Microbial xyloglucanases: A comprehensive review. Biocatal. Biotransform. 2018, 36, 280–295. [Google Scholar] [CrossRef]
- Damasio, A.R.L.; Rubio, M.V.; Gonçalves, T.A.; Persinoti, G.F.; Segato, F.; Prade, R.A.; Contesini, F.J.; de Souza, A.P.; Buckeridge, M.S.; Squina, F.M. Xyloglucan breakdown by endo-xyloglucanase family 74 from Aspergillus fumigatus. Appl. Microbiol. Biotechnol. 2017, 101, 2893–2903. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef]
- Rodrigues Mota, T.; Matias de Oliveira, D.; Marchiosi, R.; Ferrarese-Filho, O.; Dantas dos Santos, W. Plant cell wall composition and enzymatic deconstruction. AIMS Bioeng. 2018, 5, 63–77. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Chen, Y.; Wagner, E.; Cosgrove, D.J. Xyloglucan in the primary cell wall: Assessment by FESEM, selective enzyme digestions and nanogold affinity tags. Plant J. 2018, 93, 211–226. [Google Scholar] [CrossRef]
- de Souza, A.P.; Leite, D.C.C.; Pattathil, S.; Hahn, M.G.; Buckeridge, M.S. Composition and structure of sugarcane cell wall polysaccharides: Implications for second-generation bioethanol production. BioEnergy Res. 2013, 6, 564–579. [Google Scholar] [CrossRef]
- Gao, J.; Qian, Y.; Wang, Y.; Qu, Y.; Zhong, Y. Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement ofTrichoderma reesei. Biotechnol. Biofuels 2017, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Suo, Y.; Fu, H.; Ren, M.; Yang, X.; Liao, Z.; Wang, J. Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing Class I heat shock protein GroESL. Bioresour. Technol. 2018, 250, 691–698. [Google Scholar] [CrossRef] [PubMed]
- de Paula, R.G.; Antoniêto, A.C.C.; Ribeiro, L.F.C.; Carraro, C.B.; Nogueira, K.M.V.; Lopes, D.C.B.; Silva, A.C.; Zerbini, M.T.; Pedersoli, W.R.; do Nascimento Costa, M.; et al. New genomic approaches to enhance biomass degradation by the industrial fungus Trichoderma reesei. Int. J. Genom. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Borin, G.; Sanchez, C.; De Souza, A.; De Santana, E.; De Souza, A.; Leme, A.; Squina, F.; Buckeridge, M.; Goldman, G.; De Castro Oliveira, J. Comparative secretome analysis of Trichoderma reesei and Aspergillus niger during growth on sugarcane biomass. PLoS ONE 2015, 10, e0129275. [Google Scholar] [CrossRef] [PubMed]
- Grishutin, S.G.; Gusakov, A.V.; Markov, A.V.; Ustinov, B.B.; Semenova, M.V.; Sinitsyn, A.P. Specific xyloglucanases as a new class of polysaccharide-degrading enzymes. Biochim. Biophys. Acta 2004, 1674, 268–281. [Google Scholar] [CrossRef]
- Häkkinen, M.; Arvas, M.; Oja, M.; Aro, N.; Penttilä, M.; Saloheimo, M.; Pakula, T.M. Re-annotation of the CAZy genes of Trichoderma reesei and transcription in the presence of lignocellulosic substrates. Microb. Cell Fact. 2012, 11, 134. [Google Scholar] [CrossRef]
- Dos Santos Castro, L.; Pedersoli, W.; Antoniêto, A.; Steindorff, A.; Silva-Rocha, R.; Martinez-Rossi, N.; Rossi, A.; Brown, N.; Goldman, G.; Faça, V.; et al. Comparative metabolism of cellulose, sophorose and glucose in Trichoderma reesei using high-throughput genomic and proteomic analyses. Biotechnol. Biofuels 2014, 7, 41. [Google Scholar] [CrossRef]
- Arnal, G.; Stogios, P.J.; Asohan, J.; Attia, M.A.; Skarina, T.; Viborg, A.H.; Henrissat, B.; Savchenko, A.; Brumer, H. Substrate specificity, regiospecificity, and processivity in glycoside hydrolase family 74. J. Biol. Chem. 2019, 294, 13233–13247. [Google Scholar] [CrossRef] [PubMed]
- Desmet, T.; Cantaert, T.; Gualfetti, P.; Nerinckx, W.; Gross, L.; Mitchinson, C.; Piens, K. An investigation of the substrate specificity of the xyloglucanase Cel74A from Hypocrea jecorina. FEBS J. 2007, 274, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.C.; Agger, J.W.; Zhang, Z.; Wichmann, J.; Meyer, A.S. A comparative study on the activity of fungal lytic polysaccharide monooxygenases for the depolymerization of cellulose in soybean spent flakes. Carbohydr. Res. 2017, 449, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Florencio, C.; Cunha, F.M.; Badino, A.C.; Farinas, C.S.; Ximenes, E.; Ladisch, M.R. Secretome analysis of Trichoderma reesei and Aspergillus niger cultivated by submerged and sequential fermentation processes: Enzyme production for sugarcane bagasse hydrolysis. Enzyme Microb. Technol. 2016, 90, 53–60. [Google Scholar] [CrossRef]
- Kaida, R.; Kaku, T.; Baba, T.; Oyadomari, M.; Watanabe, T.; Nishida, K.; Kanaya, T.; Shani, Z.; Shoseyov, O.; Hayashi, T. Loosening xyloglucan accelerates the enzymatic degradation of cellulose in wood. Mol. Plant 2009, 2, 904–909. [Google Scholar] [CrossRef]
- Benko, Z.; Siika-aho, M.; Viikari, L.; Réczey, K. Evaluation of the role of xyloglucanase in the enzymatic hydrolysis of lignocellulosic substrates. Enzyme Microb. Technol. 2008, 43, 109–114. [Google Scholar] [CrossRef]
- Marx, I.J.; van Wyk, N.; Smit, S.; Jacobson, D.; Viljoen-Bloom, M.; Volschenk, H. Comparative secretome analysis of Trichoderma asperellum S4F8 and Trichoderma reesei Rut C30 during solid-state fermentation on sugarcane bagasse. Biotechnol. Biofuels 2013, 6, 172. [Google Scholar] [CrossRef]
- He, J.; Wu, A.M.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Tian, G. Cost-effective lignocellulolytic enzyme production by Trichoderma reesei on a cane molasses medium. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- dos Santos Castro, L.; de Paula, R.G.; Antoniêto, A.C.C.; Persinoti, G.F.; Silva-Rocha, R.; Silva, R.N. Understanding the role of the master regulator XYR1 in trichoderma reesei by global transcriptional analysis. Front. Microbiol. 2016, 7, 175. [Google Scholar] [CrossRef]
- de Paula, R.G.; Antoniêto, A.C.C.; Carraro, C.B.; Lopes, D.C.B.; Persinoti, G.F.; Peres, N.T.A.; Martinez-Rossi, N.M.; Silva-Rocha, R.; Silva, R.N. The duality of the MAPK signaling pathway in the control of metabolic processes and cellulase production in trichoderma reesei. Sci. Rep. 2018, 8, 14931. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life v2: Online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 2011, 39, W475–W478. [Google Scholar] [CrossRef]
- Derntl, C.; Kiesenhofer, D.P.; Mach, R.L.; Mach-Aigner, A.R. Novel strategies for genomic manipulation of trichoderma reesei with the purpose of strain engineering. Appl. Environ. Microbiol. 2015. [Google Scholar] [CrossRef]
- Borges, T.A.; de Souza, A.T.; Squina, F.M.; Riaño-Pachón, D.M.; Santos, R.A.C.d.; Machado, E.; Oliveira, J.V.d.C.; Damásio, A.R.L.; Goldman, G.H. Biochemical characterization of an endoxylanase from Pseudozyma brasiliensis sp. nov. strain GHG001 isolated from the intestinal tract of Chrysomelidae larvae associated to sugarcane roots. Process Biochem. 2014, 49, 77–83. [Google Scholar] [CrossRef]
- Bussink, H.J.; Buxton, F.P.; Fraaye, B.A.; de Graaff, L.H.; Visser, J. The polygalacturonases of Aspergillus niger are encoded by a family of diverged genes. Eur. J. Biochem. 1992, 208, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, M.; Valkonen, M.J.; Westerholm-Parvinen, A.; Aro, N.; Arvas, M.; Vitikainen, M.; Penttilä, M.; Saloheimo, M.; Pakula, T.M.; Hakkinen, M.; et al. Screening of candidate regulators for cellulase and hemicellulase production in Trichoderma reesei and identification of a factor essential for cellulase production. Biotechnol. Biofuels 2014, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Leung, K.T.; Qin, W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 2009, 5, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.R.; Dayanand, A. Optimization of process for the production of fungal pectinases from deseeded sunflower head in submerged and solid-state conditions. Bioresour. Technol. 2006, 97, 2340–2344. [Google Scholar] [CrossRef]
- Antoniêto, A.C.C.; de Paula, R.G.; Castro, L.D.S.; Silva-Rocha, R.; Persinoti, G.F.; Silva, R.N. Trichoderma reesei CRE1-mediated carbon catabolite repression in re-sponse to sophorose through RNA sequencing analysis. Curr. Genom. 2016, 17, 119–131. [Google Scholar] [CrossRef]
- Gupta, V.; Steindorff, A.; de Paula, R.; Silva-Rocha, R.; Mach-Aigner, A.; Mach, R.; Silva, R. The post-genomic era of trichoderma reesei: What’s next? Trends Biotechnol. 2016, 34. [Google Scholar] [CrossRef] [PubMed]
- de Paula, R.G.; Antoniêto, A.C.C.; Ribeiro, L.F.C.; Srivastava, N.; O’Donovan, A.; Mishra, P.K.; Gupta, V.K.; Silva, R.N. Engineered microbial host selection for value-added bioproducts from lignocellulose. Biotechnol. Adv. 2019, 37, 107347. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, M.S.; Souza, A.P.; Arundale, R.A.; Anderson-Teixeira, K.J.; DeLucia, E. Ethanol from sugarcane in Brazil: A ‘midway’ strategy for increasing ethanol production while maximizing environmental benefits. GCB Bioenergy 2012, 4, 119–126. [Google Scholar] [CrossRef]
- Daas, M.J.; Martínez, P.M.; van de Weijer, A.H.; van der Oost, J.; de Vos, W.M.; Kabel, M.A.; van Kranenburg, R. Biochemical characterization of the xylan hydrolysis profile of the extracellular endo-xylanase from Geobacillus thermodenitrificans T12. BMC Biotechnol. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Zavyalov, A.V.; Rykov, S.V.; Lunina, N.A.; Sushkova, V.I.; Yarotskya, S.V.; Berezinaa, O.V. Plant polysaccharide xyloglucan and enzymes that hydrolyze it (Review). Russ. J. Bioorganic Chem. 2019, 45, 845–859. [Google Scholar] [CrossRef]
- Xu, J.; Nogawa, M.; Okada, H.; Morikawa, Y. Regulation of xyn3 gene expression in Trichoderma reesei PC-3-7. Appl. Microbiol. Biotechnol. 2000, 54, 370–375. [Google Scholar] [CrossRef]
- Mach, R.L.; Zeilinger, S.; Kristufek, D.; Kubicek, C.P. Ca2+-calmodulin antagonists interfere with xylanase formation and secretion in Trichoderma reesei. Biochim. Biophys. Acta Mol. Cell Res. 1998, 1403, 281–289. [Google Scholar] [CrossRef][Green Version]
- Tisch, D.; Kubicek, C.P.; Schmoll, M. New insights into the mechanism of light modulated signaling by heterotrimeric G-proteins: ENVOY acts on gna1 and gna3 and adjusts cAMP levels in Trichoderma reesei (Hypocrea jecorina). Fungal Genet. Biol. 2011, 48, 631–640. [Google Scholar] [CrossRef]
- Martins-Santana, L.; de Paula, R.G.; Gomes Silva, A.; Christian Borges Lopes, D.; do Nascimento Silva, R.; Silva-Rocha, R. CRZ1 regulator and calcium cooperatively modulate holocellulases gene expression in Trichoderma reesei QM6a. Genet. Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Karlsson, J.; Momcilovic, D.; Wittgren, B.; Schulein, M.; Tjerneld, F.; Brinkmalm, G. Enzymatic degradation of carboxymethyl cellulose hydrolyzed by the endoglucanases Cel5A, Cel7B, and Cel45A from Humicola insolens and Cel7B, Cel12A and Cel45Acore from Trichoderma reesei. Biopolymers 2002, 63, 32–40. [Google Scholar] [CrossRef]
- Miotto, L.S.; De Rezende, C.A.; Bernardes, A.; Serpa, V.I.; Tsang, A.; Polikarpov, I. The characterization of the endoglucanase Cel12A from Gloeophyllum trabeum reveals an enzyme highly active on β-glucan. PLoS ONE 2014, 9, e0108393. [Google Scholar] [CrossRef]
- Kojima, Y.; Várnai, A.; Ishida, T.; Sunagawa, N.; Petrovic, D.M.; Igarashi, K.; Jellison, J.; Goodell, B.; Alfredsen, G.; Westereng, B.; et al. A lytic polysaccharide monooxygenase with broad xyloglucan specificity from the brown-rot fungus Gloeophyllum trabeum and its action on cellulose-xyloglucan complexes. Appl. Environ. Microbiol. 2016, 82, 6557–6572. [Google Scholar] [CrossRef]
- Shimokawa, T.; Shibuya, H.; Nojiri, M.; Yoshida, S.; Ishihara, M. Purification, molecular cloning, and enzymatic properties of a family 12 endoglucanase (EG-II) from Fomitopsis palustris: Role of EG-II in larch holocellulose hydrolysis. Appl. Environ. Microbiol. 2008, 74, 5857–5861. [Google Scholar] [CrossRef]
- Derntl, C.; Rassinger, A.; Srebotnik, E.; Mach, R.L.; Mach-Aigner, A.R. Identification of the main regulator responsible for synthesis of the typical yellow pigment produced by Trichoderma reesei. Appl. Environ. Microbiol. 2016, 82, 6247–6257. [Google Scholar] [CrossRef]
- Gruber, F.; Visser, J.; Kubicek, C.P.; de Graaff, L.H. The development of a heterologous transformation system for the cellulolytic fungus Trichoderma reesei based on a pyrG-negative mutant strain. Curr. Genet. 1990, 18, 71–76. [Google Scholar] [CrossRef]
- de Souza, W.R.; de Gouvea, P.F.; Savoldi, M.; Malavazi, I.; de Souza Bernardes, L.A.; Goldman, M.H.S.; de Vries, R.P.; de Castro Oliveira, J.V.; Goldman, G.H. Transcriptome analysis of Aspergillus niger grown on sugarcane bagasse. Biotechnol. Biofuels 2011, 4, 40. [Google Scholar] [CrossRef]
- Nogueira, K.M.V.; De Paula, R.G.; Antoniêto, A.C.C.; Dos Reis, T.F.; Carraro, C.B.; Silva, A.C.; Almeida, F.; Rechia, C.G.V.; Goldman, G.H.; Silva, R.N. Characterization of a novel sugar transporter involved in sugarcane bagasse degradation in Trichoderma reesei. Biotechnol. Biofuels 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Verbeke, J.; Coutinho, P.; Mathis, H.; Quenot, A.; Record, E.; Asther, M.; Heiss-Blanquet, S. Transcriptional profiling of cellulase and expansin-related genes in a hypercellulolytic Trichoderma reesei. Biotechnol. Lett. 2009, 31, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Farias, M.D.P.; Albuquerque, P.B.S.; Soares, P.A.G.; de Sá, D.M.A.T.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Xyloglucan from Hymenaea courbaril var. courbaril seeds as encapsulating agent of L-ascorbic acid. Int. J. Biol. Macromol. 2018, 107, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Alahuhta, M.; Adney, W.S.; Himmel, M.E.; Lunin, V.V. Structure of Acidothermus cellulolyticus family 74 glycoside hydrolase at 1.82Å resolution. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.; Stepper, J.; Davies, G.J.; Brumer, H. Functional and structural characterization of a potent GH74 endo-xyloglucanase from the soil saprophyte Cellvibrio japonicus unravels the first step of xyloglucan degradation. FEBS J. 2016, 283, 1701–1719. [Google Scholar] [CrossRef]
- Yaoi, K.; Kondo, H.; Hiyoshi, A.; Noro, N.; Sugimoto, H.; Tsuda, S.; Mitsuishi, Y.; Miyazaki, K. The structural basis for the Exo-mode of action in GH74 oligoxyloglucan reducing end-specific cellobiohydrolase. J. Mol. Biol. 2007, 370, 53–62. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER suite: Protein structure and function prediction. Nat. Methods 2014, 12, 7–8. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, D.C.B.; Carraro, C.B.; Silva, R.N.; de Paula, R.G. Molecular Characterization of Xyloglucanase cel74a from Trichoderma reesei. Int. J. Mol. Sci. 2021, 22, 4545. https://doi.org/10.3390/ijms22094545
Lopes DCB, Carraro CB, Silva RN, de Paula RG. Molecular Characterization of Xyloglucanase cel74a from Trichoderma reesei. International Journal of Molecular Sciences. 2021; 22(9):4545. https://doi.org/10.3390/ijms22094545
Chicago/Turabian StyleLopes, Douglas Christian Borges, Cláudia Batista Carraro, Roberto Nascimento Silva, and Renato Graciano de Paula. 2021. "Molecular Characterization of Xyloglucanase cel74a from Trichoderma reesei" International Journal of Molecular Sciences 22, no. 9: 4545. https://doi.org/10.3390/ijms22094545
APA StyleLopes, D. C. B., Carraro, C. B., Silva, R. N., & de Paula, R. G. (2021). Molecular Characterization of Xyloglucanase cel74a from Trichoderma reesei. International Journal of Molecular Sciences, 22(9), 4545. https://doi.org/10.3390/ijms22094545







