Amyloid Aggregates of Smooth-Muscle Titin Impair Cell Adhesion
Abstract
:1. Introduction
2. Results
2.1. SDS-PAGE and Mass Spectrometry of Purified Titin
2.2. Formation of SMT Aggregates and Confirmation of Their Amyloid Characteristics
2.3. Cytotoxicity Study of SMT Aggregates
2.4. Study of the Impact of SMT Aggregates on Actin Cytoskeleton
2.5. Atomic Force Microscopy Study of the Morphology and Nanostructure of Surfaces Coated with Titin Amyloid Aggregates
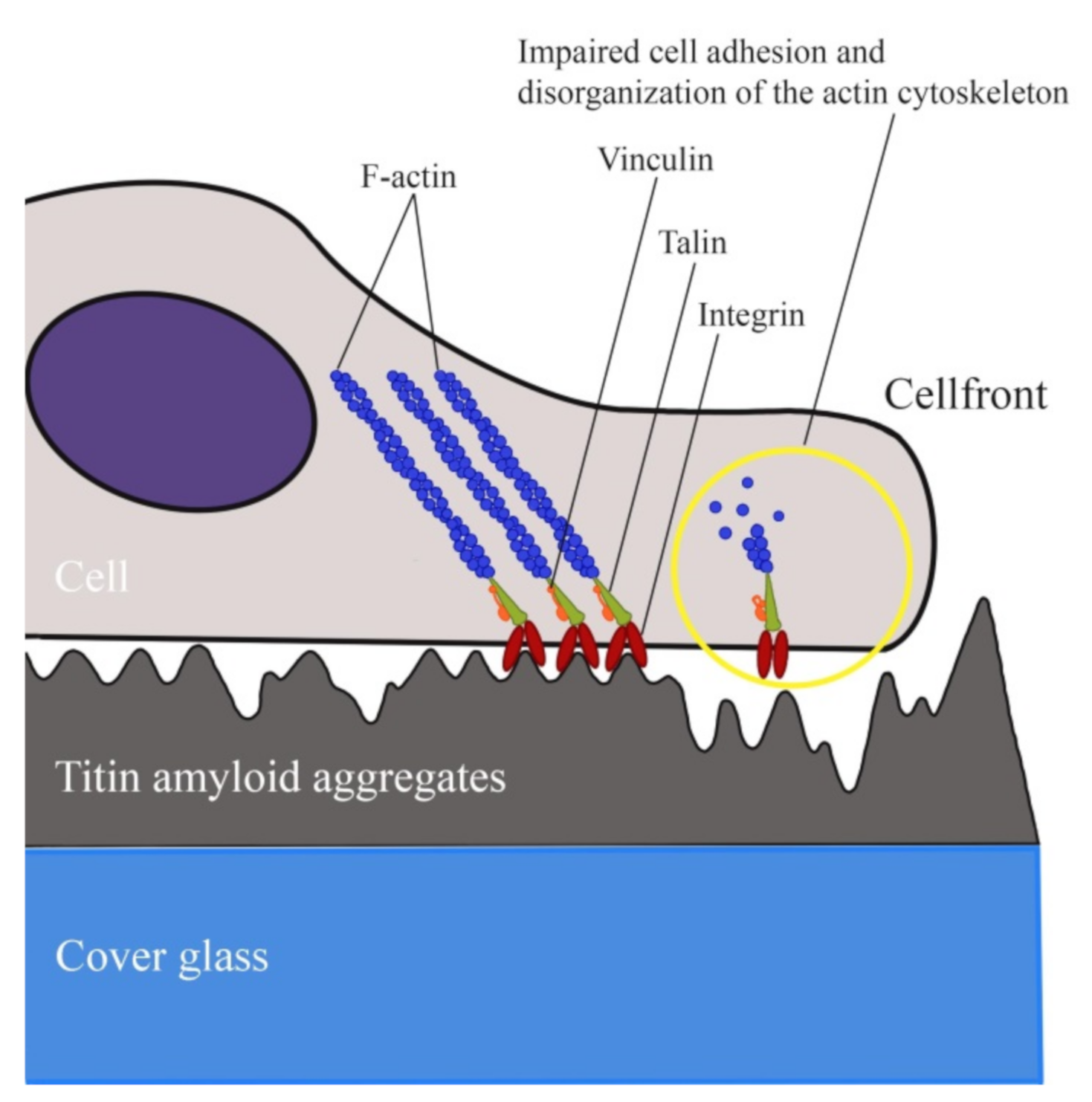
2.6. Study of the Effect of SMT-Aggregate-Coated Surface on Fibroblast Adhesion
3. Discussion
4. Materials and Methods
4.1. Purification of Chicken Gizzard SMT
4.2. SDS-PAGE and Mass Spectrometry Analysis of Titin
4.3. Isolation and Purification of Actin and Determination of the Concentration of Isolated Proteins
4.4. Formation of SMT Aggregates and F-Actin Fibrils
4.5. Fluorescence Analysis
4.6. Electron Microscopy
4.7. Cytotoxicity Assay
4.8. Confocal Microscopy
4.9. Study of the Effect Of Titin Amyloid Aggregates on Adhesion of Fibroblasts
4.10. Atomic Force Microscopy
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| FASP | filter-aided sample preparation |
| FBS | fetal bovine serum |
| FnIII | fibronectin III-like domain |
| HDF | human dermal fibroblasts |
| HPLC–MS | high performance liquid chromatography mass spectrometry |
| Ig | immunoglobulin-like domain |
| PMSF | phenylmethylsulfonyl fluoride |
| RAOSMCs | rat aortic smooth-muscle cells |
| RMS | root mean square |
| SMT | smooth-muscle titin |
| ThT | thioflavin T |
References
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, F.; Schymkowitz, J.; Itzhaki, L.S. Implications of 3D domain swapping for protein folding, misfolding and function. Adv. Exp. Med. Biol. 2012, 747, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 6, 384–396, Erratum in 2014, 15, 496. [Google Scholar] [CrossRef]
- Dobson, C.M. Experimental investigation of protein folding and misfolding. Methods 2004, 34, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.N.; Linke, R.P. A molecular history of the amyloidoses. J. Mol. Biol. 2012, 421, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 2004, 15, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Stefani, M.; Rigacci, S. Protein folding and aggregation into amyloid: The interference by natural phenolic compounds. Int. J. Mol. Sci. 2013, 14, 12411–12457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasansuklab, A.; Tencomnao, T. Amyloidosis in Alzheimer’s disease: The toxicity of Amyloid Beta (Aβ), mechanisms of its accumulation and implications of medicinal plants for therapy. Evid. Based Complement Alternat. Med. 2013, 2013, 413808. [Google Scholar] [CrossRef] [Green Version]
- Marshall, K.E.; Marchante, R.; Xue, W.F.; Serpell, L.C. The relationship between amyloid structure and cytotoxicity. Prion 2014, 8, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Bucciantini, M.; Giannoni, E.; Chiti, F.; Baroni, F.; Formigli, L.; Zurdo, J.; Taddei, N.; Ramponi, G.; Dobson, C.M.; Stefani, M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.S.; Liu, K.N.; Han, T.C. Amyloid fibrillation and cytotoxicity of insulin are inhibited by the amphiphilic surfactants. Biochim. Biophys. Acta 2010, 1802, 519–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetri, V.; Canale, C.; Relini, A.; Librizzi, F.; Militello, V.; Gliozzi, A.; Leone, M. Amyloid fibrils formation and amorphous aggregation in concanavalin A. Biophys. Chem. 2007, 125, 184–190. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Lin, Y.; Yagi, H.; Lee, Y.H.; Kitayama, H.; Sakurai, K.; So, M.; Ogi, H.; Naiki, H.; Goto, Y. Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14446–14451. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, N.; Sakae, Y.; Gouda, T.; Tsujimura, Y.; Okamoto, Y. Two major stable structures of amyloid-forming peptides: Amorphous aggregates and amyloid fibrils. Mol. Simul. 2017, 43, 1370–1376. [Google Scholar] [CrossRef]
- Wickner, S.; Maurizi, M.R.; Gottesman, S. Posttranslational quality control: Folding, refolding, and degrading proteins. Science 1999, 286, 1888–1893. [Google Scholar] [CrossRef]
- Elghetany, M.T.; Saleem, A. Methods for staining amyloid in tissues: A review. Stain Technol. 1988, 63, 201–212. [Google Scholar] [CrossRef]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.; Eisenberg, D. Recent atomic models of amyloid fibril structure. Curr. Opin. Struct. Biol. 2006, 16, 260–265. [Google Scholar] [CrossRef]
- Hashimoto, M.; Rockenstein, E.; Crews, L.; Masliah, E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromol. Med. 2003, 4, 21–36. [Google Scholar] [CrossRef]
- Qahwash, I.; Weiland, K.L.; Lu, Y.; Sarver, R.W.; Kletzien, R.F.; Yan, R. Identification of a mutant amyloid peptide that predominantly forms neurotoxic protofibrillar aggregates. J. Biol. Chem. 2003, 278, 23187–23195. [Google Scholar] [CrossRef] [Green Version]
- Sirangelo, I.; Malmo, C.; Iannuzzi, C.; Mezzogiorno, A.; Bianco, M.R.; Papa, M.; Irace, G. Fibrillogenesis and cytotoxic activity of the amyloid-forming apomyoglobin mutant W7FW14F. J. Biol. Chem. 2004, 279, 13183–13189. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.K.; Park, Y.W.; Shin, D.Y.; Mook-Jung, I.; Yoo, Y.J. Cytosolic amyloid-beta peptide 42 escaping from degradation induces cell death. Biochem. Biophys. Res. Commun. 2006, 344, 471–477. [Google Scholar] [CrossRef]
- Ghosh, S.; Pandey, N.K.; Banerjee, P.; Chaudhury, K.; Nagy, N.V.; Dasgupta, S. Copper(II) directs formation of toxic amorphous aggregates resulting in inhibition of hen egg white lysozyme fibrillation under alkaline salt-mediated conditions. J. Biomol. Struct. Dyn. 2015, 33, 991–1007. [Google Scholar] [CrossRef]
- Haass, C.; Mandelkow, E. Fyn-tau-amyloid: A toxic triad. Cell 2010, 142, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Kayed, R.; Lasagna-Reeves, C.A. Molecular mechanisms of amyloid oligomers toxicity. J. Alzheimer’s Dis. 2013, 33 (Suppl. 1), S67–S78. [Google Scholar] [CrossRef] [Green Version]
- Bobylev, A.G.; Kraevaya, O.A.; Bobyleva, L.G.; Khakina, E.A.; Fadeev, R.S.; Zhilenkov, A.V.; Mishchenko, D.V.; Penkov, N.V.; Teplov, I.Y.; Yakupova, E.I.; et al. Anti-amyloid activities of three different types of water-soluble fullerene derivatives. Colloids Surf. B Biointerfaces 2019, 183, 110426. [Google Scholar] [CrossRef]
- Freundt, J.K.; Linke, W.A. Titin as a force-generating muscle protein under regulatory control. J. Appl. Physiol. 2019, 126, 1474–1482. [Google Scholar] [CrossRef]
- Labeit, S.; Lahmers, S.; Burkart, C.; Fong, C.; McNabb, M.; Witt, S.; Witt, C.; Labeit, D.; Granzier, H. Expression of distinct classes of titin isoforms in striated and smooth muscles by alternative splicing, and their conserved interaction with filamins. J. Mol. Biol. 2006, 362, 664–681. [Google Scholar] [CrossRef]
- Vikhlyantsev, I.M.; Podlubnaya, Z.A. Nuances of electrophoresis study of titin/connectin. Biophys. Rev. 2017, 9, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorio, C.C.; Granzier, H.; Sorimachi, H.; Labeit, S. Muscle assembly: A titanic achievement? Curr. Opin. Cell Biol. 1999, 11, 18–25. [Google Scholar] [CrossRef]
- Vikhlyantsev, I.M.; Okuneva, A.D.; Shumilina, U.V.; Salmov, N.N.; Bobylev, A.G.; Molochkov, N.V.; Podlubnaya, Z.A. Method for isolation of intact titin (connectin) molecules from mammalian cardiac muscle. Biochemistry 2013, 78, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Keller, T.C., 3rd. Smitin, a novel smooth muscle titin-like protein, interacts with myosin filaments in vivo and in vitro. J. Cell Biol. 2002, 156, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UniProtkB–Q8WZ42 (TITIN_HUMAN). Available online: https://www.uniprot.org/uniprot/Q8WZ42 (accessed on 19 April 2021).
- Bobylev, A.G.; Galzitskaya, O.V.; Fadeev, R.S.; Bobyleva, L.G.; Yurshenas, D.A.; Molochkov, N.V.; Dovidchenko, N.V.; Selivanova, O.M.; Penkov, N.V.; Podlubnaya, Z.A.; et al. Smooth muscle titin forms in vitro amyloid aggregates. Biosci. Rep. 2016, 36, e00334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakupova, E.I.; Vikhlyantsev, I.M.; Bobyleva, L.G.; Penkov, N.V.; Timchenko, A.A.; Timchenko, M.A.; Enin, G.A.; Khutzian, S.S.; Selivanova, O.M.; Bobylev, A.G. Different amyloid aggregation of smooth muscles titin in vitro. J. Biomol. Struct. Dyn. 2018, 36, 2237–2248. [Google Scholar] [CrossRef]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, T.; Verkhovsky, A.B.; Svitkina, T.M.; Bershadsky, A.D.; Kozlov, M.M. Role of focal adhesions and mechanical stresses in the formation and progression of the lamellipodium-lamellum interface [corrected]. Biophys. J. 2009, 97, 1254–1264, Erratum in 2009, 97, 2115. [Google Scholar] [CrossRef] [Green Version]
- Hotulainen, P.; Lappalainen, P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006, 173, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Miners, J.S.; Kehoe, P.; Love, S. Neprilysin protects against cerebral amyloid angiopathy and Aβ-induced degeneration of cerebrovascular smooth muscle cells. Brain Pathol. 2011, 21, 594–605. [Google Scholar] [CrossRef]
- Bourassa, P.; Tremblay, C.; Schneider, J.A.; Bennett, D.A.; Calon, F. Beta-amyloid pathology in human brain microvessel extracts from the parietal cortex: Relation with cerebral amyloid angiopathy and Alzheimer’s disease. Acta Neuropathol. 2019, 137, 801–823. [Google Scholar] [CrossRef]
- Ruzali, W.A.; Kehoe, P.G.; Love, S. Influence of LRP-1 and apolipoprotein E on amyloid-β uptake and toxicity to cerebrovascular smooth muscle cells. J. Alzheimer’s Dis. 2013, 33, 95–110. [Google Scholar] [CrossRef]
- Blaise, R.; Mateo, V.; Rouxel, C.; Zaccarini, F.; Glorian, M.; Béréziat, G.; Golubkov, V.S.; Limon, I. Wild-type amyloid beta 1-40 peptide induces vascular smooth muscle cell death independently from matrix metalloprotease activity. Aging Cell 2012, 11, 384–393. [Google Scholar] [CrossRef]
- Ferrera, P.; Zepeda, A.; Arias, C. Nonsteroidal anti-inflammatory drugs attenuate amyloid-β protein-induced actin cytoskeletal reorganization through Rho signaling modulation. Cell. Mol. Neurobiol. 2017, 37, 1311–1318. [Google Scholar] [CrossRef]
- Wang, Y.W.; Ren, J.H.; Xia, K.; Wang, S.H.; Yin, T.F.; Xie, D.H.; Li, L.H. Effect of mitomycin on normal dermal fibroblast and HaCat cell: An in vitro study. J. Zhejiang Univ. Sci. B 2012, 13, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, S.G.; Brochhausen, C.; Negrão, R.; Barbosa, M.A.; Unger, R.E.; Kirkpatrick, C.J.; Soares, R.; Granja, P.L. Implanted neonatal human dermal fibroblasts influence the recruitment of endothelial cells in mice. Biomatter 2012, 2, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.; Fan, J.; Han, A.; Chen, M.; Woodley, D.T.; Li, W. Non-compensating roles between Nckalpha and Nckbeta in PDGF-BB signaling to promote human dermal fibroblast migration. J. Investig. Dermatol. 2009, 129, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Lih, E.; Oh, S.H.; Joung, Y.K.; Lee, J.H.; Han, D.K. Polymers for cell/tissue anti-adhesion. Prog. Polym. Sci. 2015, 44, 28–61. [Google Scholar] [CrossRef]
- Schakenraad, J.M.; Busscher, H.J. Cell polymer interactions—The influence of protein adsorption. Colloids Surf. 1989, 42, 331–343. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef]
- Czeslik, C.; Jackler, G.; Hazlett, T.; Gratton, E.; Steitz, R.; Wittemann, A.; Ballauff, M. Salt-induced protein resistance of polyelectrolyte brushes studied using fluorescence correlation spectroscopy and neutron reflectometry. Phys. Chem. Chem. Phys. 2004, 6, 5557–5563. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.F.; Li, L.Y.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zheng, Q.; Wang, Y.W.; Chen, H. Combining surface topography with polymer chemistry: Exploring new interfacial biological phenomena. Polym. Chem. 2014, 5, 14–24. [Google Scholar] [CrossRef]
- Vladkova, T.G. Surface engineered polymeric biomaterials with improved biocontact properties. Int. J. Polym. Sci. 2010, 2010, 296094. [Google Scholar] [CrossRef] [Green Version]
- Vagaská, B.; Bacáková, L.; Filová, E.; Balík, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2010, 59, 309–322. [Google Scholar] [CrossRef]
- Geiger, B.; Yamada, K.M. Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005033. [Google Scholar] [CrossRef] [Green Version]
- Sackmann, E.; Bruinsma, R.F. Cell adhesion as wetting transition? ChemPhysChem 2002, 3, 262–269. [Google Scholar] [CrossRef]
- Ventre, M.; Causa, F.; Netti, P.A. Determinants of cell-material crosstalk at the interface: Towards engineering of cell instructive materials. J. R. Soc. Interface 2012, 9, 2017–2032. [Google Scholar] [CrossRef]
- Carre, A.; Lacarriere, V. How substrate properties control cell adhesion. A physical–chemical approach. J. Adhes. Sci. Technol. 2010, 24, 815–830. [Google Scholar] [CrossRef]
- Schakenraad, J.M.; Kuit, J.H.; Arends, J.; Busscher, H.J.; Feijen, J.; Wildevuur, C.R. In vivo quantification of cell-polymer interactions. Biomaterials 1987, 8, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Dalsin, J.L.; Hu, B.H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Pompe, T.; Amin, I.; Luxenhofer, R.; Werner, C.; Jordan, R. Tailored poly(2-oxazoline) polymer brushes to control protein adsorption and cell adhesion. Macromol. Biosci. 2012, 12, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability influences cell behavior on superhydrophobic surfaces with different topographies. Biointerphases 2012, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulangara, K.; Leong, K.W. Substrate topography shapes cell function. Soft Matter 2009, 5, 4072–4076. [Google Scholar] [CrossRef]
- Pot, S.A.; Liliensiek, S.J.; Myrna, K.E.; Bentley, E.; Jester, J.V.; Nealey, P.F.; Murphy, C.J. Nanoscale topography-induced modulation of fundamental cell behaviors of rabbit corneal keratocytes, fibroblasts, and myofibroblasts. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Dang, Y.; Quan, M.; Xing, C.M.; Wang, Y.B.; Gong, Y.K. Biocompatible and antifouling coating of cell membrane phosphorylcholine and mussel catechol modified multi-arm PEGs. J. Mater. Chem. B 2015, 3, 2350–2361. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Clark, R.; Moses, R.L. Cell adhesion to biomaterials: Correlations between surface charge, surface roughness, adsorbed protein, and cell morphology. J. Long Term Eff. Med. Implant. 1995, 5, 209–231. [Google Scholar]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Fritz, J.D.; Swartz, D.R.; Greaser, M.L. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal. Biochem. 1989, 180, 205–210. [Google Scholar] [CrossRef]
- PubMed.gov. Available online: https://pubmed.ncbi.nlm.nih.gov/32103137/ (accessed on 19 April 2021).
- PubMed.gov. Available online: https://pubmed.ncbi.nlm.nih.gov/19377485/ (accessed on 19 April 2021).
- Pardee, J.D.; Spudich, J.A. Purification of muscle actin. Methods Enzymol. 1982, 85, 164–181. [Google Scholar] [CrossRef]
- Rees, M.K.; Young, M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J. Biol. Chem. 1967, 242, 4449–4458. [Google Scholar] [CrossRef]
- Trinick, J.; Knight, P.; Whiting, A. Purification and properties of native titin. J. Mol. Biol. 1984, 180, 331–356. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Nomizu, M.; Kim, W.H.; Yamamura, K.; Utani, A.; Song, S.Y.; Otaka, A.; Roller, P.P.; Kleinman, H.K.; Yamada, Y. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J. Biol. Chem. 1995, 270, 20583–20590. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobylev, A.G.; Fadeev, R.S.; Bobyleva, L.G.; Kobyakova, M.I.; Shlyapnikov, Y.M.; Popov, D.V.; Vikhlyantsev, I.M. Amyloid Aggregates of Smooth-Muscle Titin Impair Cell Adhesion. Int. J. Mol. Sci. 2021, 22, 4579. https://doi.org/10.3390/ijms22094579
Bobylev AG, Fadeev RS, Bobyleva LG, Kobyakova MI, Shlyapnikov YM, Popov DV, Vikhlyantsev IM. Amyloid Aggregates of Smooth-Muscle Titin Impair Cell Adhesion. International Journal of Molecular Sciences. 2021; 22(9):4579. https://doi.org/10.3390/ijms22094579
Chicago/Turabian StyleBobylev, Alexander G., Roman S. Fadeev, Liya G. Bobyleva, Margarita I. Kobyakova, Yuri M. Shlyapnikov, Daniil V. Popov, and Ivan M. Vikhlyantsev. 2021. "Amyloid Aggregates of Smooth-Muscle Titin Impair Cell Adhesion" International Journal of Molecular Sciences 22, no. 9: 4579. https://doi.org/10.3390/ijms22094579
APA StyleBobylev, A. G., Fadeev, R. S., Bobyleva, L. G., Kobyakova, M. I., Shlyapnikov, Y. M., Popov, D. V., & Vikhlyantsev, I. M. (2021). Amyloid Aggregates of Smooth-Muscle Titin Impair Cell Adhesion. International Journal of Molecular Sciences, 22(9), 4579. https://doi.org/10.3390/ijms22094579







