The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia
Abstract
:1. Introduction
2. Results
2.1. Serum Leptin Levels in the Yakut Population
2.2. Association between Leptin Levels and 14 SNP Markers of the 10 Genes, Potentially Related to Nonshivering Thermogenesis Processes
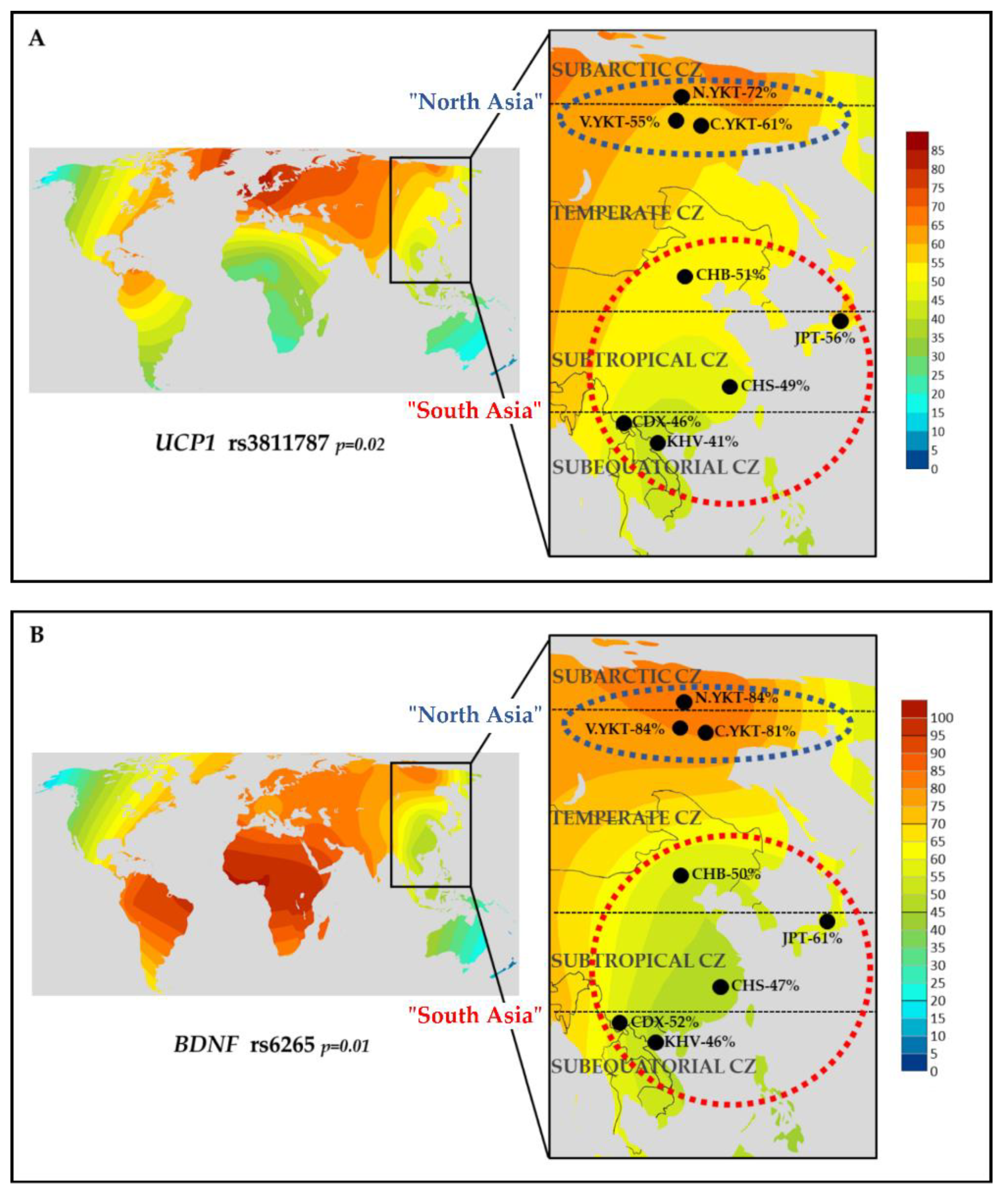
2.3. Search for Indicators of Natural Selection for Cold Climate Adaptation
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Anthropometric Measurements
4.3. Serum Leptin Analyses
4.4. DNA Analysis
4.5. Stratification of the Sample of Yakuts
4.6. The Search for Indicators of Natural Selection
4.7. Statistical Analysis
5. Conclusions
- (1)
- Our analyses showed the strong positive correlation of body mass index (BMI) and serum leptin levels in the Yakut population, both in females (r2 = 0.2152; p < 0.01) and in males (r2 = 0.1913; p < 0.01). Serum leptin was significantly higher in females (18.87 ± 12.31 ng/mL) than in males (6.56 ± 8.47 ng/mL). We found that the TT genotype of rs3811787 (UCP1) and the GG genotype of rs6265 (BDNF) were associated with the elevated leptin levels in females with a normal BMI (p < 0.05).
- (2)
- The rs3811787 (UCP1) and rs6265 (BDNF) were studied for possible indicators of natural selection towards cold climate adaptation. Among eight East Asian populations, the high prevalence of the T-allele of rs3811787 (UCP1) and the G-allele of rs6265 (BDNF) was found in populations living in subarctic and temperate climatic zones, in comparison with populations from subtropical and subequatorial climate (p < 0.05).
- (3)
- Subsequent analysis of worldwide data showed that the T-allele of rs3811787 (UCP1) gradient increases from the south to the north in Eurasia, along the shore of the Arctic Ocean, while the G-allele of rs6265 (BDNF) less strongly correlates with cold climates and is probably more related to other adaptation mechanisms. These results demonstrate the potential involvement of the UCP1 gene in the leptin-mediated thermoregulation mechanism, while the distribution of its allelic variants is probably related to human adaptation to the cold climate.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Zhang, F.; Basinski, M.B.; Beals, J.M.; Briggs, S.L.; Churgay, L.M.; Clawson, D.K.; Di Marchi, R.D.; Furman, T.C.; Hale, J.E.; Hsiung, H.M.; et al. Crystal Structure of the Obese Protein Ieptin-E100. Nature 1997, 387, 206–209. [Google Scholar] [CrossRef]
- Green, E.D.; Maffei, M.; Braden, V.V.; Proenca, R.; De Silva, U.; Zhang, Y.; Chua, S.C.; Leibel, R.L.; Weissenbach, J.; Friedman, J.M. The Human Obese (OB) Gene: RNA Expression Pattern and Mapping on the Physical, Cytogenetic, and Genetic Maps of Chromosome 7. Genome Res. 1995, 5, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef]
- Elmquist, J.K.; Elias, C.F.; Saper, C.B. From Lesions to Leptin: Hypothalamic Control of Food Intake and Body Weight. Neuron 1999, 22, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Baicy, K.; London, E.D.; Monterosso, J.; Wong, M.-L.; Delibasi, T.; Sharma, A.; Licinio, J. Leptin Replacement Alters Brain Response to Food Cues in Genetically Leptin-Deficient Adults. PNAS 2007, 104, 18276–18279. [Google Scholar] [CrossRef] [Green Version]
- Garfield, A.S.; Patterson, C.; Skora, S.; Gribble, F.M.; Reimann, F.; Evans, M.L.; Myers, M.G.; Heisler, L.K. Neurochemical Characterization of Body Weight-Regulating Leptin Receptor Neurons in the Nucleus of the Solitary Tract. Endocrinology. 2012, 153, 4600–4607. [Google Scholar] [CrossRef] [Green Version]
- Ahima, R.S.; Bjorbaek, C.; Osei, S.; Flier, J.S. Regulation of Neuronal and Glial Proteins by Leptin: Implications for Brain Development. Endocrinology. 1999, 140, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin Activates Anorexigenic POMC Neurons through a Neural Network in the Arcuate Nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.R.; Mayer, J. Imperfect Homeothermia in the Hereditary Obese-Hyperglycemic Syndrome of Mice. Am. J. Physiol. 1954, 177, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Joosten, H.F.; van der Kroon, P.H. Role of the Thyroid in the Development of the Obese-Hyperglycemic Syndrome in Mice (Ob Ob). Metabolism 1974, 23, 425–436. [Google Scholar] [CrossRef]
- Trayhurn, P.; Thurlby, P.L.; James, W.P. A Defective Response to Cold in the Obese (Obob) Mouse and the Obese Zucker (Fafa) Rat [Proceedings]. Proc. Nutr. Soc. 1976, 35, 133A. [Google Scholar]
- Rezai-Zadeh, K.; Münzberg, H. Integration of Sensory Information via Central Thermoregulatory Leptin Targets. Physiol. Behav. 2013, 121, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Halaas, J.; Gajiwala, K.; Maffei, M.; Cohen, S.; Chait, B.; Rabinowitz, D.; Lallone, R.; Burley, S.; Friedman, J. Weight-Reducing Effects of the Plasma Protein Encoded by the Obese Gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the Obese Gene Product on Body Weight Regulation in Ob/Ob Mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef]
- Campfield, L.; Smith, F.; Guisez, Y.; Devos, R.; Burn, P. Recombinant Mouse OB Protein: Evidence for a Peripheral Signal Linking Adiposity and Central Neural Networks. Science 1995, 269, 546–549. [Google Scholar] [CrossRef]
- Harris, R.B.; Zhou, J.; Redmann, S.M.; Smagin, G.N.; Smith, S.R.; Rodgers, E.; Zachwieja, J.J. A Leptin Dose-Response Study in Obese (Ob/Ob) and Lean (+/?) Mice. Endocrinology. 1998, 139, 8–19. [Google Scholar] [CrossRef]
- Fischer, A.W.; Cannon, B.; Nedergaard, J. Leptin: Is It Thermogenic? Endocr. Rev. 2020, 41. [Google Scholar] [CrossRef]
- Mackintosh, R.M.; Hirsch, J. The Effects of Leptin Administration in Non-Obese Human Subjects. Obes. Res. 2001, 9, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, I.S.; Keogh, J.M.; Kamath, S.; Jones, S.; Gibson, W.T.; Trussell, R.; Jebb, S.A.; Lip, G.Y.H.; O’Rahilly, S. Partial Leptin Deficiency and Human Adiposity. Nature 2001, 414, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Kaiyala, K.J.; Ogimoto, K.; Nelson, J.T.; Muta, K.; Morton, G.J. Physiological Role for Leptin in the Control of Thermal Conductance. Mol. Metab. 2016, 5, 892–902. [Google Scholar] [CrossRef]
- Kaiyala, K.J. Energy Homeostasis: Thermoregulation. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 1043–1052. ISBN 978-0-08-045046-9. [Google Scholar]
- Bjerregaard, P.; Dewailly, E.; Young, T.K.; Blanchet, C.; Hegele, R.A.; Ebbesson, S.E.O.; Risica, P.M.; Mulvad, G. Blood Pressure among the Inuit (Eskimo) Populations in the Arctic. Scand. J. Public Health. 2003, 31, 92–99. [Google Scholar] [CrossRef]
- Luginbuehl, I.; Bissonnette, B.; Davis, P.J. Thermoregulation: Physiology and perioperative disturbances. Smith’s Anesthesia for Infants and Children E-Book: Expert Consult Premium; Mosby: Maryland Heights, MO, USA, 2010; p. 157. ISBN 978-0-323-08169-6. [Google Scholar]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High Incidence of Metabolically Active Brown Adipose Tissue in Healthy Adult Humans: Effects of Cold Exposure and Adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomskiy, M.I.; Chinti, S.; Tikhonov, D.G.; Loskutova, K.S.; Isakov, E.A. Brown Adipose Tissue and Extremely Cold Climate. Yakut Med. J. 2015, 49, 44–45. [Google Scholar]
- Leonard, W.R.; Snodgrass, J.J.; Sorensen, M.V. Metabolic Adaptation in Indigenous Siberian Populations. Annu. Rev. Anthropol. 2005, 34, 451–471. [Google Scholar] [CrossRef] [Green Version]
- Shephard, R.J.; Lavallée, H. Effects of Enhanced Physical Education on Lung Volumes of Primary School Children. J. Sports Med. Phys. Fit. 1996, 36, 186–194. [Google Scholar]
- Snodgrass, J.J.; Leonard, W.R.; Sorensen, M.V.; Tarskaia, L.A.; Mosher, M.J. The Influence of Basal Metabolic Rate on Blood Pressure among Indigenous Siberians. Am. J. Phys. Anthropol. 2008, 137, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Romanova, A.N.; Klimova, T.M.; Egorova, A.G.; Kuzmina, A.A.; Malogulova, I.S.; Arkhipova, N.S. Prevalence and treatment of arterial hypertension in the native rural population of Yakutia. Yakut Med. J. 2019, 67, 6–9. [Google Scholar]
- Leonard, W.R.; Levy, S.B.; Tarskaia, L.A.; Klimova, T.M.; Fedorova, V.I.; Baltakhinova, M.E.; Krivoshapkin, V.G.; Snodgrass, J.J. Seasonal Variation in Basal Metabolic Rates among the Yakut (Sakha) of Northeastern Siberia. Am. J. Hum. Biol. 2014, 26, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Nikanorova, A.A.; Barashkov, N.A.; Nakhodkin, S.S.; Pshennikova, V.G.; Solovyev, A.V.; Romanov, G.P.; Kuzmina, S.S.; Sazonov, N.N.; Burtseva, T.E.; Odland, J.Ø.; et al. The Role of Leptin Levels in Adaptation to Cold Climates. Int. J. Environ. Res. Public Health. 2020, 17, 1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, L.; Pedersen, L.M.; Lillefosse, H.H.; Fjaere, E.; Bronstad, I.; Hao, Q.; Petersen, R.K.; Hallenborg, P.; Ma, T.; De Matteis, R.; et al. UCP1 Induction during Recruitment of Brown Adipocytes in White Adipose Tissue Is Dependent on Cyclooxygenase Activity. PLoS ONE 2010, 5, e11391. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Bomberg, E.; Billington, C.J.; Levine, A.S.; Kotz, C.M. Brain-Derived Neurotrophic Factor (BDNF) in the Hypothalamic Ventromedial Nucleus Increases Energy Expenditure. Brain Res. 2010, 1336, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Hancock, A.M.; Clark, V.J.; Qian, Y.; Di Rienzo, A. Population Genetic Analysis of the Uncoupling Proteins Supports a Role for UCP3 in Human Cold Resistance. Mol. Biol. Evol. 2011, 28, 601–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.C.; McAninch, E.A. The Role of Thyroid Hormone and Brown Adipose Tissue in Energy Homoeostasis. Lancet Diabetes Endocrinol. 2013, 1, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Irving, B.A.; Still, C.D.; Argyropoulos, G. Does IRISIN Have a BRITE Future as a Therapeutic Agent in Humans? Curr. Obes. Rep. 2014, 3, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, S.; Saito, M. A New Era in Brown Adipose Tissue Biology: Molecular Control of Brown Fat Development and Energy Homeostasis. Annu. Rev. Physiol. 2014, 76, 225–249. [Google Scholar] [CrossRef] [Green Version]
- Koksharova, E.O.; Mayorov, A.Y.; Shestakova, M.V.; Dedov, I.I. Metabolic characteristics and therapeutic potential of brown and “beige” adipose tissues. Diabetes Mellit. 2014, 17, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Sazzini, M.; Schiavo, G.; De Fanti, S.; Martelli, P.L.; Casadio, R.; Luiselli, D. Searching for Signatures of Cold Adaptations in Modern and Archaic Humans: Hints from the Brown Adipose Tissue Genes. Heredity 2014, 113, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Reynés, B.; García-Ruiz, E.; Oliver, P.; Palou, A. Gene Expression of Peripheral Blood Mononuclear Cells Is Affected by Cold Exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R824–R834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, C.Y.; Acuña, M.P.; Harb, Z. Sociogenetic gradient in the Chilean population. Rev. Med. Chil. 1987, 115, 295–299. [Google Scholar]
- Sonoda, S.; Arce-Gomez, B.; Satz, M.L.; Gorodezky, C.; Juarez, V.; Olivo, A.; Hayami, M. Ethnic report on native Americans in South America and Mexico. HLA 1991, 1, 685–688. [Google Scholar]
- Couillard, C.; Mauriège, P.; Prud’homme, D.; Nadeau, A.; Tremblay, A.; Bouchard, C.; Després, J.-P. Plasma Leptin Concentrations: Gender Differences and Associations with Metabolic Risk Factors for Cardiovascular Disease. Diabetologia 1997, 40, 1178–1184. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bravo, F.; Albala, C.; Santos, J.L.; Yañez, M.; Carrasco, E. Leptin Levels Distribution and Ethnic Background in Two Populations from Chile: Caucasian and Mapuche Groups. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.S.; Esparza, J.; Nicolson, M.; Bennett, P.H.; Schulz, L.O.; Valencia, M.E.; Ravussin, E. Is a Low Leptin Concentration, a Low Resting Metabolic Rate, or Both the Expression of the “Thrifty Genotype”? Results from Mexican Pima Indians. Am. J. Clin. Nutr. 1998, 68, 1053–1057. [Google Scholar] [CrossRef] [Green Version]
- Wauters, M.; Mertens, I.; Considine, R.; De Leeuw, I.; Van Gaal, L. Are Leptin Levels Dependent on Body Fat Distribution in Obese Men and Females? Eat Weight Disord. 1998, 3, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The Role of Falling Leptin Levels in the Neuroendocrine and Metabolic Adaptation to Short-Term Starvation in Healthy Men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef] [Green Version]
- Bribiescas, R.G. Serum Leptin Levels and Anthropometric Correlates in Ache Amerindians of Eastern Paraguay. Am. J. Phys. Anthropol. 2001, 115, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Nelson, D. Lehninger Principles of Biochemistry; Anybook Ltd: Lincoln, UK, 2000; Volume 5. ISBN 978157259 9314.
- Esteghamati, A.; Khalilzadeh, O.; Ashraf, H.; Zandieh, A.; Morteza, A.; Rashidi, A.; Meysamie, A.; Nakhjavani, M. Physical Activity Is Correlated with Serum Leptin Independent of Obesity: Results of the National Surveillance of Risk Factors of Noncommunicable Diseases in Iran (SuRFNCD-2007). Metabolism 2010, 59, 1730–1735. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating Irisin in Healthy, Young Individuals: Day-Night Rhythm, Effects of Food Intake and Exercise, and Associations with Gender, Physical Activity, Diet, and Body Composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen-Torvik, L.J.; Wassel, C.L.; Ding, J.; Carr, J.; Cushman, M.; Jenny, N.; Allison, M.A. Associations of Body Mass Index and Insulin Resistance with Leptin, Adiponectin, and the Leptin-to-Adiponectin Ratio across Ethnic Groups: The Multi-Ethnic Study of Atherosclerosis (MESA). Ann. Epidemiol. 2012, 22, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, D.D.; Marchau, L.A.M.; Reyes, J.L.; Castañeda, V.L.; Macías, M.S.; Vivas, J.G.; Asseff, I.L. Leptin Levels and Nutritional Status of Indigenous Tepehuán and Mestizo Subjects in Durango, Mexico. Dis. Markers. 2014, 974503. [Google Scholar] [CrossRef]
- MacIver, N.J.; Thomas, S.M.; Green, C.L.; Worley, G. Increased Leptin Levels Correlate with Thyroid Autoantibodies in Nonobese Males. Clin. Endocrinol. 2016, 85, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Koca, T.T.; Berk, E.; Seyithanoğlu, M.; Koçyiğit, B.F.; Demirel, A. Relationship of Leptin, Growth Hormone, and Insulin-like Growth Factor Levels with Body Mass Index and Disease Severity in Patients with Fibromyalgia Syndrome. Acta Neurol. Belg. 2020, 120, 595–599. [Google Scholar] [CrossRef]
- Garlid, K.D.; Orosz, D.E.; Modrianský, M.; Vassanelli, S.; Jezek, P. On the Mechanism of Fatty Acid-Induced Proton Transport by Mitochondrial Uncoupling Protein. J. Biol. Chem. 1996, 271, 2615–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commins, S.P.; Watson, P.M.; Levin, N.; Beiler, R.J.; Gettys, T.W. Central Leptin Regulates the UCP1 and Ob Genes in Brown and White Adipose Tissue via Different Beta-Adrenoceptor Subtypes. J. Biol. Chem. 2000, 275, 33059–33067. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A Leptin–BDNF Pathway Regulating Sympathetic Innervation of Adipose Tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef]
- Elias, C.F.; Aschkenasi, C.; Lee, C.; Kelly, J.; Ahima, R.S.; Bjorbaek, C.; Flier, J.S.; Saper, C.B.; Elmquist, J.K. Leptin Differentially Regulates NPY and POMC Neurons Projecting to the Lateral Hypothalamic Area. Neuron 1999, 23, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Kindblom, J.M.; Ohlsson, C.; Ljunggren, Ö.; Karlsson, M.K.; Tivesten, Å.; Smith, U.; Mellström, D. Plasma Osteocalcin Is Inversely Related to Fat Mass and Plasma Glucose in Elderly Swedish Men. J. Bone Miner. Res. 2009, 24, 785–791. [Google Scholar] [CrossRef]
- Andersson, J.; Karpe, F.; Sjöström, L.-G.; Riklund, K.; Söderberg, S.; Olsson, T. Association of Adipose Tissue Blood Flow with Fat Depot Sizes and Adipokines in Females. Int. J. Obes. 2012, 36, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Suyila, Q.; Cui, H.; Yang, L.; Zhao, L.; Zhang, R.; Su, X. Serum Leptin Concentrations in Mongolian Females. Obes. Res. Clin. Pract. 2013, 7, e75–e80. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central Neural Regulation of Brown Adipose Tissue Thermogenesis and Energy Expenditure. Cell Metab. 2014, 19, 741–756. [Google Scholar] [CrossRef] [Green Version]
- Efremova, A.; Senzacqua, M.; Venema, W.; Isakov, E.; Di Vincenzo, A.; Zingaretti, M.C.; Protasoni, M.; Thomski, M.; Giordano, A.; Cinti, S. A Large Proportion of Mediastinal and Perirenal Visceral Fat of Siberian Adult People Is Formed by UCP1 Immunoreactive Multilocular and Paucilocular Adipocytes. J. Physiol. Biochem. 2019, 2, 185–192. [Google Scholar] [CrossRef]
- International Obesity Task Force. Obesity: Managing the Global Epidemic: Report of the World Health Organization (WHO) Consultation; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]



| Characteristics | Underweight (n = 37) | p 1 | Normal Weight (n = 215) | p2 | Overweight/Obese (n = 29) | p 1 | |||
|---|---|---|---|---|---|---|---|---|---|
| F (n = 26) | M (n = 11) | F (n = 144) | M (n = 71) | F (n = 16) | M (n = 13) | ||||
| Age (years) | 19.12 ± 1.45 | 18.91 ± 0.83 | 0.84 | 19.81 ± 2.1 | 20.1 ± 2.09 | 0.33 | 20.56 ± 2.37 | 20.38 ± 1.61 | 0.97 |
| Weight (kg) | 44.88 ± 3.64 | 50.45 ± 3.42 | 0.01 | 55.53 ± 5.8 | 66.11 ± 7.41 | 0.01 | 72.75 ± 11.13 | 81.46 ± 8.3 | 0.01 |
| Height (cm) | 160.23 ± 5.04 | 170.36 ± 5.89 | 0.01 | 160.92 ± 6.03 | 173.39 ± 5.96 | 0.01 | 162.19 ± 4.96 | 174.69 ± 6.64 | 0.01 |
| BMI (kg/m2) | 17.46 ± 0.72 | 17.39 ± 0.91 | 0.87 | 21.42 ± 1.62 | 21.96 ± 1.9 | 0.03 | 27.56 ± 2.88 | 26.64 ± 1.49 | 0.54 |
| Leptin (ng/mL) | 13.35 ± 8.2 | 5.64 ± 1.58 | 0.0006 | 18.18 ± 10.96 | 4.87 ± 5.5 | 0.0001 | 34 ± 17.59 | 16.62 ± 16.01 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikanorova, A.A.; Barashkov, N.A.; Pshennikova, V.G.; Nakhodkin, S.S.; Gotovtsev, N.N.; Romanov, G.P.; Solovyev, A.V.; Kuzmina, S.S.; Sazonov, N.N.; Fedorova, S.A. The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia. Int. J. Mol. Sci. 2021, 22, 4657. https://doi.org/10.3390/ijms22094657
Nikanorova AA, Barashkov NA, Pshennikova VG, Nakhodkin SS, Gotovtsev NN, Romanov GP, Solovyev AV, Kuzmina SS, Sazonov NN, Fedorova SA. The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia. International Journal of Molecular Sciences. 2021; 22(9):4657. https://doi.org/10.3390/ijms22094657
Chicago/Turabian StyleNikanorova, Alena A., Nikolay A. Barashkov, Vera G. Pshennikova, Sergey S. Nakhodkin, Nyurgun N. Gotovtsev, Georgii P. Romanov, Aisen V. Solovyev, Sargylana S. Kuzmina, Nikolay N. Sazonov, and Sardana A. Fedorova. 2021. "The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia" International Journal of Molecular Sciences 22, no. 9: 4657. https://doi.org/10.3390/ijms22094657
APA StyleNikanorova, A. A., Barashkov, N. A., Pshennikova, V. G., Nakhodkin, S. S., Gotovtsev, N. N., Romanov, G. P., Solovyev, A. V., Kuzmina, S. S., Sazonov, N. N., & Fedorova, S. A. (2021). The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia. International Journal of Molecular Sciences, 22(9), 4657. https://doi.org/10.3390/ijms22094657






